Abstract
Pancreatic cancer (PC) is the 5th leading cause of cancer related death in the developed world with more than 260,000 deaths annually worldwide and with a dismal 5-year survival. Surgery is the only potential hope of cure for PC, but, unfortunately, only 20% PC patients is resectable at the time of diagnosis.
Therapeutic research efforts have mainly focused on improvements in radio/ chemo treatments and to date, there are only a few chemotherapeutic agents that have shown to be effective against PC, including gemcitabine with or without abraxane as well as a combination of 5-FU, leucovorin, oxaliplatin and irinotecan (the so-called FOLFIRINOX regimen). The survival of patients treated with these regimens is marginal and hence we are in urgent need of novel therapeutic approaches to treat pancreatic cancer. The success of immunotherapeutic strategies in other cancers and various evidences that pancreatic adenocarcinoma elicits antitumor immune responses, suggest that immunotherapies can be a promising alternative treatment modality for this deadly disease.
PC immunotherapy treatments include passive immunotherapeutic approaches, such as the use of effector cells generated in vitro, and active immunotherapeutic strategies, which goal is to stimulate an antitumor response in vivo, by means of vaccination.
In this review, we describe the immune suppressive mechanisms of pancreatic cancer and discuss recent preclinical and clinical efforts toward PC immunotherapy, including passive approaches, such as the use of antibodies and active strategies (vaccination), with a special mention of most recent treatment with CRS-207 and GVAX.
Abbreviations
| PC | = | pancreatic cancer |
| CTL | = | Cytotoxic CD8 T cells |
| Th | = | T helper |
| DCs | = | Dendritic Cells |
| NK | = | Natural Killer |
| Tregs | = | Regulatory T cells |
| APC | = | Antigen Presenting Cells |
| mAbs | = | monoclonal antibodies |
| IDO | = | Indoleamine 2,3-dioxygenase |
| MUC1 | = | Mucin-1 |
| CEA | = | carcinoembryonic antigen |
| ENO1 | = | a-Enolasi |
Introduction
Pancreatic cancer (PC) is the 5th leading cause of cancer related death in the developed world with more than 260,000 deaths annually worldwideCitation1 and with a dismal 5-year survival (5%). Surgery is the only potential hope of cure for PC patients. Advantageous tumor characteristics and complete tumor resection are the factors most relevant for a positive prognosis, so detection of pre-malignant or early invasive lesions, combined with safe and oncologically adequate surgery, is an important goal but, unfortunately, only 20% PC patients is resectable at the time of diagnosis.
The PC lethality is due to its aggressive nature and its tendency to remain asymptomatic until the tumor is fairly advanced, limiting the likelihood of early diagnosis. At the time of initial presentation, most pancreatic cancers are locally advanced or metastatic and need multimodal therapy.Citation2 The prognosis for these patients is poor, with overall survival being measured in months.
Therapeutic research efforts have mainly focused on improvements in radio/ chemo treatments and to date, there are only a few chemotherapeutic agents that have shown to be effective against PC, including gemcitabine with or without abraxaneCitation3 as well as a combination of 5-FU, leucovorin, oxaliplatin and irinotecan (the so-called FOLFIRINOX regimen).Citation4,5 The survival of patients treated with these regimens is marginal and hence we are in urgent need of novel therapeutic approaches to treat pancreatic cancer.
Both innate and specific immune response are active against human cancersCitation6 and several studies have shown the significant impact of the immune system on cancer progression. The effective anticancer function of the immune system requires cytotoxic CD8 T cells (CTL), T helper-1 (Th1) cells, mature dendritic cells (DCs), activated pro-inflammatory macrophages (M1) and NK (Natural Killer) cells. However, cancer cells induce local and systemic immune dysfunction thus avoiding detection by the immune system.Citation7 The modulating mechanisms used by the cancer cells are essentially 3: contact dependent factors (expression of immune system checkpoint ligands such as PD-L1), secretion of soluble immunosuppressive factors (such as IL-,Citation10 TGF-b and VEGF) and interference with MHC class I peptide presentation (through down-regulation of MHC class I expression or disabling of the antigen degradation or antigen insertion into the MHC class I grove).
The success of immunotherapeutic strategies in other cancers and various evidences that pancreatic adenocarcinoma evokes antitumor immune responses,Citation8,9 allow us to assert that immunotherapies can be a promising alternative treatment modality for this deadly disease. PC patients generate B and T cells specific to antigens expressed on pancreatic tumor cells, such as Wilms’ tumor gene 1 (WT1) (75%),Citation10 mucin 1 (MUC1) (over 85%),Citation11 human telomerase reverse transcriptase (hTERT) (88%),Citation12 mutated K-RAS (73%),Citation13 survivin (77%),Citation14 carcinoembryonic antigen (CEA) (over 90%),Citation15 HER-2/neu (61.2%),Citation16 p53 (67%),Citation17 and α-enolase.Citation18 Furthermore, the analysis of immune infiltrates in human tumors has demonstrated a positive correlation between prognosis and the presence of humoral response to pancreatic antigens (MUC-1 and mesothelin)Citation19 or of tumor-infiltrating T cells.Citation20
To date, PC immunotherapy treatments include passive immunotherapeutic approaches, such as the use of antibodies or effector cells generated in vitro, and active immunotherapeutic strategies, which goal is to stimulate an antitumor response in vivo, by means of vaccination. But for the effectiveness of immunotherapeutic treatments is essential to overcome 2 big obstacles: i) finding specific markers for pancreatic cancer cells and ii) mitigate the immune suppressive effects of tumor cells.
In this review, we describe the immune suppressive mechanisms of pancreatic cancer and discuss recent preclinical and clinical efforts toward PC immunotherapy, including passive approaches, such as the use of antibodies and active strategies (vaccination), with a special mention of most recent treatment with CRS-207 and GVAX ().
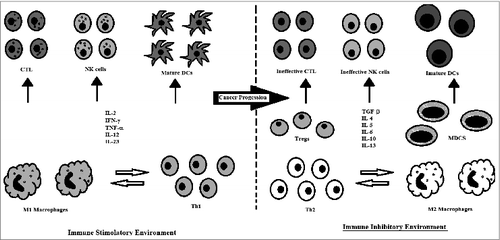
Immune Cells With Pro-cancer Activity
Different type of innate and specific immune response can support pancreatic cancer development, promoting an immunosuppressive and anti-inflammatory environment ().
Myeloid derived suppressor cells (MDSCs) are immature myeloid cells that suppress both innate and adaptive immunity with various mechanisms such as the sequestration of cysteine (essential for T cell activation) or expression of high levels of arginase (with depletion of L-arginine, necessary for protein synthesis by T cells).Citation21 Other MDSCs's strategies include TGF-b secretion increased production of reactive oxygen species and impairment of T cell homing to lymph nodes. These factors promote the development of regulatory T cells (Tregs) and inhibit the function of effector T cells and NK cells. In PC patients increased levels of circulating MDSC is an independent poor prognostic factorCitation22 and MDSCs levels correlate with levels of the Th2 cytokine IL-13 and Treg cell numbers. Also, in a mouse model the progression from premalignant lesions to PC is associated with increased degree of immune suppression of MDSCs.Citation23
The presence of tumor associated macrophages (TAMs) is associated with worse prognosis in multiple cancers. Macrophages, due to stimuli from the tumor microenvironment, especially IL-10 and TGF-b, switch their differentiation from M1 (pro-inflammatory) with anticancer role) to M2 (anti-inflammatory) with pro-tumor properties, such as angiogenesis promotion, matrix remodeling and tumor metastasis, as well as suppression of specific immune response.Citation24,25 TAMs interact with the immune system by multiple mechanisms such as through secretion of IL-10 and TGF-b, or by expression of immune inhibitory ligands such as PD-L1. In pancreatic cancer TAMs are significantly increased in tumor tissueCitation26 and the M2 presence is associated with worse prognosis in PC patients.Citation27
The number of Tregs is very high in the tumor microenvironment. In physiological status, by expression of CTLA-4 and secretion of IL-10 and TGF-b, among others, Tregs suppress exaggerated immune responses and have a key role in the prevention of auto-immune diseases. In cancer, however, Tregs produce a local immunosuppressive environment that favors the tumor progression.Citation28,29
PC patients have increased numbers of Tregs both in the circulationCitation30 and at the tumor site.Citation31 Moreover, the presence of Tregs at the tumor site correlates with more advanced presentation of diseaseCitation30,32 and a worse survival after resection,Citation33 while the low Treg percentage of the circulation one year post resection correlates with improved survival.Citation33
Dysfunctional Immune Effector Cells in Cancer
Usually the cancer cells use varied mechanisms to provoke an altered function of immune cells directly involved in the effective anti-cancer response ().
The dendritic cells play a critical role in the anti-tumor response because are the most professional APCs (antigen-presenting cells), having a very efficient machinery to internalize antigens, degrade them into peptides and present them on both MHC class I and II molecules to CD4+ and CD8+ T cells respectively. They can prime tumor specific effector T cells to start attacking cancer cells. However, due to the immunosuppressive tumor microenvironment their maturation and survival in cancer is significantly impaired. In PC patients while the presence of peripheral or intra-tumoral DCs is associated with prolonged survival,Citation34 DCs also display maturation defects,Citation35 suggesting that therapy aimed at improving DC functionality could be beneficial.
The NK cells represent a first line of defense against pathogens and tumor cells. The activation of NK cells is regulated by the integration of signals deriving from activating and inhibitory receptors expressed on their surface.
Through MHC class I loss, which is a cancer common event, pancreatic cancer cells become the target of NK cells.Citation36 However, pancreatic cancer cells can escape control of this system. Indeed, NK cell activity is diminished in patients with pancreatic cancer.Citation37 Also, activating receptors, such as NKG2D, which are necessary for the NK activation, are reduced in NK cells and reduced levels are associated with advanced PC disease.Citation38 On the other hand, higher absolute levels of NK cells in the circulation are associated with enhanced survival,Citation39 indicating that the immune system, through NK cells, still exerts some control on cancer growth despite disease progression.
In general both the number and function of CTL and Th cells is known to be affected in various cancers. In general, the presence of increased numbers of cytotoxic T cells in the tumor is associated with a better prognosis, while both CTL and Th cells are functionally impaired under the influence of immunosuppressive cytokines, leading to predominately Th2 (tumor tolerating) rather than Th1 (tumor killing) responses.
In PC patients, effector CTL can be detected as in the circulation as in the bone marrow,Citation40 while the presence of both CD8+ and CD4+ T cells in the tumor correlates with better prognosis.Citation41 In addition, when pancreatic lesions progress from premalignant to malignant, CTL decrease in number while the presence of Tregs is increased.Citation32 At the same time, circulating CTL and Th cells from PC patients have impaired function while Th2, rather than Th1, responses predominate.Citation42,43
Immunosuppresive Mechanisms of PC Cells
T cells are activated by a complex interaction of ligands and receptors. Specifically, APCs present antigenic peptides on MHC molecules to the T cell receptor (TCR). Binding of the MHC molecule to the TCR is not sufficient to initiate T cell activation, which requires additional ligand binding to co-stimulatory receptors (CD28, CD40, OX40, and 4–1BB). Activated CD4+T cells express CD40-ligand, which activates APC via ligation of CD40, thus forming a stimulatory loop between APC and T cells. On the contrary, receptors such as CTLA-4 and programmed death 1 (PD-1) expressed on the surface of activated T cells inhibit T cell activation upon binding to their ligands CD80/CD86 and PD-L1/PD-L2 respectively. In cancer, this mechanism of immune co-stimulation and co-inhibition is extremely important.Citation44
PC cells express a number of ligands that are meant to inactivate cytotoxic T-cells in the local tumor microenvironment. For example the ligand for PD-1 (PD-L1) is expressed by PC cells and its expression is associated with reduced cytotoxic T-cell infiltration, advanced stage of disease and poor prognosis.Citation45,46
In addition, PC cells express both CD40 and CD40L resulting in the secretion of several pro- and anti-inflammatory cytokines in the tumor microenvironment.Citation47 High expression of CD40L on tumor cells has been associated with good prognosis in pancreatic cancer patients.Citation47 Other immune inhibitory ligands with a role in pancreatic cancer are B7-H3 and B7-H4, the receptors of which are still unknown. The expression, for example, of the immune inhibitory ligand B7-H3 has been associated with improved prognosis in PC patients,Citation48 while blockade of the B7-H3 ligand interaction leads to tumor shrinkage in animal models.Citation49 Even the direct expression of FoxP3 by PC cells, mimicking thus Tregs, has been detected.Citation50
Direct secretion of immunosuppressive factors by PC cells is another mechanism of escaping the immune system. TGF-b induces tumors to secrete VEGF and matrix metaloprotein-2 which are associated with advanced stage of disease and metastasis.Citation51,50 Tumor derived TGF-b and IL-10 inhibit the development of Th1 responses, whereas they promote Th2 responses.Citation52 In general, secretion of multiple cytokines by PC cells contributes to the general immunosuppressive micro-environment of pancreatic cancer by switching the balance from a Th1 to a Th2 state.Citation53
Indoleamine 2,3-dioxygenase (IDO) is an enzyme upregulated in PC cells that catabolizes tryptophan into kynurenin. The kynurenin accumulation in the tumor microenvironment, inhibits T cell activation and stimulates Treg differentiation.Citation54 In other words, the PC expression of IDO attracts Tregs to the tumor microenvironmentCitation55 and inhibitors of IDO are already under phase I investigation.Citation56
Galectins are soluble immunomodulating glycoproteins that are involved in T-cell homeostasis, preservation of fetal-maternal tolerance and suppression of autoimmunity. In cancer, galectins have been shown to contribute to the immunosuppressive tumor microenvironment and evasion of immune responses. The best studied galectins in cancer immunomodulation are Gal-1, Gal-3, and Gal-9. In particular, Gal-1, is known to promote a Th2 cytokine profile in cancer, induce the IL-10 production in Tregs and is important in immune cell trafficking and DC physiology.Citation57 In PC Gal-1 is overexpressed by tumor cellsCitation58 and has been identified as a proteomic biomarker highly correlated with the stage of disease.Citation59
While Gal-9, partly through its complex interaction with TIM-3, modulates T cell, NK cell and MDSC activityCitation60 but the specific role in PC has not yet been investigated. Another important immune modulator galectin is Gal-3,Citation61 that is overexpressed in PC cells, secreted in the serum of PC patients and is associated with tumor differentiation.Citation62,63 However, not much is known about the role of Gal-3 as an immune modulator in pancreatic cancer.
Passive Immunotherapy: the Role of Monoclonal Antibodies
Antibodies can target antigens differentially expressed in tumor cells (tumor associated antigens, TAAs) or can be used to block molecules involved in cancer progression or angiogenesis. The immunoglobulins can invoke tumor cell death by blocking ligand-receptor growth and survival pathways. In addition, innate immune effector mechanisms: antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cytotoxicity (CMC), and antibody-dependent cellular phagocytosis (ADCP) - are emerging as equally important.Citation64
Although unconjugated antibodies have had efficacy, molecular genetics and chemical modifications to monoclonal antibodies (mAbs) have advanced their clinical utility. For example, modification of immune effector engagement has improved pharmacokinetic profiles, and conjugating cytotoxic agents to mAbs has enhanced targeted therapeutic delivery to tumors. The increasing facility of antibody modifications has made it possible to construct diverse and efficacious mAb-based therapies.
The humoral immune response to mesothelin (detected in 90–100% of pancreatic adenocarcinomas), has been found to be a favorable prognostic factor for pancreatic cancer.Citation65,66 Different antibodies to mesothelin have been studied and in particular SS1P, a murine single-chain Fv, specific for human mesothelin, which has been fused to PE38, a 38kDa portion of Pseudomonas exotoxin A (PE-A), that inhibits protein synthesis and results in apoptosis.Citation67 In Phase I clinical studies SS1P was found to be well tolerated, with self-limiting pleuritis as the dose-limiting toxicity. Also, the administration of a version of SS1P with releasable PEGylation resulted in complete regression of a mesothelin-expressing human carcinoma in mice with only a single dose.Citation67,68,69 MORAb-009, a monoclonal antibody against mesothelin is being tested in a phase I trial of 11 patients (3 with pancreatic cancer).Citation70 One of them who had previously progressed on gemcitabine showed disease stabilization and a drop in CA19–9 (carbohydrate antigen 19–9). Two fully human, antihuman mesothelin antibodies, M912 and HN1, have been developed, which bind mesothelin-positive cells and result in their lysis via ADCC.Citation71,72 Similar to SS1P, HN1 has been fused to truncated PE-A immunotoxin, although its binding site on mesothelin probably binds a distinct but overlapping epitope to that of SS1P.Citation72
MUC1 (mucin-1, CD227) is a polymorphic, glycosylated type I transmembrane protein presents on glandular epithelium of different tissues (pancreas, breast, lung) and over-expressed (aberrantly glycosylated) in 90% of pancreatic cancers.Citation73,74 It inhibits cell-cell and cell-stroma interactions and functions as a signal transducer in the cancer progression, including tumor invasion and metastasis.Citation75 Downregulation of MUC1 expression in human PC cell line S2–013 significantly decreased proliferation in vitro and in nude mice .Citation76 In a murine model, the use of MUC1-specific 90Yttrium-labeled moAb PAM4 in combination with gemcitabineCitation77 increased inhibition of tumor growth and prolonged animal survival. To date, it is undergoing phase I trial for stage III or IV PC patients.
In vitro study showed that 213Bi-C595 was specifically cytotoxic to MUC1-expressing PC cells in a concentration-dependent manner compared to controls. 213Bi-C595 is a moAb targeting the protein core of MUC1, conjugated with the α-particle-emitting 213bismuth.Citation74
PankoMab™ (Glycotope, Germany) is a murine anti-human MUC-1 antibody that binds to a carbohydrate induced conformational tumor epitope of MUC-1, greatly increasing its tumor specificity.Citation78 PankoMab can induce ADCC of MUC-1 positive cells and can also induce death following internalization by inhibition of RNA polymerase when linked to β-amanitin. The humanized version of PankoMab has been shown to react to the tumor expressed MUC-1 in multiple human carcinomas, although no clinical trials have been published.Citation79
The epidermal growth factor receptor 2 (HER2), is overexpress in up to 45% of pancreatic cancer. An anti-Her-2/neu antibody, known as Herceptin® (Genetech Inc., CA, USA) or trastuzumab, has been used with some success to treat PC murine models. Treatments with trastuzumab prolonged survival and reduced liver metastasis in nude mice orthotopically challenged with human pancreatic tumor cell lines that expressed Her-2/neu at low levels. The pancreatic lines were sensitive to ADCC lysis by trastuzumab in vitro.Citation80 Similar results were found when nude mice (challenged with Her-2/neu high expressing human PC cell lines) were treated with both trastuzumab and 5-fluorouracil.Citation81 The combination of treatments significantly inhibited tumor growth compared with either treatment alone. When combined with matuzumab, an anti-EGFR antibody, trastuzumab treatment resulted in inhibited PC growth in a nude mouse .Citation82 Also, this combined treatment was more effective than treatment with either antibody alone or combined with gemcitabine.Citation83
Carcinoembryonic antigen (CEA) is frequently overexpressed in various types of human cancers. Many anti-CEA antibodies have been used for immunotherapy, such as hMN-14 (labetuzumab), which has been shown to induce ADCC in vitro with CEA+ colon tumor cells and inhibited growth of lung metastases in nude mice.Citation84 A Phase I/II trial with hMN-14 in PC patients has been completed, but the results have not been published.Citation85
EGFR is a transmembrane glycoprotein receptor, over-expressed in 90% of pancreatic tumors,Citation86 where induces tumor cell proliferation and neovascularization; also his expression is associated with worse prognosis.Citation87,88 Blocking EGFR signaling decreases growth and metastasis of pancreatic tumor in animal models and enhance the effects of gemcitabine.Citation89,90
Cetuximab (Erbitux or IMC-C225) is a chimeric monoclonal antibody generated from the fusion of the variable region of the murine anti-EGFR monoclonal antibody M225 and the human IgG1 constant region. Promising laboratory results have led cetuximab to be tested in clinical trials. A phase III randomized study by the Southwestern Oncology Group (SWOG) tested the efficacy of cetuximab and gemcitabine combination in patients with advanced PC. The median survival was 6 months in the gemcitabine arm and 6.5 months in the combination arm for an overall hazard ratio (HR) of 1.09 (P = 0. Fourteen). The corresponding progression free survival was 3 months and 3.5 months, respectively. The study failed to demonstrate a clinically significant advantage of the addition of cetuximab to gemcitabine.Citation91 In an ongoing phase II trial with the trimodal therapy of cetuximab, gemcitabine and intensity modulated radiotherapy (IMRT) for patients with advanced PC, there was no increase in toxicity profile.Citation92 One-year survival was 57%, while median survival has not been reached.
Matuzumab (EMD72000) is a humanized IgG1 monoclonal antibody to the human EGFR. Laboratory studies have shown promising inhibitory effects on tumor growth and angiogenesis, including L3.6pl in an orthotopic rat model.Citation93 In a phase I study of combined treatment with matuzumab and gemcitabine, 8 out of 12 patients with advanced pancreatic adenocarcinoma showed partial response or stable disease.Citation94
Vascular endothelial growth factor (VEGF) plays a pivotal role in the control of angiogenesis, tumor growth and metastasis.Citation95 VEGF and its receptors are overexpressed in the PC and have been demonstrated to be a poor prognostic factor. There is a suggestion that elevated serum VEGF levels correlate with tumor stage, disease recurrence and survival.Citation96 Development of therapeutic strategies directed toward the VEGF mediated signaling axis has been extensively tested in patients with advanced PC.
Bevacizumab (Avastin) is a recombinant humanized anti-VEGF monoclonal antibody. A pilot study demonstrated that bevacizumab, when added to gemcitabine in patients with metastatic PC resulted in a significant improvement in response, survival, and progression-free survival.Citation97 This was immediately followed by a phase III trial by CALGB comparing gemcitabine plus bevacizumab to gemcitabine plus placebo and showed no benefit for the bevacizumab addition.Citation98 The AviTa phase III trial that examined treatment with gemcitabine plus erlotinib with either bevacizumab or placebo, has been closed. Bevacizumab however, may have a role in palliative treatment of chemotherapy-resistant PC. In a case report, a patient with stage IV disease initially unresponsive to gemcitabine, 5-FU, irinotecan and cisplatin subsequently responded with the addition of bevacizumab.Citation99
Basic Mechanisms for Vaccine-Based Immunotherapy
Vaccination involves administering tumor antigen/s with the aim of stimulating tumor-specific immunity. Antigens could be delivered in the form of DNA or peptides, as well as tumor cells or antigen-pulsed DCs. To be considered an ideal tumor vaccine candidate, expression of the antigen must be restricted to the tumor or only minimally expressed elsewhere in the body. Cancer cells are derived from their normal counterparts owing to genetic and epigenetic alterations that de facto underpin their malignant phenotype and lead to the expression of TAAs, i.e., proteins that are generally not expressed by non-transformed cells. But unfortunately, at the moment there aren't antigens expressed only by PC cells and so the TAAs () used as the target of immunotherapy treatments are self protein or over-expressed in tumor cells are present in acetylated form, with the risk of ineffective treatments (enrollment of immune suppressive cells such as Tregs) or autoimmune phenomena.
Table 1. Most significant antigens associated with pancreatic cancer
Thus, the goal of anticancer vaccination is to activate and expand tumor-specific T cells as a means of eliciting novel or boosting pre-existent anticancer immune responses and, as said earlier, overcoming all the barriers raised by malignant cells against immune activation.
Vaccination against tumor antigens is an attractive approach to adjuvant treatment post-surgery, when tumor induced immune suppression is minimal.Citation100–102 Also, Additional synergistic help is added to elicit a more vigorous and effective immune response, such as cytokines and immunostimulating compounds.
Vaccine Therapy Against Pancreatic Cancer
Whole cancer cell-based vaccines
The simplest vaccine approach that has been applied to cancer is the inoculation of patients with irradiated tumor cells. This approach remains a potent vehicle for generating antitumor immunity because tumor cells express all relevant candidate TAAs, including both known and unidentified. In the clinical setting, the use of autologous tumor cell depends on the availability of an adequate number of them. As only 10–15% of PC patients diagnosed are eligible for surgical, autologous PC cells may not be provided in most of the patients. Moreover, even if the patients are treated by surgical resection, it is difficult to prepare a sufficient number of tumor cells due to the length of culture time and risk of contamination.Citation103 To elude this problem, allogeneic tumor cell lines may be used instead of autologous tumor cells.Citation104 This strategy has many advantages: 1) specific TAAs do not need to be identified for vaccination, 2) allogeneic tumor cell lines are well characterized as TAAs source, 3) allogeneic tumor cell lines can grow well in vitro, 4) it is not necessary to determine HLA typing of patients and allogeneic tumor cells, because autologous DCs can process and present multiple TAAs from allogeneic tumor cells owing to cross presentation in the context of appropriate MHC class I and II alleles,Citation105 5) polyclonal antigen-specific T cells (CD4+ /CD8+) can be generated, which may protect against tumor escape variants and 6) the tumor cell vaccine platform can be easily modified. For example, tumor cells can be transduced to express immunomodulatory cytokines such as granulocyte macrophage colony-stimulating factor (GM-CSF), which has shown significant anti-tumor effect in vivo.Citation106
Two allogeneic whole cell based anticancer vaccines are currently being investigate for their safety and antineoplastic effects in PC patients.Citation107,108
Allogeneic GM-CSF-secreting vaccines
The first whole cell-based anticancer vaccine developed for the PC treatment (pancreatic GVAX) comprised 2 allogeneic human pancreatic cancer cell lines, both of which were engineered to express GM-CSF. This vaccine was developed based on preclinical studies demonstrating that the elicitation of effective antitumor immune responses by anticancer vaccines required the secretion of high levels of GM-CSF at the site of vaccination for several days.Citation109
GM-CSF-expressing cancer cells indeed prime the immune system very efficiently as they boost the capacity of DCs to present TAAs, which in this setting are several. Jaffee and colleagues have conducted multiple Phase I/II clinical trials to test the safety and efficacy of irradiated, allogeneic GM-CSF-secreting pancreatic cancer cell lines in patients with resected PCs or metastatic PC.Citation107,108
In the context of 2 completed Phase I/II clinical trialsCitation110,111 and one ongoing study,Citation112 resectable PC patients underwent vaccination after surgery, before the initiation of adjuvant chemotherapy or radiation therapy, and received 4 additional vaccine doses only once they had completed chemotherapy and radiation therapy.
Such a sequential design was intended to avoid as much as possible the immunosuppressive effects of chemoradiation on vaccine elicited immune responses. In the Phase I study, 3 out of 8 patients who received the highest 2 doses of vaccine still remains disease free, now for more than 15 y (y).Citation107
In the Phase II study, 60 patients with resected PC were treated with the vaccine in combination with standard chemoradiation in a similar schedule as in the Phase I study.Citation110
The median disease-free survival is 17.3 months (mo) with median overall survival of 24.8 mo. The post-vaccination induction of mesothelin-specific CD8+ T cells in HLA-A1+ and HLA-A2+ patients correlates with their disease-free survivals. Comparing the Kaplan-Meier curved of patients treated with the vaccine plus adjuvant chemoradiation in this clinical trial and a historical cohort of PC patients treated at the Johns Hopkins Hospital with adjuvant chemoradiation alone suggests that the vaccine may provide clinical benefits over chemoradiation in the first 2 y after surgery.
However, there was no significant difference in the median overall survival of the 2 cohorts, suggesting that the immune responses elicited by the vaccine may have weaned off after stopping vaccination.
In light of these results, 2 new studies are currently being conducted in which PC patients who remain disease-free in response to the pancreatic GVAX vaccine are treated with boost vaccinations every 6 mo after they have completed the first cycle of immunization.
The pancreatic GVAX vaccine has also been tested in patients with metastatic PC.Citation108 Two patient cohorts were enrolled in this open-label Phase II study: cohort A, including 30 patients who received a maximum of 6 doses of GVAX at 21-d intervals; and cohort B, including 20 patients who were treated with intravenous cyclophosphamide at a low dose (250 mg/m2) to inhibit regulatory T cells (one day prior to the administration of GVAX). The median survival of cohort A and cohort B was 2.3 and 4.7 mo, respectively. These findings supported the initiation of additional studies to evaluate the effects of low-dose cyclophosphamide (CY), which was shown to deplete Tregs in preclinical settings, in combination with anticancer vaccines.Citation108
Mesothelin-specific T-cell responses were shown to be of higher avidity in patients receiving cyclophosphamide/GVAX as compared with subjects treated with GVAX only, and these responses correlated with prolonged patient survival. This observation stimulated the launch of a multi-institutional study that has recently been completed.Citation113 In this setting, patients were enrolled with metastatic pancreatic cancer who received or refused ≥ 1 prior chemotherapy, had ECOG ≤ 1 and adequate organ function. Patients were randomized 2:1 to receive 2 doses of CY/GVAX followed by 4 doses of CRS-207 (Arm A) or 6 doses of CY/GVAX (Arm B) every 3 weeks. Clinically stable patients were offered an additional 20-week courses. CRS-207 is a live-attenuated Listeria monocytogenes engineered to express human mesothelin. CRS-207 stimulates potent innate and adaptive immunity and has shown synergy with GVAX in mouse tumor models. The anecdotal survival benefit was observed in the CRS-207 phase I study in patients who received prior GVAX.
The primary endpoint was a comparison of OS between treatment arms. Secondary endpoints were to evaluate safety, clinical and immune responses.
In total, 90 patients were treated (Arm A: 61, Arm B: 29). As of Jan 2013, 27 patients completed 1 course (A: 24, B: 3) and 17 patients (A: 15, B: 2) initiated a 2nd course. Median age was 63. Median number of prior regimens was 3. No treatment-related serious adverse events (SAEs) or unexpected toxicities were observed. The most frequent Grade (G) 3/4 related toxicities were fever, lymphopenia, hypophosphatemia, elevated liver enzymes, and fatigue following CRS-207 in <5 % of subjects. Of 51 patients evaluated post-treatment, 34% had stable disease in Arm A vs. Nineteen% in Arm B. OS (overall survival) for all patients treated was 6 months in Arm A vs. Three.4 months in Arm B (2-sided, p = 0. 0114).
In conclusion, combined CY/GVAX pancreas and CRS-207 was generally well-tolerated with no treatment-related SAEs or unexpected G3/4 toxicities. The significant difference in OS between treatment arms met the criteria for early stopping. This indicates that the combination immunotherapy may extend OS for metastatic PC patients with minimal toxicity and should continue to be developed as an effective therapy. This prime boost approach therefore constitutes a vaccination platform that warrants further investigation.
Algenpantucel-L
Algenpantucel-L (also known as hyperacute-PC vaccine) consists of 2 irradiated, live, human allogeneic pancreatic cancer cell lines that express murine α-1,3-galactosyltransferase, which is responsible for the synthesis of α-galactosylated epitopes on cell surface proteins. A recent multi-institutional Phase II clinical trial have investigated Algenpantucel-L in combination with standard adjuvant chemoradiotherapy for the treatment of resected PC patients.Citation114 The first cycle of treatment consisted of Algenpantucel-L on days 1 and 8. One week after the second vaccination, gemcitabine was administered weekly for 3 weeks, on days 1, 8, and 15, in conjunction with Algenpantucel-L on days 1 and 15 of cycle 2.
Radiotherapy was initiated 1 to 2 weeks after the completion of cycle 2. Vaccination continued along with radiation therapy on days 1, 15, 29, and 43. After a median follow-up of 21 mo, the 12-mo disease-free survival was 62%, and the 12-mo overall survival was 86%. At the moment, the addition of Algenpantucel-L to standard adjuvant therapy for the treatment of resected PC patients is being investigated in a Phase III clinical study.
Antigen/peptide specific vaccines
The natural starting point for vaccines against PC was represented by tumor markers such as carcinoembryonic antigen (CEA), mucin 1 (MUC1)Citation115 as well as by proteins that play an early and prominent role in PC initiation or progression, including KRAS and telomerase and a-Enolasi (ENO1).Citation116,117
Peptide and protein-based vaccines were the first form of anti-PC vaccinations investigated, attempting to use immunodominant tumor epitopes to stimulate antitumor T-cell responses.
Peptide-based cancer vaccines are preparations made from antigenic epitopes, that represent the minimal immunogenic region of antigens,Citation118,119 designed to enhance the T cell response, especially the CD8+. Induction of CTLs needs peptides derived from TAAs to be presented on the surface of APCs (Antigens Presenting Cells), such as DCs, in the context of HLA molecules. The major advantages of peptide vaccines are that they are simple, stable, safe, economical, and don't require manipulation of patient tissues, whose availability may be limited. However, there are also several obstacles that limit the widespread usefulness of peptide vaccines and, especially, a limited number of known synthesized short peptides cannot be available in many HLA molecules.Citation120-122
In we have reported a list of various pancreatic cancer-associated antigens, targets for different PC vaccine-based immunotherapy.
KRAS-targeting vaccines
The gene KRAS, that is mutated in more than 90% of PC patients, is a good target for the immunotherapeutic treatments. In addition, the first peptide-based vaccine investigated in patients targeted KRAS, showing that this immunotherapeutic approach is safe.Citation123 In a Phase I/II study, the administration of synthetic KRAS-derived peptides in unresectable PC patients resulted in an immune response in 2 out of 5 individuals.Citation124 In another Phase I/II clinical trial, synthetic peptides derived from mutant KRAS were administered together with GM-CSF in 48 PC patients (10 surgically resected and 38 with advanced disease) on an outpatient basis.Citation125 Peptide specific immune responses were induced in 25 of 43 (58%) evaluable patients, indicating that this protocol is potent enough to elicit immune responses even in patients with end stage disease. Patients with advanced PC manifesting an immune response to vaccination exhibited a prolonged survival as compared with immunological non-responders (median survival 148 d vs. 61 d; p = 0.0002).
In an independent study, patients with resected pancreatic cancers harboring KRAS mutations at codon 12 were vaccinated once monthly for 3 mo with a 21-mer epitope encompassing the patient specific mutation.Citation126
About 200 μg of the vaccine were injected intradermally on day 7 of a 10-d course involving intradermal GM-CSF. Of 62 screened patients, 24 were vaccinated. Median recurrence-free survival time was 8.6 mo and median overall survival time was 20.3 mo. Thus, KRAS-targeting vaccines proved to be well tolerated by patients with resectable PC. Although these preparations demonstrated some immunogenicity, however, their clinical efficacy remains unproven.
Telomerase-targeting vaccines
Telomerase, which is reactivated in more than 85% of PC cells, has also been used to develop peptide-based vaccines against pancreatic cancer. The telomerase-targeting vaccine (GV1001) consist a 16-aa peptide derived from human TERT that binds to multiple MHC molecules. In a Phase I/II clinical study, which GV1001 was well tolerated by PC patients and prolonged survival,Citation127 4eight patients with non-resectable PC received intradermal injections of GV1001 (at 3 dose levels) in combination with GM-CSF for 10 weeks. Immune responses were observed in 24 of 38 evaluable patients, with the highest proportion of immunological responders belonging to the intermediate-dose group. The median survival of patients receiving intermediate doses of GV1001 was 8.6 mo, which was significantly longer than that of subjects in the low and high dose groups. One-year survival among evaluable patients of the intermediate-dose group was 25%. Two Phase III studies tested GV1001 in patients with nonresectable PC, namely, the PrimoVax and TeloVac trials. The PrimoVax trial examined the efficacy of GV1001 monotherapy vs. gemcitabine, but was terminated owing to a lack of survival advantage.Citation128 The TeloVac study investigated the efficacy of GV1001 in sequential combination with gemcitabine vs. gemcitabine alone in subjects with locally advanced and metastatic pancreatic adenocarcinoma.Citation128
It should be noted that the vaccine cycles overlapped with the gemcitabine cycles in the combinational arm of this study, raising concerns about the effectiveness of vaccination in the setting of chemotherapy-induced immunosuppression. The results of the TeloVac study have recently been reported, demonstrating no survival benefit for the combination of GV1001 and gemcitabine as compared with gemcitabine alone.Citation128
Gastrin-based vaccines
Gastrin and cholecystokinin B receptor (CCKBR, best known as CCK-2) are upregulated and co-expressed in both pancreatic cell lines and human PC specimens and have been implicated in autocrine, paracrine, and endocrine growth pathways.Citation129,130 In a multi-institutional, double-blinded, placebo-controlled clinical trial, the administration of a gastrin-based vaccine to chemotherapy-refractory advanced-stage PC patients resulted in a nearly fold2- increase in median overall survival, as compared with placebo (151 vs. 82 d, respectively; p = 0.03) (130). Gastrin-based vaccines appear therefore to be well tolerated by and could represent a new therapeutic option for pancreatic cancer.
Survivin-targeting vaccines
Survivin is a member of the inhibitor of apoptosis family and is known to exert robust anti-apoptotic effects. Survivin is expressed to high levels by a majority of human carcinomas, including pancreas cancer, but not by non-transformed adult tissues.Citation131 A 72-y-old patient suffering from gemcitabine-refractory PC experienced a complete remission (with a duration of 8 mo) upon receiving a survivin-targeting that consisted of a modified HLA-A2+-restricted survivin epitope (residues 96–104) in Montanide.Citation132 Immunological monitoring revealed a strong vaccine-induced immunoreactivity against survivin, and when the patient was weaned from vaccination, he developed recurrent disease. In another study, a survivin-derived peptide (AYACNTSTL) was used in combination with IFN-α to vaccinate 6 patients with advanced PC. More than half of the patients had manifested immunological responses to vaccination, which were often accompanied by clinical benefits.Citation133 Nonetheless, this vaccine was tested in a limited number of individuals and its application would be limited to HLA-A2+ patients.
HSP-peptide complex-based vaccines
HSPs exists ubiquitously across all species and their function as chaperones can be harnessed to stabilize peptides for ex vivo and in vivo delivery to APCs. HSP-peptide complexes can be presented on MHC class I molecules on the cell surface. Tumor-derived HSP peptide complexes have been shown to induce antitumor immune responses in preclinical studies.Citation134 HSP96-peptides's complexes produced from resected tumor tissues were the first to be employed in anticancer vaccines. In a Phase I clinical trial, 10 resected PC patients who did not receive adjuvant chemoradiation were vaccinated with autologous HSP96-peptide complexes weekly, for a total number of 4 vaccination, exhibiting a median overall survival of 2.2 y.Citation135 Although this pilot study demonstrated the feasibility of preparing HSP96-peptide complexes from resected tumors, it would be a technical challenge to produce such an autologous complex for a large number of patients.
CEA- and MUC1-targeting vaccines
Clinical trials testing recombinant CEA- and MUC1- targeting vaccines in PC patients have shown little efficacy.
TRICOM is a poxvirus-based vaccine encoding a combination 3 distinct T-cell co-stimulatory molecules: B7–1, ICAM1 and CD58 (best known as LFA-3). In a Phase I study, viral vaccines targeting CEA and MUC1 were tested in 10 advanced PC patients.Citation136 The vaccination regimen consisted of a vaccinia virus expressing CEA and MUC1 (PANVAC-V) coupled to a fowlpox virus expressing the same antigens and co-stimulatory molecules (PANVAC-F). Patients were primed with PANVAC-V followed by 3 booster vaccinations with PANVAC-F.
GM-CSF was used as a local adjuvant after each vaccination and for 3 consecutive days thereafter. The median overall survival of vaccinated patients was 6.3 me and a significant increase in overall survival was noted among those individuals, patients who developed CEA- and/or MUC-1-specific immune responses as compared with those who did not (15.1 vs. Three.9 mo, respectively; p = 0.002). In a Phase III, randomized clinical trial involving 255 patients with metastatic PC, PANVAC-V was compared with standard gemcitabine-based chemotherapy. Regrettably, the vaccine failed to ameliorate overall survival in this setting.Citation137
α-Enolasi and PC vaccination
ENO1 localizes to the cytoplasm, where it functions as a glycolytic enzyme, as well as to the plasma membrane, where operates as a plasminogen receptor and plays an important role in cell migration.Citation138 ENO1-specific T-cell responses can be detected in PC patients who bear ENO1-specific autoantibodies, but not in those who do not.Citation138 Antibodies against ENO1 can be detected in over 60% of PC patients.Citation139 Upon transfer into immunocompromised mice, ENO1-specific T cells inhibit the growth of xenotransplanted human pancreatic tumors. Despite the ubiquitous expression of ENO1, normal cells are spared by ENO1-specific CTLs, presumably because they express low levels of this enzyme.Citation138 These results have led us to develop a DNA vaccine targeting ENO1.
There are strains of genetically engineered mice (GEM) that spontaneously develop PC, which are already being exploited for the development of novel diagnostic and therapeutic strategies.Citation140 A recent study used 2 of these strains to assess both the prophylactic and therapeutic potential of a ENO1-targeting DNA vaccine. Genetically engineered KrasG12DPdx1-Cre (KC) andKrasG12DTrp53R172HPdx1-Cre (KPC) mice spontaneously develop lethal pancreatic carcinomas with different kinetics.Citation141 Both KC and KPC mice were vaccinated with a plasmid encoding human ENO1, which displays more than 95% identity (99% homology) with its mouse ortholog, resulting in the induction of a specific immune response that significantly prolonged survival: from 336–474 d for KC mice (representing the longest overall survival for these animals ever reported), and from 203–245 d for KPC mice. The ENO1-targeting DNA vaccine activated several immune effector mechanisms, including the production of high levels of anti-ENO1 IgG antibodies, the activation of ENO1-specific Th1 and Th17 cells, as well as an intense recruitment of CD3+ cells to the tumor bed. Notably, anti-ENO1 IgGs were able to bind to murine PC cells and induce their killing via complement-dependent cytotoxicity, while Th1/Th17 cytokines favored the switching to effector antibody subclasses. Furthermore the ENO1-targeting DNA vaccine significantly decreased the abundance of immunosuppressive cells in the tumor microenvironment, including MDSCs and Tregs. Of note, when these immunosuppressive cells rebounded to levels similar to those of control mice, tumor progression was no longer counteracted and animals died. Still, the therapeutic efficacy of the ENO1-targeting DNA vaccine appeared to be very promising, especially when the administration protocol started at 8–9 mo of age.Citation142
Dendritic cell-based vaccines
DCs are the most potent APCs being very efficient at priming naïve T cells to generate memory T cells and B cells that mediate robust antigen-specific immune responses. Several groups have attempted to harness these characteristics by isolating DCs, loading them with TAAs as well as with TAA-coding or tumor-derived mRNA ex vivo, and subsequently re-infusing them in patients. The safety and efficacy of DC-based vaccines in PC patients have been tested in 2 clinical trials only.
The first of these studies is a Phase I/II clinical trial in which 12 PC patients were treated upon tumor resection with DCs loaded ex vivo with a MUC1-derived peptide.Citation143 These patients have been followed for >4 y after vaccination, and 4 of them are alive, all without evidence of recurrence.
In the second study, a DC-based vaccine alone or combined with LAK cells was administered together with gemcitabine and/or S-1 to 49 patients with inoperable pancreatic cancer.Citation144 Of these patients, 2 manifested a complete remission, 5 a partial remission, and 10 had stable disease. The median survival of these individuals was 360 days, which appeared to be longer than what could be achieved with gemcitabine and/or S-1. Thus, the combination of DC-based immunotherapy and chemotherapy was well tolerated by advanced PC patients and warrants further investigation.
Conclusions and Future Perspectives
Pancreatic cancer is a dismal disease that has a high morbidity and mortality and at present there aren't effective chemotherapeutic treatments, especially for patients with advanced and metastatic diseases. For all these reasons, it is of prime importance to investigate new pancreatic cancer treatments. In this review, we have analyzed the various strategies of the immunotherapeutic vaccine approach, some of which are still used in animal models, others are already being exploited in clinical trials. Immunotherapy is certainly a promising treatment for pancreatic cancer, because is highly specific for cancer cells and therefore without the side effects associated with traditional chemotherapy. But, although clinical trials testing anticancer vaccines in PC patients have generated promising results, most of these studies have failed to demonstrate a robust efficacy and a statistically significant improvement in patient survival. Moreover, most of these clinical studies identified a number of critical aspects that must be carefully considered in the design the next generation of cancer vaccines, such as the lack, for the present, of antigens expressed only by PC cells; in fact the antigens used as the target of immunotherapic treatments are self-protein or overexpressed (Table 1) in tumor cells or present in acetylated form, with the risk of autoimmune phenomena.
Most often, cancer patients have developed a state of immunological tolerance against TAAs that we could be used in immunotherapeutic strategies, because TAAs are essentially self protein, that can be overexpressed in cancer cells. Indeed, although mutated oncogenes may produce neo-antigens, the expression of these potentially antigenic epitopes occurs at a specific stage of tumorigenesis.Citation145 For example, KRAS is mutated in the early stage of pancreatic oncogenesis, implying that the tolerance to mutated KRAS may be established long before invasive PC develops. KRAS-targeting vaccines may therefore have a potential for the PC prevention, but not for the treatment of established pancreatic neoplasms.
Along similar lines, vaccines that target other proteins harboring tumor-associated mutations would have difficulty in overcoming the state of immunological tolerance that develops relative to these proteins in patients with established tumors. Recent advances in high throughput genome sequencing may provide the opportunity to develop patient-specific vaccines that target multiple, as opposed to just one, cancer-associated mutant proteins.Citation146
Essentially all the clinical trials performed so far to compare anticancer vaccines with standard chemotherapy failed to demonstrate the superiority of the former. Apparently, vaccine therapy would not be able to replace chemotherapy and radiation therapy.
Moreover, although recent clinical studied have investigated the sequential administration of anticancer vaccines and chemoradiation, the immunosuppressive effects of these standard antineoplastic regimens may compromise the efficacy of immunotherapy.
Therefore, it will be critical to identify an optimal way to combine anticancer vaccination with chemotherapy and/or radiation therapy. It will also be interesting to test vaccines as a maintenance therapy for patients who are grossly disease-free upon, or whose disease is at least stabilized, upon chemotherapy and/or radiation therapy. Prime-boost vaccination strategies, in particular those that use listerial vaccines for boosting, are a promising approach for maintenance therapy.
The PC immunotherapy will greatly benefit from the identification of PC-specific TAAs. However, as mentioned above, cancer cells often exploit immune checkpoints to evade detection by cytotoxic T cells. Reciprocally, although immune checkpoint blockers are effective as single agents against specific malignancies,Citation147-149 this is not the case of the PC, which elicits limited adaptive immune responses owing to a high degree of immunological tolerance at baseline.Citation150,151 Indeed, the efficiency of immune checkpoint-targeting agents is dependent on adaptive immune responses.Citation152 Thus, it is conceivable to combine immune checkpoint blockers with anticancer vaccines, presumably resulting in the elicitation of robust antigen-specific adaptive immune responses. This notion is supported by the results of a recent clinical study investigating the clinical profile of ipilimumab plus GVAX as compared with ipilimumab alone in previously treated locally advanced or metastatic PC patients.Citation153
A large proportion of the immunological infiltrate of PC lesions exerts pro-inflammatory functions. However, these proinflammatory components are insufficient to elicit adequate antitumor immune responses. Cytokines such as TGF-β or IL-10, are produced by the pro-inflammatory infiltrate of PC lesions and can stimulate tumor growth. Once the tumor is established, the microenvironment is skewed toward a highly immunosuppressive state characterized by a high frequency of tumor-associated macrophages with an M2 phenotype, increased Th2 immune responses, and abundant Tregs, which further contribute to immune evasion.Citation151
In the presence of such an immunosuppressive microenvironment, immune effector cells are unable to mediate cytotoxic functions even when they have been fully activated in the periphery. Therefore, for anticancer vaccines to elicit therapeutically relevant tumor-specific immune responses, new strategies must be designed that convert the highly immunosuppressive microenvironment of pancreatic tumors into an immunostimulatory one.Citation154,155
In accord with this assumption, very interesting are the results obtained using of low-dose cyclophosphamide, which was shown to deplete Tregs in preclinical settings, in combination with anticancer vaccines.Citation108 In particular, the use of CY/GVAX and CRS-207 have shown anecdotal survival benefit generating promising hopes for future treatment of PC patients. So, in conclusion we can say that immunotherapy may be included among the future treatments for pancreatic cancer, especially for inoperable patients, but for the effectiveness of this innovative treatment is essential to overcome some obstacles: (a) finding specific markers for pancreatic cancer cells, (b) mitigating the immune suppressive effects of tumor cells, (c) early diagnosis of the tumor so as to act in a timely manner before the cancer spreads in other locations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Giulia Nannini for assistance in manuscript editing.
Funding
This work was supported by grants from the Italian Ministry of University and Research and the University of Florence
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol 2013; 14:476-85; PMID:24079875; http://dx.doi.org/10.1016/S1470-2045(13)70172-4
- Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011 Dec 1;29(34):4548-54; PMID:21969517; http://dx.doi.org/10.1200/JCO.2011.36.5742
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al; Groupe Tumours Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364:1817-25; PMID:21561347; http://dx.doi.org/10.1056/NEJMoa1011923
- Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, et al. Second-Line Oxaliplatin, Folinic Acid, and Fluorouracil Versus Folinic Acid and Fluorouracil Alone for Gemcitabine-Refractory Pancreatic Cancer: Outcomes From the CONKO-003 Trial. J Clin Oncol 2014 Jun 30; 32(23):2423-9. pii: JCO.2013.53.6995.
- Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 2012; 22:33-40; PMID:22210179; http://dx.doi.org/10.1016/j.semcancer.2011.12.005
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5:263-74; PMID:15776005
- Johnston FM, Tan MC, Tan BR Jr, Porembka MR, Brunt EM, Linehan DC, Simon PO JR, Plambeck-Suess S, Eberlein TJ, Hellstrom KE, et al. Circulating mesothelin protein and cellular antimesothelin immunity in patients with pancreatic cancer. Clin Cancer Res 2009; 15:6511-18; PMID:19843662
- Kubuschok B, Neumann F, Breit R, Sester M, Schormann C, Wagner C, Sester U, Hartmann F, Wagner M, Remberger K, et al. Naturally occurring T-cell response against mutated p21 ras oncoprotein in pancreatic cancer. Clin Cancer Res 2006; 12:1365-1372; PMID:16489095
- Oji Y, Nakamori S, Fujikawa M, Nakatsuka S, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, et al. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci 2004; 95:583-87; PMID:15245594
- Ueda M, Miura Y, Kunihiro O, Ishikawa T, Ichikawa Y, Endo I, Sekido H, Togo S, Shimada H. MUC1 overexpression is the most reliable marker of invasive carcinoma in intraductal papillary-mucinous tumor (IPMT). Hepato-Gastroenterology 2005; 52:398-403; PMID:15816444
- Seki K, Suda T, Aoyagi Y, Sugawara S, Natsui M, Motoyama H, Shirai Y, Sekine T, Kawai H, Mita Y, et al. Diagnosis of pancreatic adenocarcinoma by detection of human telomerase reverse transcriptasemessenger RNA in pancreatic juice with sample qualification. Clin Cancer Res 2001; 7:1976-81; PMID:11448913
- Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Søreide O, Thorsby E, Gaudernack G. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 1995; 346:1399-1400, 1995; PMID:7475823
- Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen surviving. Cancer Immunol Immunother 2006; 55:1294-1298; PMID:16315030
- Yamaguchi K, Enjoji M, Tsuneyoshi M. Pancreatoduodenal carcinoma: a clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19-9. J Surg Oncol 1991; 47:148-54; PMID:2072697
- Komoto M, Nakata B, Amano R, Yamada N, Yashiro M, Ohira M, Wakasa K, Hirakawa K. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer Sci 2009; 100:1243-1247; PMID:19432892
- Maacke H, Kessler A, Schmiegel W, Roeder C, Vogel I, Deppert W, Kalthoff H. Overexpression of p53 protein during pancreatitis. Br J Cancer 1997; 75:1501-04; PMID:9166944
- Cappello P, Tomaino B, Chiarle R, Ceruti P, Novarino A, Castagnoli C, Migliorini P, Perconti G, Giallongo A, Milella M, et al. An integrated humoral and cellular response is elicited in pancreatic cancer by alpha-enolase, a novel pancreatic ductal adenocarcinoma-associated antigen. Int J Cancer 2009; 125:639-48; PMID:19425054
- Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer 2003; 103:97-100; PMID:12455059
- Tewari N, Zaitoun AM, Arora A, Madhusudan S, Ilyas M, Lobo DN. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer 2013; 13:436; PMID:24063854
- Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 2010; 59:1593-600; http://dx.doi.org/10.1007/s00262-010-0855-8; PMID:20414655
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60:1419-30; PMID:21644036.; http://dx.doi.org/10.1007/s00262-011-1028-0
- Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, Greten TF. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology 2009; 128:141-9; PMID:19689743; http://dx.doi.org/10.1111/j.1365-2567.2009.03105.x
- Schmieder A, Michel J, Schönhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol 2012; 22:289-97; PMID:22349514; http://dx.doi.org/10.1016/j.semcancer.2012.02.002
- Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA, Sarukhan A. Mechanisms Driving Macrophage Diversity and Specialization in Distinct Tumor Microenvironments and Parallelisms with Other Tissues. Front Immunol 2014; 5:127; PMID:24723924
- Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol 2004; 57:630-6; PMID:15166270
- Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res 2011; 167:e211-9; PMID:19765725; http://dx.doi.org/10.1016/j.jss.2009.05.026
- Nurieva R, Wang J, Sahoo A. T-cell tolerance in cancer. Immunotherapy 2013; 5:513-31; PMID:23638746; http://dx.doi.org/10.2217/imt.13.33
- Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol 2013; 34:33-40; PMID:22999714; http://dx.doi.org/10.1016/j.it.2012.08.005
- Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, Maekawa Y, Yasutomo K, Shimada M. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas 2006; 33:386-90; PMID:17079944
- Amedei A, Niccolai E, Benagiano M, Della Bella C, Cianchi F, Bechi P, Taddei A, Bencini L, Farsi M, Cappello P, et al. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother 2013; 62:1249-60; PMID:23640603; http://dx.doi.org/10.1007/s00262-013-1429-3
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3 +regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12:5423-34; PMID:17000676
- Yamamoto T, Yanagimoto H, Satoi S, Toyokawa H, Hirooka S, Yamaki S, Yui R, Yamao J, Kim S, Kwon AH. Circulating CD4+CD25 +regulatory T cells in patients with pancreatic cancer. Pancreas 2012; 41:409-15; PMID:22158072; http://dx.doi.org/10.1097/MPA.0b013e3182373a66
- Yamamoto T, Yanagimoto H, Satoi S, Toyokawa H, Yamao J, Kim S, Terakawa N, Takahashi K, Kwon AH. Circulating myeloid dendritic cells as prognostic factors in patients with pancreatic cancer who have undergone surgical resection. J Surg Res 2012; 173:299-308; PMID:21195425; http://dx.doi.org/10.1016/j.jss.2010.09.027
- Tjomsland V, Spångeus A, Sandström P, Borch K, Messmer D, Larsson M. Semi mature blood dendritic cells exist in patients with ductal pancreatic adenocarcinoma owing to inflammatory factors released from the tumor. PLoS One 2010; 5:e13441; PMID:20976171; http://dx.doi.org/10.1371/journal.pone.0013441
- Pandha H, Rigg A, John J, Lemoine N. Loss of expression of antigen presenting molecules in human pancreatic cancer and pancreatic cancer cell lines. Clin Exp Immunol 2007; 148:127-35; PMID:17302733
- Aparicio-Pagés MN, Verspaget HW, Peña AS, Lamers CB. Natural killer cell activity in patients with adenocarcinoma in the upper gastrointestinal tract. J Clin Lab Immunol 1991; 35:27-32; PMID:1668287
- Duan X, Deng L, Chen X, Lu Y, Zhang Q, Zhang K, Hu Y, Zeng J, Sun W. Clinical significance of the immunostimulatory MHC class I chain-related molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med Oncol 2011; 28:466-74; PMID:20354827; http://dx.doi.org/10.1007/s12032-010-9480-9
- Davis M, Conlon K, Bohac GC, Barcenas J, Leslie W, Watkins L, Lamzabi I, Deng Y, Li Y, Plate JM. Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother 2012; 35:629-40; PMID:22996369
- Schmitz-Winnenthal FH, Volk C, Z’graggen K, Galindo L, Nummer D, Ziouta Y, Bucur M, Weitz J, Schirrmacher V, Büchler MW, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res 2005; 65:10079-87; PMID:16267034
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004; 28:e26-31; PMID:14707745
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011; 208:469-78; PMID:21339327; http://dx.doi.org/10.1084/jem.20101876
- Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, Doglioni C, Braga M, Di Carlo V, Protti MP. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol 2008; 181:6595-603; PMID:19798410
- Inman BA, Frigola X, Dong H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer Drug Targets 2007; 7:15-30; PMID:17305475
- Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, Hu Z, Zhang J, Jiang G, Zheng S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol 2008; 134:1021-7; PMID:18347814; http://dx.doi.org/10.1007/s00432-008-0364-8
- Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007; 13:2151-7; PMID:17404099
- Shoji Y, Miyamoto M, Ishikawa K, Yoshioka T, Mishra R, Ichinokawa K, Matsumura Y, Itoh T, Shinohara T, Hirano S, et al. The CD40-CD154 interaction would correlate with proliferation and immune escape in pancreatic ductal adenocarcinoma. J Surg Oncol 2011; 103:230-8; PMID:21337550; http://dx.doi.org/10.1002/jso.21812
- Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, Kleeff J, Friess H. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 2009; 9:463; PMID:20035626; http://dx.doi.org/10.1186/1471-2407-9-463
- Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H, Nakajima Y. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer 2009; 101:1709-16; PMID:19844235; http://dx.doi.org/10.1038/sj.bjc.6605375
- Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C, Ungefroren H, Saeger HD, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 2007; 67:8344-50; PMID:17804750
- Teraoka H, Sawada T, Yamashita Y, Nakata B, Ohira M, Ishikawa T, Nishino H, Hirakawa K. TGF-beta1 promotes liver metastasis of pancreatic cancer by modulating the capacity of cellular invasion. Int J Oncol 2001; 19:709-15; PMID:11562745
- Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G, Rodeck U. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol 1999; 155:537-47; PMID:10433946
- Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecchi A, Pirisi M, Emanuelli G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother 2006; 55:684-98; PMID:16094523
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006; 176:6752-61; PMID:16709834
- Witkiewicz A, Williams TK, Cozzitorto J, Durkan B, Showalter SL, Yeo CJ, Brody JR. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 2008; 206:849-54; PMID:18471709; http://dx.doi.org/10.1016/j.jamcollsurg.2007.12.014
- Söderlund J, Erhardt S, Kast RE. Acyclovir inhibition of IDO to decrease Tregs as a glioblastoma treatment adjunct. J Neuroinflammation 2010; 7:44; PMID:20691089; http://dx.doi.org/10.1186/1742-2094-7-44
- Cooper D, Ilarregui JM, Pesoa SA, Croci DO, Perretti M, Rabinovich GA. Multiple functional targets of the immunoregulatory activity of galectin-1: Control of immune cell trafficking, dendritic cell physiology, and T-cell fate. Methods Enzymol 2010; 480:199-244; PMID:20816212; http://dx.doi.org/10.1016/S0076-6879(10)80011-4
- Kuramitsu Y, Taba K, Ryozawa S, Yoshida K, Zhang X, Tanaka T, Maehara S, Maehara Y, Sakaida I, Nakamura K. Identification of up- and down-regulated proteins in gemcitabine-resistant pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res 2010; 30:3367-72; PMID:20944110
- Chung JC, Oh MJ, Choi SH, Bae CD. Proteomic analysis to identify biomarker proteins in pancreatic ductal adenocarcinoma. ANZ J Surg 2008; 78:245-51; PMID:18366394; http://dx.doi.org/10.1111/j.1445-2197.2008.04429.x
- Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol 2010; 185:1383-92; PMID:20574007; http://dx.doi.org/10.4049/jimmunol.0903275
- Peng W, Wang HY, Miyahara Y, Peng G, Wang RF. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res 2008; 68:7228-36; PMID:18757439; http://dx.doi.org/10.1158/0008-5472.CAN-08-1245
- Xie L, Ni WK, Chen XD, Xiao MB, Chen BY, He S, Lu CH, Li XY, Jiang F, Ni RZ. The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol 2012; 138:1035-43; PMID:22367363; http://dx.doi.org/10.1007/s00432-012-1178-2
- Song S, Ji B, Ramachandran V, Wang H, Hafley M, Logsdon C, Bresalier RS. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signalling. PLoS One 2012; 7:e42699; PMID:22900040; http://dx.doi.org/10.1371/journal.pone.0042699
- Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov 2011; 10:101-111; PMID:21283105.; http://dx.doi.org/10.1038/nrd3365
- Hellstrom I, Friedman E, Verch T, Yang Y, Korach J, Jaffar J, Swisher E, Zhang B, Ben-Baruch G, Tan MC, et al. Anti-mesothelin antibodies and circulating mesothelin relate to the clinical state in ovarian cancer patients. Cancer Epidemiol, Biomarkers & Prevention 2008; 17:1520-1526; PMID:18559570; http://dx.doi.org/10.1158/1055-9965.EPI-08-0039
- Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, Pastan I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res 2005; 11:3814-3820; PMID:15897581; http://dx.doi.org/10.1158/1078-0432.CCR-04-2304
- Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelinexpressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007; 13:5144-5149; PMID:17785569; http://dx.doi.org/10.1158/1078-0432.CCR-07-0869
- Filpula D, Zhao H. Releasable PEGylation of proteins with customized linkers. Adv Drug Delivery Rev 2008; 60:29-49; PMID:17884239; http://dx.doi.org/10.1016/j.addr.2007.02.001
- Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion antimesothelin recombinant immunotoxin SS1P. Clin Cancer Res 2009; 15:5274-5279; PMID:19671873; http://dx.doi.org/10.1158/1078-0432.CCR-09-0062
- Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010 Dec 15;16(24):6132-8; PMID:21037025; http://dx.doi.org/10.1158/1078-0432.CCR-10-2275. Epub 2010 Oct 29
- Feng Y, Xiao X, Zhu Z, Streaker E, Ho M, Pastan I, Dimitrov DS. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol Cancer Therap 2009; 8:1113-1118; PMID:19417159; http://dx.doi.org/10.1158/1535-7163.MCT-08-0945
- Ho M, Feng M, Fisher RJ, Rader C, Pastan I. A novel high affinity human monoclonal antibody to mesothelin. Int J Cancer 2011; 128:2020-2030; PMID:20635390; http://dx.doi.org/10.1002/ijc.25557
- Yamasaki H, Ikeda S, Okajima M, Miura Y, Asahara T, Kohno N, Shimamoto F. Expression and localization of MUC1, MUC2, MUC5AC and small intestinal mucin antigen in pancreatic tumors. Int J Oncol 2004; 24:107-113; PMID:14654947; http://dx.doi.org/10.3892/ijo.24.1.107
- Qu CF, Li Y, Song YJ, Rizvi SM, Raja C, Zhang D, Samra J, Smith R, Perkins AC, Apostolidis C, et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. British J Cancer 2004; 91:2086-2093; PMID:15599383; http://dx.doi.org/10.1038/sj.bjc.6602232
- Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol 2004; 57:456-462; PMID:15113850; http://dx.doi.org/10.1136/jcp.2003.013292
- Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, Yonezawa S, Hollingsworth MA. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res 2006; 12:2976-2987; PMID:16707592; http://dx.doi.org/10.1158/1078-0432.CCR-05-1197
- Gold DV, Modrak DE, Schutsky K, Cardillo TM. Combined 90Yttrium-DOTA-labeled PAM4 antibody radioimmunotherapy and gemcitabine radiosensitization for the treatment of a human pancreatic cancer xenograft. Int J Cancer 2004; 109:618-626; PMID:14991585; http://dx.doi.org/10.1002/ijc.20004
- Danielczyk A, Stahn R, Faulstich D, Löffler A, Märten A, Karsten U, Goletz S. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol, Immunother 2006; 55: 1337-1347; PMID:16485130; http://dx.doi.org/10.1007/s00262-006-0135-9
- Fan XN, Karsten U, Goletz S, Cao Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TA-MUC1) with various human carcinomas. Pathol, Res Practice 2010; 206:585-589; PMID:20400237; http://dx.doi.org/10.1016/j.prp.2010.03.006
- Pratesi G, Petrangolini G, Tortoreto M, Addis A, Zunino F, Calcaterra C, Merlo A, Tagliabue E, Menard S, Balsari A. Antitumor efficacy of trastuzumab in nude mice orthotopically xenografted with human pancreatic tumor cells expressing low levels of HER-2/neu. J Immunother 2008; 31:537-544; PMID:18528301; http://dx.doi.org/10.1097/CJI.0b013e31817c37ff
- Saeki H, Yanoma S, Takemiya S, Sugimasa Y, Akaike M, Yukawa N, Rino Y, Imada T. Antitumor activity of a combination of trastuzumab (Herceptin) and oral fluoropyrimidine S-1 on human epidermal growth factor receptor 2- overexpressing pancreatic cancer. Oncol Rep 2007; 18:433-439; PMID:17611667; http://dx.doi.org/10.3892/or.18.2.433
- Larbouret C, Robert B, Navarro-Teulon I, Thèzenas S, Ladjemi MZ, Morisseau S, Campigna E, Bibeau F, Mach JP, Pèlegrin A, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas,” Clin Cancer Res 2007; vol. 13, no. 11, pp. 3356-3362; PMID:17545543; http://dx.doi.org/10.1158/1078-0432.CCR-06-2302
- Larbouret C, Robert B, Bascoul-Mollevi C, Penault-Llorca F, Ho-Pun-Cheung A, Morisseau S, Navarro-Teulon I, Mach JP, Pèlegrin A, Azria D. Combined cetuximab and trastuzumab are superior to gemcitabine in the treatment of human pancreatic carcinoma xenografts. Ann Oncol 2010; 21:98-103; PMID:19889608; http://dx.doi.org/10.1093/annonc/mdp496
- Blumenthal RD, Osorio L, Hayes MK, Horak ID, Hansen HJ, Goldenberg DM. Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft. Cancer Immunol, Immunother 2005; 54: 315-327; PMID:15592930; http://dx.doi.org/10.1007/s00262-004-0597-6
- A Phase I/II Study of Radioimmunotherapy With 90Y-Humanized MN-14 IgG Administered as a Single Dose to Patients With Refractory Advanced/Metastatic Pancreatic Carcinoma (NCT00041639). Available at http:clinicaltrials.gov
- Lemoine NR, Hughes CM, Barton CM, Poulsom R, Jeffery RE, Klöppel G, Hall PA, Gullick WJ. The epidermal growth factor receptor in human pancreatic cancer. J Pathol 1992; 166:166:7-12; PMID:1538276
- Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res 1993; 13:565-569; PMID:8317885
- Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res 2000; 60:2926-2935; PMID:10850439
- Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Therap 2002; 1:777-783; PMID:12492110
- Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. Int J Oncol 2005; 23:2445-2459; PMID:15753456; http://dx.doi.org/10.1200/JCO.2005.11.890
- Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, et al. Phase III Study Comparing Gemcitabine Plus Cetuximab Versus Gemcitabine in Patients With Advanced Pancreatic Adenocarcinoma: Southwest Oncology Group- Directed Intergroup Trial S0205. J Clin Oncol 2010; 28: 3605-3610; PMID:20606093; http://dx.doi.org/10.1200/JCO.2009.25.7550
- Krempien R, Muenter MW, Huber PE, Nill S, Friess H, Timke C, Didinger B, Buechler P, Heeger S, Herfarth KK, et al. Cetuximab in combination with intensity modulated radiotherapy (IMRT) and gemcitabine for patients with locally advanced pancreatic cancer: A prospective phase II trial [PARC-Study ISRCTN56652283]. J Clin Oncol 2007; 25:4573; PMID:16219105; http://dx.doi.org/10.1186/1471-2407-5-131
- Bangard C, Gossmann A, Papyan A, Tawadros S, Hellmich M, Bruns CJ. Magnetic resonance imaging in an orthotopic rat model: blockade of epidermal growth factor receptor with EMD72000 inhibits human pancreatic carcinoma growth. Int J Cancer 2005; 114:131-138; PMID:15523683; http://dx.doi.org/10.1002/ijc.20696
- Graeven U, Kremer B, Südhoff T, Killing B, Rojo F, Weber D, Tillner J, Unal C, Schmiegel W. Phase I study of the humanised anti-EGFR monoclonal antibody matuzumab (EMD 72000) combined with gemcitabine in advanced pancreatic cancer. British J Cancer 2006; 94:1293-1299; PMID:16622465; http://dx.doi.org/10.1038/sj.bjc.6603083
- Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer 2003; 2:8; PMID:12556241; http://dx.doi.org/10.1186/1476-4598-2-8
- Karayiannakis AJ, Bolanaki H, Syrigos KN, Asimakopoulos B, Polychronidis A, Anagnostoulis S, Simopoulos C. Serum vascular endothelial growth factor levels in pancreatic cancer patients correlate with advanced and metastatic disease and poor prognosis. Cancer Lett 2003; 194:119-124; PMID:12706865; http://dx.doi.org/10.1016/S0304-3835(03)00047-8
- Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2005; 23:8033-8040; PMID:16258101; http://dx.doi.org/10.1200/JCO.2005.01.9661
- Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2007; 28:3617-3622; PMID:20606091; http://dx.doi.org/10.1200/JCO.2010.28.1386
- Bruckner HW, Hrehorovich VR, Sawhney HS. Bevacizumab as treatment for chemotherapyresistant pancreatic cancer. Anticancer Res 2005; 25:3637-3639; PMID:16101193
- Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W, Wagner H, Siewert JR, Holzmann B. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun 1997; 65:2283-91; PMID:9169765
- Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients?. Ann Surg Oncol 2003; 10:972-92; PMID:14527919
- Weighardt H, Heidecke CD, Emmanuilidis K, Maier S, Bartels H, Siewert JR, Holzmann B. Sepsis after major visceral surgery is associated with sustained and interferon-γ resistant defects of monocyte cytokine production. Surgery. 2000; 127:309-15; PMID:10715987
- Koido S, Hara E, Homma S, Namiki Y, Komita H, Takahara A, Nagasaki E, Ito M, Sagawa Y, Mitsunaga M, et al. Dendritic/pancreatic carcinoma fusions for clinical use: comparative functional analysis of healthy- versus patient-derived fusions. Clin Immunol 2010; 135:384-400; PMID:20226739
- Koido S, Hara E, Homma S, Torii A, Toyama Y, Kawahara H, Watanabe M, Yanaga K, Fujise K, Tajiri H, et al. Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer-specific antigens and induce antitumor immunity against autologous tumor cells. Clin Cancer Res 2005; 11:7891-900; PMID:16278414
- Saito H, Dubsky P, Dantin C, Finn OJ, Banchereau J, Palucka AK. Cross-priming of cyclin B1, MUC-1 and survivin-specific CD8+ T cells by dendritic cells loaded with killed allogeneic breast cancer cells. Breast Cancer Res 2006; 8:R65; PMID:17129372
- Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19:145-56; PMID:11134207
- Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19:145-56; PMID:11134207
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 2008; 14:1455-63; PMID:18316569; http://dx.doi.org/10.1158/1078-0432.CCR-07-0371
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993; 90:3539-43; PMID:8097319
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011; 253:328-35; PMID:21217520; http://dx.doi.org/10.1097/SLA.0b013e3181fd271c
- Dung TLe, Andrea Wang-Gillam, Vincent J Picozzi, Tim F Greten, Todd S Crocenzi, Gregory M Springett, Michael Morse, Herbert Zeh, Deirdre Jill Cohen, Robert Lance Fine, et al. Brockstedt, Elizabeth M. Jaffee; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD; Washington University School of Medicine in St. Louis, St. Louis, MO; Virginia Mason Medical Center, Seattle, WA; National Cancer Institute, Bethesda, MD; Providence Cancer Center, Portland, OR; H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL; Duke University Medical Center, Durham, NC; University of Pittsburgh Medical Center, Pittsburgh, PA; New York University Cancer Institute, New York, NY; Columbia University, New York, NY; Aduro BioTech, Inc., Berkeley, CA Interim safety and efficacy analysis of a phase II, randomized study of GVAX pancreas and CRS-207 immunotherapy in patients with metastatic pancreatic cancer. J Clin Oncol 31, 2013 (suppl; abstr 4040^).
- ClinicalTrials.gov identifier:NCT00727441.
- Dung T, Wang-Gillam A, Picozzi Jr V, Greten TF, Crocenzi TS, Springett GM, Morse M, Zeh H, Cohen DJ, Fine RL, et al. A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. J Clin Oncol 2014; 32(suppl 3):Abstract 177.
- Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg 2013; 17:94-100; PMID:23229886; http://dx.doi.org/10.1007/s11605-012-2064-6
- Adsay NV, Basturk O, Cheng JD, Andea AA. Ductal neoplasia of the pancreas: nosologic, clinicopathologic, and biologic aspects. Semin Radiat Oncol 2005; 15:254-64; PMID:16183479.; http://dx.doi.org/10.1016/j.semradonc.2005.04.001
- Gaudernack G. Prospects for vaccine therapy for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006; 20:299-314; PMID:16549329
- Capello M, Ferri-Borgogno S, Cappello P, Novelli F. α-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J 2011; 278:1064-74; PMID:21261815; http://dx.doi.org/10.1111/j.1742-4658.2011.08025.x
- Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov 2007; 6:404-14; PMID:17473845
- Bijker MS, Melief CJ, Offringa R, van der Burg SH. Design and development of synthetic peptide vaccines: past, present and future. Expert Rev Vaccines 2007; 6:591-603; PMID:17669012
- Kanodia S, Kast WM. Peptide-based vaccines for cancer: realizing their potential. Expert Review of Vaccines 2008; 7:1533-45; PMID:19053209
- Voskens CJ, Strome SE, Sewell DA. Synthetic peptide-based cancer vaccines: lessons learned and hurdles to overcome. Current Molecular Medicine 2009; 9:683-93; PMID:19689295
- Mocellin S, Pilati P, Nitti D. Peptide-based anticancer vaccines: recent advances and future perspectives. Curr Med Chem 2009; 16:4779-96; PMID:19929787
- Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Gedde-Dahl T 3rd, Stokke KT, Sølheim BG, Egge TS, Søreide O, Thorsby E, et al. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer 1996; 65:450-3; PMID:8621226
- Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Søreide O, Thorsby E, Gaudernack G. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 1995; 346:1399-400; PMID:7475823
- Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Søreide O, Eriksen JA, Møller M, Baksaas I, Lothe RA, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 2001; 92:441-50; PMID:11291084
- Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP, et al. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol 2011; 34:321-5; PMID:20686403; http://dx.doi.org/10.1097/COC.0b013e3181e84b1f
- Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer 2006; 95:1474-82; PMID:17060934
- Gunturu KS, Rossi GR, Saif MW. Immunotherapy updates in pancreatic cancer: are we there yet? Ther Adv Med Oncol 2013; 5:81-9; PMID:23323149; http://dx.doi.org/10.1177/1758834012462463
- Monstein HJ, Ohlsson B, Axelson J. Differential expression of gastrin, cholecystokinin-A and cholecystokinin-B receptor mRNA in human pancreatic cancer cell lines. Scand J Gastroenterol 2001; 36:738-43; PMID:11444473
- Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, Whitehead A, Takhar A, Rowlands BJ, Beckingham IJ. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas 2012; 41:374-9; PMID:22228104; http://dx.doi.org/10.1097/MPA.0b013e31822ade7e
- Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer 2001; 92(2):271-8; PMID:11466679
- Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother 2006; 55:1294-8; PMID:16315030
- Kameshima H, Tsuruma T, Kutomi G, Shima H, Iwayama Y, Kimura Y, Imamura M, Torigoe T, Takahashi A, Hirohashi Y, et al. Immunotherapeutic benefit of α-interferon (IFNα) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci 2013; 104:124-9; PMID:23078230; http://dx.doi.org/10.1111/cas.12046
- Fałkowska-Podstawka M, Wernicki A. Heat shock proteins in health and disease. Pol J Vet Sci 2003; 6:61-70; PMID:12675471
- Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, Srivastava PK, Brennan MF. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci 2007; 52:1964-72; PMID:17420942
- Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med 2007; 5:60; PMID:18039393
- Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev Immunol 2007; 27:451-62; PMID:18197807
- Cappello P, Tomaino B, Chiarle R, Ceruti P, Novarino A, Castagnoli C, Migliorini P, Perconti G, Giallongo A, Milella M, et al. An integrated humoral and cellular response is elicited in pancreatic cancer by alpha-enolase, a novel pancreatic ductal adenocarcinoma-associated antigen. Int J Cancer 2009; 125:639-48; PMID:19425054; http://dx.doi.org/10.1002/ijc.24355
- Tomaino B, Cappello P, Capello M, Fredolini C, Sperduti I, Migliorini P, Salacone P, Novarino A, Giacobino A, Ciuffreda L, et al. Circulating autoantibodies to phosphorylated α-enolase are a hallmark of pancreatic cancer. J Proteome Res 2011; 10:105-12; PMID:20455595; http://dx.doi.org/10.1021/pr100213b
- Pérez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology 2012; 142:1079-92; PMID:22406637; http://dx.doi.org/10.1053/j.gastro.2012.03.002
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005; 7:469-83; PMID:15894267
- Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, Feo S, Giovarelli M, Novelli F. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology 2013; 144:1098-106; PMID:23333712; http://dx.doi.org/10.1053/j.gastro.2013.01.020
- Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther 2008; 6:955-64; PMID:19129927
- Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas 2012; 41:195-205; PMID:21792083; http://dx.doi.org/10.1097/MPA.0b013e31822398c6
- Goggins M, Kern SE, Offerhaus JA, Hruban RH. Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol 1999; 4:4-8; PMID:10436774
- Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008; 68:889-92; PMID:18245491; http://dx.doi.org/10.1158/0008-5472.CAN-07-3095
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013; 144:1230-40; PMID:23622132; http://dx.doi.org/10.1053/j.gastro.2012.12.042
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010; 33:828-33; PMID:20842054; http://dx.doi.org/10.1097/CJI.0b013e3181eec14c
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207-12; PMID:22236695; http://dx.doi.org/10.1016/j.coi.2011.12.009
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013; 36:382-9; PMID:23924790; http://dx.doi.org/10.1097/CJI.0b013e31829fb7a2
- Bazhin AV, Shevchenko I, Umansky V, Werner J, Karakhanova S. Two immune faces of pancreatic adenocarcinoma: possible implication for immunotherapy. Cancer Immunol Immunother 2014; 63:59-65; PMID:24129765; http://dx.doi.org/10.1007/s00262-013-1485-8
- Niccolai E, Prisco D, D’Elios MM, Amedei A. What is recent in pancreatic cancer immunotherapy? Biomed Res Int 2013; 2013:492372; PMID:23509731; http://dx.doi.org/10.1155/2013/492372