Abstract
In a blinded randomized trial, preoperative receipt of the Merck V710 Staphylococcus aureus vaccine was associated with a higher mortality rate than placebo in patients who later developed postoperative S. aureus infections. Of the tested patients, all 12 V710 recipients (but only 1 of 13 placebo recipients) with undetectable serum IL2 levels prior to vaccination and surgery died after postoperative S. aureus infection. The coincidence of 3 factors (low prevaccination IL-2 levels, receipt of V710, and postoperative S. aureus infection) appeared to substantially increase mortality in our study population after major cardiothoracic surgery. Furthermore, 9 of the 10 V710 recipients with undetectable preoperative IL17a levels and postoperative S. aureus infections died. Although the current study is hypothesis-generating and the exact pathophysiology remains speculative, these findings raise concern that immune predispositions may adversely impact the safety and efficacy of staphylococcal vaccines actively under development. The potential benefits of an effective vaccine against S. aureus justify continued but cautious pursuit of this elusive goal.
A Phase IIB/III randomized placebo-controlled trial of the Merck V710 Staphylococcus aureus vaccine containing non-adjuvanted iron-regulated surface determinant B (IsdB) demonstrated significant seroconversion rates in surgical patients following a single injection 14–60 d prior to elective cardiothoracic surgery.Citation1 However, the vaccine was not efficacious in preventing postoperative bacteremia or deep sternal wound infection, and appeared to be associated with multi-organ system failure. Although all-cause mortality rates did not differ between V710 and placebo recipients overall (5.7% and 5.0%, respectively), more patients with postoperative S. aureus infections died in the V710 vaccine group (15/73, 23.0%) than in the placebo group (4/96, 4.2%). A causal relationship explaining the observed difference in mortality in this subgroup of patients has not been established. Aberrant Th2 responses to pathogens that require Th1 and Th17 responses for control can result in life-threatening infections.Citation2-4 We designed a post-hoc hypothesis-generating analysis to explore possible associations between serum immune markers at baseline and death after postoperative S. aureus infection in V710 recipients.
Trial participants were classified into 4 groups based on vaccination status and study outcome.Citation1 Samples were selected so as to include all S. aureus-infected patients who had died during the study in both the V710 and placebo groups. As controls, an unmatched convenience sample of approximately the same numbers of S. aureus-infected survivors was chosen. The study groups consisted of (1) V710 recipient/S. aureus-infected/survived, n = 14; (2) V710 recipient/S. aureus-infected/died, n = 15; (3) placebo recipient/S. aureus-infected/survived, n = 19; (4) placebo recipient/S. aureus-infected/died, n = 4. Frozen sera from up to 4 time-points (day of vaccination; day of hospital admission for cardiothoracic surgery; postoperative day 45; and postoperative day 90) if available were assayed for 10 cytokines (interferon-γ, IL1β, IL1ra, IL2, IL4, IL6, IL8, IL10, IL17a, and TNF-α). Cytokine levels were compared between patients who survived and died in each group using Mann-Whitney tests corrected for multiplicity.
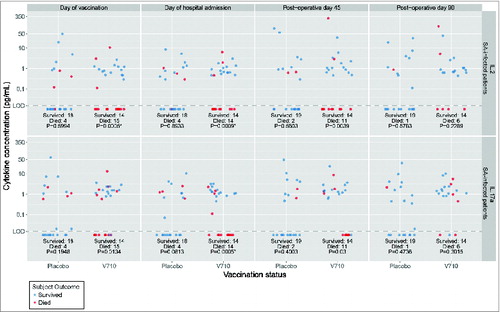
Undetectable IL2 and IL17a levels preoperatively were associated with mortality in V710 recipients after postoperative S. aureus infection (). Comparable associations were not evident among placebo recipients. IL2 levels just prior to vaccination and on the day of hospital admission in V710 recipients who postoperatively developed S. aureus infections were significantly lower in patients who died than in surviving patients (). IL17a concentration on the day of hospital admission was likewise significantly associated with death after postoperative S. aureus infection exclusively in V710 recipients.
Table 1. Relative mortality rates in patients with postoperative S. aureus infections by vaccination group and preoperative IL2 and IL17a levels
Figure 1. Log-plot of IL2 and IL17a levels over time in V710 and placebo recipients with postoperative S. aureus infections by survival versus death. Top Panels: IL2 levels. Bottom Panels: IL17a levels. Interleukin (IL) levels were measured with the Milliplex MAP human cytokine assay (EMD Millipore Corp, Merck KGaA, Darmstadt, Germany) having an approximate lower limit of quantification of 0.2–0.6 pg/mL and lower limit of detection (LOD) of ∼0.1 pg/mL. Rank values were compared between patients who survived or died in each group using Mann-Whitney tests adjusted for multiplicity with a Bonferroni correction of 40 (10 cytokines x 4 time points). Accordingly, α = 0.00125 was set as the threshold for statistical significance. The unadjusted p-values are shown at the bottom of each column and designated with an asterisk as significant when ≤0.00125. As summarized in , IL-values were arbitrarily dichotomized as detectable or undetectable. V710 recipients having preoperative IL-levels below the level of assay detection (both before and after vaccination) were more likely to succumb following postoperative S. aureus infections than similar patients with detectable levels. A comparable relationship between IL levels and death after postoperative S. aureus infections was not apparent in the placebo group, where in contrast to the V710 recipients, the mortality rate was numerically lower in placebo recipients with undetectable IL levels than in similar patients with detectable levels.
V710-induced IgG-antibody responses against IsdB were not protective in the pivotal trial.Citation1 A clear mechanism by which the anti-IsdB antibody response induced by preoperative receipt of V710 could have aggravated the outcome of postoperative staphylococcal infections in our patients, despite appearing safe and efficacious in the early clinical studies and preclinical models,Citation5-8 remains to be determined. In this retrospective analysis, persistently undetectable IL2 levels before and after vaccination and a low IL17a level at hospital admission were predictors of postoperative mortality in V710 (but not placebo) recipients after subsequent S. aureus infection.
The striking observation that low serum IL2 and IL17a concentrations preoperatively were associated with postoperative death after S. aureus infections only in V710 recipients represents a biologically plausible finding.Citation9-11 IL2 differentially stimulates Th1 and Th17 cells, and its apparent absence at the time of vaccination may lead to ineffectual or misdirected CMI responses to IsdB. Furthermore, IL17a appears to be critical in eradicating S. aureus once the host is infected or colonized.Citation4,12,13 Low levels of circulating IL17a after vaccination may indicate ongoing susceptibility to life-threatening S. aureus infection.Citation3 Alternatively, interpretation of the observed reduction in survival rates among V710 recipients after a postoperative S. aureus infection could be confounded by chance or lurking covariates, such as the site or severity of infection. Among the limitations of our retrospective exploratory study, information regarding preexistent comorbidities of the patient, details of the surgical procedure, perioperative management, intraoperative complications, virulence of the infecting S. aureus strain, treatment history, superinfection with other pathogens, and the proximate cause of death was neither systematically collected nor logistically incorporated into the current analysis.
The paradoxical finding of worse outcomes after vaccination has been previously encountered.Citation14,15 As an instructive historical analogy, the formalin-inactivated respiratory syncytial virus vaccine induced primarily Th2-dominant responses in many infants which were associated with more severe disease after later wild-type infection than Th1-dominant responses.Citation15,16 The basal level of specific interleukins may determine (or at least identify) the immune predisposition of the host to certain vaccines. Immunization with IsdB in a predisposed host without circulating IL2 could lead to commission of an original antigenic sin fostering a misdirected and suboptimal Th2-biased immune response to subsequent natural infection.Citation11,15-18
The coincidence of 3 factors (low prevaccination IL-2 levels, receipt of V710, and postoperative S. aureus infection) appeared to be necessary and sufficient to substantially increase the mortality rate in our study population after major cardiothoracic surgery. All 12 V710 recipients (but only 1/13 placebo recipients) in our sample with undetectable serum IL2 concentrations prior to vaccination who then developed a postoperative S. aureus infection died. In contrast, 14 of the 17 V710 recipients with detectable IL2 concentrations at baseline and a postoperative S. aureus infection survived. If confirmed, our results reinforce the need for innovative approaches to broaden testing of S. aureus vaccines in development. Although the implications remain speculative, these findings raise the concern that discoverable immune predispositions can adversely influence the safety and efficacy of investigational staphylococcal (and other) vaccines. The potential benefits of an effective vaccine against S. aureus infections justify continued but cautious pursuit of this elusive goal, especially for high-risk chronic hemodialysis patients and patients requiring major orthopedic and cardiothoracic procedures.Citation19-22
Disclosure of Potential Conflicts of Interest
Merck had been developing V710, but the S. aureus vaccine program has since been discontinued. A penultimate version of this paper was reviewed by the sponsor. Present and former Merck employees may own stock and/or stock options in the company. Dr. Florea Lupu had been compensated by Merck for performing the assays, but was not paid for his participation in writing this report. Every author had full access to all pertinent data. Each co-author approved an essentially final version of the manuscript. The opinions expressed in this report represent the consensus of the authors and do not necessarily reflect the formal positions of Merck or the Oklahoma Medical Research Foundation.
Author Contributions
Study concept and design by TBM and AJ.
The acquisition of datawas by FL, RSK, and AJ. Analysis and interpretation of data was completed by all authors. Drafting of the manuscript was done by MJD and TBM. Critical revision of the manuscript for important intellectual content was completed by all authors. The statistical analysis was done by NS, AF, and JSH.
Acknowledgments
The authors are deeply indebted to all the patients, healthcare providers, and investigators involved with the parent study. We thank Karyn Davis of Merck for technical assistance during the preparation of this manuscript.
Funding
The parent study and the current analysis were sponsored and funded by Merck.
References
- Fowler VG, Allen KB MD, Moreira ED Jr, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery. A randomized trial. JAMA 2013; 309:1368-78; PMID:23549582; http://dx.doi.org/10.1001/jama.2013.3010
- Joshi A, Pancari G, Cope L, Bowman EP, Cua D, Proctor RA, McNeely T. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother 2012; 8:336-46; PMID:22327491; http://dx.doi.org/10.4161/hv.18946
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008; 452:773-6; PMID:18337720; http://dx.doi.org/10.1038/nature06764
- Archer NK, Harro JM, Shirtliff ME. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect Immun 2013; 81:2070-5; PMID:23529621; http://dx.doi.org/10.1128/IAI.00084-13
- Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, Noble L, Brown MJ, Zorman JK, Wang XM, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun 2006; 74:2215-23; PMID:16552052; http://dx.doi.org/10.1128/IAI.74.4.2215-2223.2006
- Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, Schneewind O. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 2010; 28:6382-92; PMID:20226248; http://dx.doi.org/10.1016/j.vaccine.2010.02.097
- Harro C, Betts R, Orenstein W, Kwak EJ, Greenberg HE, Onorato MT, Hartzel J, Lipka J, DiNubile MJ, Kartsonis N. Safety and immunogenicity of a novel Staphylococcus aureus vaccine: results from a first-in-human dose-ranging study. Clin Vaccine Immunol 2010; 17:1868-74; PMID:20943877; http://dx.doi.org/10.1128/CVI.00356-10
- Harro C, Betts R, Hartzel J, Onorato MT, Lipka J, Smugar SS, Kartsonis NA. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two Phase I studies. Vaccine 2012; 30:1729-36; PMID:22192849; http://dx.doi.org/10.1016/j.vaccine.2011.12.045
- Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:1179-86; PMID:22354924; http://dx.doi.org/10.1093/cid/cis033
- Kelly-Quintos C, Kropec A, Briggs S, Ordonez CL, Goldmann DA, Pier GB. The role of epitope specificity in the human opsonic antibody response to the staphylococcal surface polysaccharide poly N-acetyl glucosamine. J Infect Dis 2005; 192:2012-9; PMID:16267775; http://dx.doi.org/10.1086/497604
- Haralambieva IH, Ovsyannikova IG, Pankratz VS, Kennedy RB, Jacobson RM, Poland GA. The genetic basis for interindividual immune response variation to measles vaccine: new understanding and new vaccine approaches. Expert Rev Vaccines 2013; 12:57-70; PMID:23256739; http://dx.doi.org/10.1586/erv.12.134
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med 2009; 361:888-98; PMID:19710487; http://dx.doi.org/10.1056/NEJMra0707449
- Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 2011; 1:505-18; PMID:21720387; http://dx.doi.org/10.1038/nri3010
- Choi YS, Baek YH, Kang W, Nam SJ, Lee J, You S, Chang DY, Youn JC, Choi YK, Shin EC. Reduced antibody responses to the pandemic (H1N1) 2009 vaccine after recent seasonal influenza vaccination. Clin Vaccine Immunol 2011; 18:1519-23; PMID:21813667; http://dx.doi.org/10.1128/CVI.05053-11
- Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res 2007; 39:225-39; PMID:17917067; http://dx.doi.org/10.1007/s12026-007-0071-6
- Olson MR1, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol 2007; 179:5415-24; PMID:17911628; http://dx.doi.org/10.4049/jimmunol.179.8.5415
- Berry JD, Peeling RW, Brunham RC. Analysis of the original antigenic sin antibody response to the major outer membrane protein of Chlamydia trachomatis. J Infect Dis 1999; 179:180-6; PMID:9841837; http://dx.doi.org/10.1086/314538
- Wipasa J, Xu H, Liu X, Hirunpetcharat C, Stowers A, Good MF. Effect of Plasmodium yoelii exposure on vaccination with the 19-kilodalton carboxyl terminus of merozoite surface protein 1 and vice versa and implications for the application of a human malaria vaccine. Infect Immun 2009; 77:817-24; PMID:19015251; http://dx.doi.org/10.1128/IAI.01063-08
- Pier GB. Will there ever be a universal Staphylococcus aureus vaccine? Hum Vaccin Immunother 2013; 9:1865-76; PMID:23793522; http://dx.doi.org/10.4161/hv.25182
- Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346:491-6; PMID:11844850; http://dx.doi.org/10.1056/NEJMoa011297
- Lee BY, Wiringa AE, Bailey RR, Lewis GJ, Feura J, Muder RR. Staphylococcus aureus vaccine for orthopedic patients: an economic model and analysis. Vaccine 2010; 28:2465-71; PMID:20064479; http://dx.doi.org/10.1016/j.vaccine.2009.12.075
- Chen LF, Arduino JM, Sheng S, Muhlbaier LH, Kanafani ZA, Harris AD, Fraser TG, Allen K, Corey GR, Fowler VG. Epidemiology and outcome of major post-operative infections following cardiac surgery: risk factors and impact of pathogen type. Am J Infect Control 2012; 40:963-8; PMID:22609237; http://dx.doi.org/10.1016/j.ajic.2012.01.012
