Abstract
In a previous study in end-stage renal disease (ESRD) hemodialysis patients, a single dose of Staphylococcus aureus type 5 and 8 capsular polysaccharides (T5/T8) conjugated to nontoxic recombinant Pseudomonas aeruginosa exotoxin A investigational vaccine showed no efficacy against S. aureus bacteremia 1 year post-vaccination, but a trend for efficacy was observed over the first 40 weeks post-vaccination. Vaccine efficacy (VE) of 2 vaccine doses was therefore evaluated. In a double-blind trial 3359 ESRD patients were randomized (1:1) to receive vaccine or placebo at week 0 and 35. VE in preventing S. aureus bacteremia was assessed between 3–35 weeks and 3–60 weeks post-dose-1. Anti-T5 and anti-T8 antibodies were measured. Serious adverse events (SAEs) were recorded for 42 days post-vaccination and deaths until study end. No significant difference in the incidence of S. aureus bacteremia was observed between vaccine and placebo groups between weeks 3–35 weeks post-dose 1 (VE -23%, 95%CI: -98;23, p = 0.39) or at 3–60 weeks post-dose-1 (VE -8%, 95%CI: -57;26, p = 0.70). Day 42 geometric mean antibody concentrations were 272.4 μg/ml and 242.0 μg/ml (T5 and T8, respectively) in vaccinees. SAEs were reported by 24%/25.3% of vaccinees/placebo recipients. These data do not show a protective effect of either 1 or 2 vaccine doses against S. aureus bacteremia in ESRD patients. The vaccine induced a robust immune response and had an acceptable safety profile. Further investigation suggested possible suboptimal vaccine quality (manufacturing) and a need to expand the antigen composition of the vaccine. This study is registered at www.clinicaltrials.gov NCT00071214.
Abbreviations:
Introduction
Staphylococcus aureus, a human commensal frequently carried in the nose and on the skin, is the most common nosocomial pathogen, responsible for approximately 16% of healthcare-associated infections.Citation1 Patients with end-stage renal disease (ESRD) who are receiving hemodialysis are at high long-term risk of S. aureus infection as a result of being immune compromised and the need for regular vascular access.Citation2,3 The incidence of culture-confirmed S. aureus bacteremia in more than 293000 patients receiving chronic hemodialysis in the United States was 4.0 per 100 outpatient-years.Citation4 In the United States, between one-third and one-half of S. aureus bacteremias in hemodialysis patients are due to multi-resistant S. aureus strains.Citation1,4-6 Healthcare costs associated with S. aureus bacteremia in hemodialysis patients are substantial.Citation5 Complications include endocarditis, osteomyelitis, discitis and soft tissue abscesses, and the case fatality rate is as high as 20%.Citation5,7 In hemodialysis patients the type of vascular access may predispose toward bacteremia, with higher rates of S. aureus bacteremia observed in patients with venous catheters in situ than in patients with arteriovenous fistulas.Citation8,9
S. aureus produces a range of virulence factors and toxins that contribute to its invasive capacity and ability to evade host defense systems.Citation10 Several virulence factors have been investigated as possible candidates for active or passive vaccination against S. aureus. However as yet, none have been licensed for use. Several immunotherapy candidates failed to show efficacy in humans: Altastaph (Nabi Biopharmaceuticals) containing S.aureus capsular polysaccharides type 5 (T5) and type 8 (T8) antibodies purified from subjects vaccinated with StaphVAX™ (investigational vaccine originally developed by Nabi Biopharmaceuticals, Rockville, MD, USA); Veronate (Inhibitex), polyclonal antibodies targeting S. aureus clumping factor A (ClfA) and S. epidermidis adhesion SdrG; Aurexis (Tefibazumab, Inhibitex), monoclonal antibodies targeting ClfA; Aurograb (NeuTec Pharma), single chain antibodies against an ATP-binding cassette transporter; and Pagibaximab (Biosynexus), a monoclonal anti-lipoteichoic acid antibody.Citation11,12
More recently, the V710 IsdB vaccine (Merck &Co) which contains an iron scavenging protein, failed to demonstrate efficacy against staphylococcal bacteremia and/or deep sternal wound infections in cardiothoracic surgery patients.Citation13 The vaccine was associated with increased mortality among patients who developed S. aureus infections.Citation13
The S. aureus capsular polysaccharides prevent opsonophagocytotic killing by neutrophils, resulting in bacterial clearance by the host.Citation14 S. aureus capsular T5 and T8 account for over 85% of clinical isolates.Citation15-17 StaphVAX™ is an investigational bivalent S. aureus T5 and T8 capsular polysaccharide conjugate vaccine. In a phase III study in ESRD patients on hemodialysis (study 1356, www.clinicaltrials.gov NCT00071214), a single dose of StaphVAX™ showed partial efficacy (57%, 95% confidence interval [CI] 10; 81) against S. aureus bacteremia over the first 40 weeks of follow-up, although no efficacy was shown 1 year post-vaccination.Citation18 Rapid antibody decline during the study suggested that potential benefits in prolonging protection could be gained by administration of a second dose. We therefore evaluated the immunogenicity, safety and efficacy of StaphVAX™ in preventing S. aureus bacteremia in ESRD patients for up to 35 weeks after a single dose, and for up to 60 weeks after 1 or 2 doses (study 1371, www.clinicaltrials.gov NCT00071214). Evaluation of health economic outcomes of patients with S. aureus bacteremia enrolled in this study has been reported elsewhere.Citation5
Results
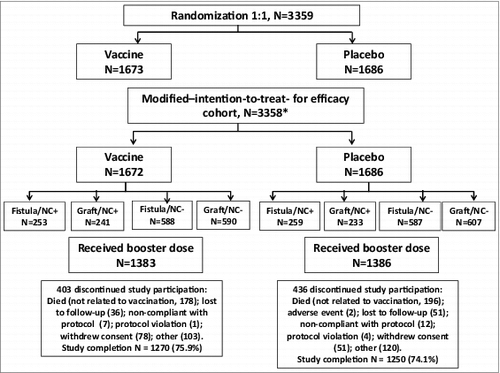
There were 3359 patients who were randomized (1:1) to receive 2 doses of the investigational vaccine or placebo (phosphate-buffered saline) at weeks 0 and 35. Of these, 3358 were included in the modified-intention-to-treat-for-efficacy cohort (). One quarter of subjects (25.8% in the placebo group and 24.1% in the vaccine group) withdrew from the study, mostly due to death unrelated to vaccination (). The two groups were balanced with regard to age, sex and race ().
Table 1. Summary of demographic characteristics (all randomized subjects)
Figure 1. Study flow Randomization was stratified by (1) the use of a native-vessel fistula or synthetic/heterologous graft for vascular access, and (2) the presence (NC+) or absence (NC-) of S. aureus nasal carriage. N = total number of patients within treatment group *One patient was not included in the modified intent-to-treat-or-efficacy cohort due to known serious S. aureus infection within 3 months of injection. Vaccine = S. aureus polysaccharide conjugate vaccine Placebo = phosphate-buffered saline.
The primary efficacy study endpoint was the incidence of culture-proven first-time S. aureus bacteremia that occurred during the 3-35 week period following dose 1. Strains that were typed but were not T5 or T8 were excluded from the primary analyses. Strains not received by Nabi Pharmaceuticals for typing were classified as “not typed."
Efficacy
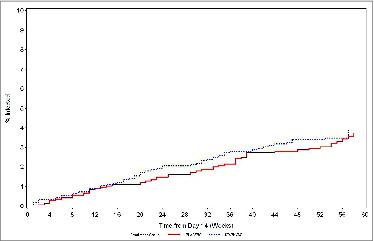
There was no protective effect of the vaccine on the incidence of first time S. aureus bacteremia between weeks 3-35 (primary endpoint), or between weeks 3-60 either overall, or considering intravenous access type (native-vessel fistula or synthetic/heterologous graft, but not catheter) or S.aureus nasopharyngeal carriage status (, ). Between weeks 3–35, 12 (32%) T5, 12 (32%) T8, and 14 (37%) “not typed” S. aureus bacteremia cases were reported in the vaccine group. In the same period, 13 (42%) T5, 5 (16%) T8 and 13 (42%) “not typed” S. aureus bacteremia cases were reported in the placebo group. Between weeks 3-60, 19 (34%) T5, 17 (30%) T8 and 20 (36%) “not typed” S. aureus bacteremia cases were reported in the vaccine group, versus 21 (40%) T5, 12 (23%) T8, and 19 (37%) “not typed” S. aureus bacteremia cases reported in the placebo group. There was no significant difference between the vaccine and placebo groups in the distribution of time-to-S. aureus bacteremia (2-sided stratified logrank test, p=0.39 for week 3–35, and p=0.7 for week 3–60 follow-up periods; ).
Table 2. Person-time rates of S. aureus bacteremia and vaccine efficacy by dialysis access mode and nasal carriage between weeks 3-60 (modified intention-to-treat-for-efficacy cohort).
Figure 2. Kaplan-Meier estimate of time-to-S. aureus bacteremia in the vaccine and placebo groups (weeks 3–60, Modified–intention-to-treat-for-efficacy cohort).
None of the evaluated risk factors (age, gender, presence of diabetes or prior hemodialysis infection experience) influenced the effect (or lack thereof) of the study vaccine (p > 0.05 for all risk factors).
Among the S. aureus bacteremias reported but not included in the primary efficacy analyses, 4 were episodes reported before week 3 (all in the vaccine group and all T5), and 18 were strain 336 episodes reported between weeks 3-60 (10 in the vaccine group, 8 in the placebo group).
Immunogenicity
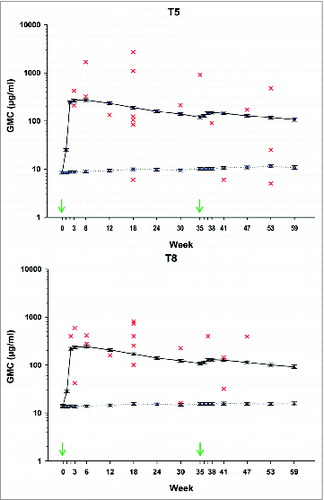
Anti-S. aureus antibodies to capsular polysaccharides T5 (“anti-T5”) and T8 (“anti-T8”) geometric mean antibody concentrations (GMCs) peaked at day 42 following the first vaccine dose (). At day 42, the anti-T5 GMC was 272.4 μg/ml in the vaccine group and 8.9 μg/ml in the placebo group (), and the anti-T8 GMC was 242.0 μg/ml in the vaccine group and 13.8 μg/ml in the placebo group (). Antibody GMCs increased after dose 2 (GMC at day 266 in the vaccine group 149.1 μg/ml for anti-T5 and 130.1 μg/ml for anti-T8) but were lower than after dose 1. Thus, the observed booster effect was minimal.
Figure 3. Anti-T5 (A) and anti-T8 (B) capsular polysaccharide geometric mean antibody concentrations (GMC) in vaccine and placebo groups (Modified–intention-to-treat-for-efficacy cohort). Individual antibody concentrations in patients reporting S. aureus T5 (A) or T8 (B) bacteremia are shown for the vaccine group. Red crosses represent individual anti-T5 or anti-T8 antibody concentrations of patients in the vaccine group reporting S. aureus Type 5 or Type 8, bacteremia, respectively at the closest time point prior to the onset of the disease. Black = GMC in the vaccine group, blue = GMC in the placebo group. Error bars: 95% confidence interval. Arrows indicate vaccination time points.
Assessment of vaccine efficacy failure
Capsular expression of infection isolates collected during the study was assessed. The vast majority were either T5 or T8, or non-capsulated strains. Some of the T5 or T8 isolates reacted with both capsular-specific and 336 antibodies, indicating that these isolates were partially encapsulated.Citation19
The antibody concentration against the capsular serotype of the infecting S. aureus strain measured at the closest time point prior to bacteremia was displayed for all vaccine failures (). No trend for lower responses in cases of vaccine failure was observed.
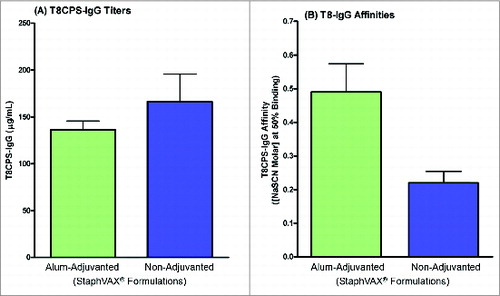
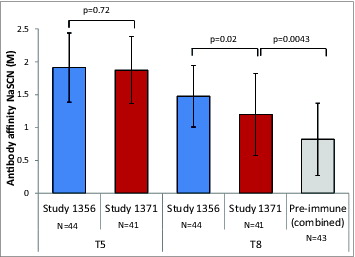
To assess the reasons for failure to demonstrate efficacy, the immune responses induced by vaccination were further characterized and compared with those induced by the vaccine lot used in the previous phase III study in patients with ESRD (study 1356).Citation18 Comparison of the individual sera from patients enrolled in the previous study 1356 with those from patients enrolled in the present study (1371) showed no significant difference in terms of antibodies when measured by enzyme-linked immunosorbent assay (ELISA) and opsonophagocytic killing assay (OPK) (). Affinity of antibodies to T8 but not T5 was lower in the present study compared to study 1356 (T8 mean 1.199M vs. 1.477M, p=0.02; T5 mean 1.873M versus 1.913M, p=0.72, unpaired t-test, ). However, T8 antibody affinity was higher after vaccination in study 1371 compared to the pooled group of pre-vaccination sera and sera from unvaccinated healthy subjects (1.199M vs. 0.822M, P < 0.0043, unpaired t-test, ).
Figure 4. Opsonophagocytosis of S. aureus type 5 and type 8 mediated by antibodies from the present study and study 1356Citation18 (day 42 samples).
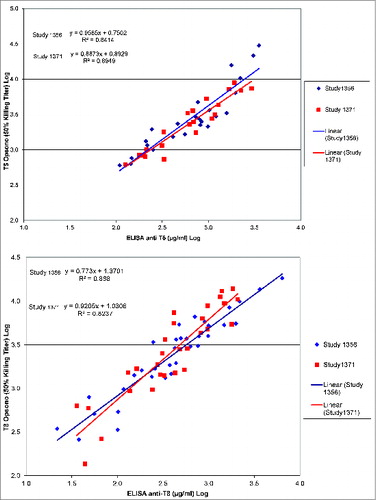
Figure 5. Geometric mean affinity of anti-T5 and anti-T8 antibodies from the present study (1371), study 1356Citation18 (day 42 samples), and compared to pooled pre-immune sera in ESRD patients and unvaccinated healthy individuals. Footnote to figure: NaSCN (M) = Sodium Thiocyanate concentration (Molar). Vertical lines represent standard deviation. N = number of samples.
In the mouse lethality model, animals injected with antibodies collected from patients in the present study (1371) exhibited sub-optimal protection (40% survival) against a T8 challenge as compared to antibodies derived from patients in study 1356 (91% survival) (). Protection against T5 lethal challenge was similar between lots.
Table 3. Passive protection in mice afforded by antibodies from patients given bivalent S. aureus conjugate vaccine against lethal challenge with S. aureus T5 or T8
Similarly, animals vaccinated with the same lots as those used in the present study (1371) and the previous study (study 1356) showed that the 1371 lot afforded sub-optimal protection (37% survival) against T8 challenge as compared to the lot used in study 1356 (83% survival) (). As for passive immunization experiments, protection against T5 lethal challenge following active immunization was similar between lots.
Table 4. Protection in mice afforded by vaccination by vaccine lots used in studies 1356 and 1371 against lethal challenge with S. aureus T5 or T8 isolates
Taken together, we hypothesized that the vaccine used in the present study may have been suboptimal, generating antibodies of lower quality and functionality than the vaccine used in the previous 1356 study, at least for T8. This hypothesis was substantiated by testing the re-formulated 1371-alum-adjuvanted vaccine against the same vaccine in PBS in the animal challenge model. There were 73% (11/15) of animals that received the alum-formulated vaccine who survived the T8 challenge compared to only 7% (1/15) in the group that received the vaccine in PBS, despite the induction of similar levels of anti-T8-specific IgG induced by both vaccines (). Furthermore, the formulation in alum restored antibody affinity generated by the 1371-vaccine ().
Safety
SAEs were reported by 829 of patients (24.7%) of whom 402 (24%) were in the vaccine group and 427 (25.3%) were in the placebo group. The SAEs reported encompassed 19 MedDRA System Organ Classes. Across both treatment groups, SAEs occurred most frequently under “Cardiac Disorders” (7.8% of vaccines and 8.7% of placebo recipients). The second most common was “Infections and Infestations” (6.0% and 5.7%, respectively). The four most frequent Preferred Terms were “cardiac arrest” (2.0% of vaccinees and 2.4% of placebo recipients), “congestive cardiac failure” (1.3% and 1.2%, respectively), “pneumonia” (1.1% and 0.9%, respectively) and acute myocardial infarction (1.0% in each group). Two SAEs in each treatment group were considered to be possibly related to study vaccine/placebo: one case of pyrexia in each treatment group, limb abscess in one vaccine recipient, and angioneurotic edema in one placebo recipient. There was one case of multi-organ failure (0.1%) in the placebo group and 4 cases (0.2%) in the vaccine group (p > 0.05). No cases of multi-organ failure occurred in patients with S. aureus bacteremia.
There were 374 deaths reported during the entire study, 178 (10.6%) in the vaccine group and 196 (11.6%) in the placebo group (). No deaths were considered related to the study vaccine/placebo. Among patients that reported at least one S. aureus bacteremia, 15/52 (29%) died in the placebo group and 14/59 (24%) died in the vaccine group.
Table 5. Summary table of deaths
Discussion
Despite robust antibody responses to primary vaccination, this study failed to demonstrate VE of the bivalent S. aureus T5 and T8 conjugate vaccine in preventing S. aureus bacteremia in ESRD patients receiving hemodialysis at any time interval studied, or in any strata considered. These results contrast with a previous clinical trial (1356) conducted in a similar population, which suggested short term (to 40 weeks) efficacy against S. aureus bacteremia after a single dose of StaphVAX™.Citation18 Of note, the positive results for short-term efficacy were not the primary objective of the 1356 trial and were observed after post-hoc analyses.
One possible contributing factor to the failure of the vaccine is the immunological impairment associated with ESRD and hemodialysis that may result from hyper-uremia syndrome, underlying disease such as diabetes, and complement depletion due to the dialysis process. Of importance, neutrophils of ESRD patients have reduced phagocytotic and killing ability. While vaccination successfully induced functional antibodies, patients with ESRD typically show deficiencies in antigen-presenting cells and T-lymphocytes,Citation3,20Citation-22 which may also have been important for protection. Comparison of the population enrolled in this study to that of the population enrolled in study 1356 failed to identify differences in the health status, frequency of underlying disease, dialysis procedures, or routine treatment provided for these ESRD patients. Although S. aureus infections appeared to occur more frequently in patients with nasal S. aureus colonization, VE did not differ between colonized or non-colonized subjects. We did not assess the effect of vaccination on nasophayngeal carriage, but another study showed similar immune responses to StaphVAX™ vaccination in subjects with and without nasal colonization with S. aureus, and no impact of vaccination on nasal colonization.Citation23
Polysaccharide capsule expression may vary according to S aureus strain, and strains not expressing a capsule may express a cell-wall polysaccharide 336.Citation19 About one third of bacteremia cases included in the primary analysis relate to strains that were unavailable for typing, raising questions on the capsulation of these strains. However as expected,Citation15 approximately 80% of all typed strains in our study were T5 or T8. We therefore extrapolated that the same proportion of T5 or T8 strains would apply to the bacteremia cases classified as “not typed." If we except US300 epidemic strain, the majority of human infection S. aureus strains express capsule in humans,Citation19 and we consider it unlikely that the lack of efficacy in our study can be attributed solely to uncapsulated S. aureus strains.
Studies of isogenic T5 and T8 S. aureus strains indicate that T5 strains are more virulent than T8 strains, inducing less opsonophagocytic killing and longer survival in infected mice.Citation24 The efficacy against S. aureus in study 1356 was mostly directed toward T8 strainsCitation17 and we observed no VE against T5 in the present study. These data may indicate that patients with ESRD are less able to clear more virulent (T5) infections than less virulent strains. We are unable to test this hypothesis in the framework of the present study as we did not assess the virulence of the T5 versus the T8 strains isolated from patients.
We undertook additional investigations to explore possible reasons for the lack of efficacy in the present study. ELISA antibody responses induced by vaccination were robust and of a similar magnitude to those observed in study 1356. In the majority of vaccinated patients who experienced T5 or T8 S. aureus bacteremia, the antibody concentration measured at the time point just prior to the onset of the disease was substantially higher than in the placebo group. After the second dose given at week 40, the booster effect was only minimal, possibly due to the short period between dose 1 and dose 2 and high levels of antibodies persisting at the time of the second dose.
Comparison of the individual sera from patients enrolled in each study showed no significant difference in terms of OPK. However, affinity of antibodies to T8 (but not T5) tended to be lower than those generated by the lot used in the previous study, and passive or active immunization of mice with the present vaccine lot resulted in suboptimal protection against a lethal T8 challenge. When the vaccine used in this study was adjuvanted with alum, it generated significantly higher affinity antibodies in mice and restored protection of vaccinated mice against T8 challenge. Altogether, the data suggest that the quality of the vaccine lot (for T8) used in the present study may have been suboptimal. Nevertheless, the lack of efficacy against both T5 and T8 strains argues against this being the only factor responsible for the failure of the vaccine.
Another explanation could be that anti-capsular antibodies do not protect against S. aureus infections. In view of the results in animals and in the previous clinical trial, we consider this unlikely, especially for T8. However, although opsonophagocytosis is important in preventing gram positive infections, anti-capsular polysaccharide opsonic antibodies alone may not be sufficient for protection against invasive S. aureus isolates.Citation24 Failure to protect study subjects could not be correlated to a lack of vaccine-induced antibodies in either study 1356,Citation18 or the present study. It was recently suggested that although opsonization of S aureus enhanced phagocytosis by neutrophils in suspension, there was a weak correlation between uptake of S aureus and subsequent killing of the bacteria by neutrophils.Citation25 In view of the importance of toxins in S. aureus infections, toxin neutralizing antibodies may be needed to protect against infection.Citation26 Additionally, the role of T-cell mediated immunity may be important in protection against S.aureus bacteremia.Citation27-30 The ability of S. aureus to evade immune-protective mechanisms and induce immune-tolerance needs also to be considered when designing new vaccines.Citation31,32
Consistent with the previous study (1356), the vaccine was well-tolerated, with no significant differences between vaccine or placebo groups in terms of nature or incidence of SAEs or deaths.
In conclusion, the bivalent conjugate vaccine did not protect against T5 or T8 bacteremia even though vaccination induced a robust antibody response to both T5 and T8 S. aureus capsular polysaccharides. No differences in the study populations were identified. Factors that may have contributed to the results include aspects of vaccine quality (for the T8 component) and the target of only one virulence factor (the polysaccharide capsule) by the vaccine. Given the multi-factorial nature of the pathogenesis of staphylococcal infections, a successful staphylococcal vaccine should include in addition to the polysaccharide conjugates generating opsonic antibodies, a component that would generate toxin neutralizing antibodies. Alpha toxin, the most prominent and widespread toxin, was selected as a first choice. Assessment of a bivalent α toxin and PVL (panton valentine leukocidin) vaccine induced antibody responses with neutralizing activity in healthy adults.Citation33,34 Finally, T-cell-mediated immunity may also play a role in protection against disease.
Methods
Study objectives
The primary study objective was to demonstrate vaccine efficacy (VE) in reducing the incidence of S. aureus bacteremia for up to 8 months (i.e., from weeks 3-35 post-vaccination) following a first dose of vaccine in patients with ESRD receiving hemodialysis. Secondary objectives included assessment of cumulative efficacy after 1 or 2 vaccine doses from week 3–60 post-dose 1 (the second dose was administered at week 35); assessment of efficacy in sub-strata defined by fistula/graft status, nasopharyngeal carriage of S. aureus, and immunogenicity after one and 2 vaccine doses. Assessment of vaccine safety was also a secondary objective. In view of the recent termination of the Merck & Co study of V710,Citation13 a post-hoc analysis of safety in terms of episodes of multi-organ failure and death was performed.
Study subjects and design
The phase III study was prospective, randomized, placebo-controlled and double-blind, conducted at 163 dialysis centers in the United States between 29 September 2003 and 23 September 2005. The study was conducted according to the Declaration of Helsinki (1996). The protocol and associated documents were reviewed and approved by the Schulman Associates Institutional Review Board, Cincinnati, Ohio, as well as local institutional review boards when required. All patients gave written informed consent prior to enrolment.
Patients were to be at least 18 years of age with a diagnosis of chronic ESRD receiving maintenance hemodialysis for at least 8 weeks prior to enrollment. Hemodialysis access using native vessel fistula or synthetic/heterologous graft was allowed. Women of childbearing potential were to have a negative serum pregnancy test within 7 days prior to vaccination.
Patients were excluded from participation if they had known serious S. aureus infection within 3 months prior to study entry, or known recurrent S. aureus infection of the current graft. Patients were also excluded if they had active viral or bacterial infection or symptoms/signs consistent with infection within 2 weeks prior to vaccination. Subjects known to be positive for human immunodeficiency virus, subjects who had known hypersensitivity or previous anaphylaxis to polysaccharides or polysaccharide-conjugate vaccines, or to components of such vaccines, and subjects with known/suspected drug abuse in the past year were also excluded. Current use of immunosuppressive or immunomodulatory drugs (10 mg of prednisone or equivalent per day was allowed), known malignancy or treatment for malignancy within the past 6 months, and previous receipt of the study vaccine were exclusion criteria.
Randomization was stratified by the type of venous access (native-vessel fistula or synthetic/heterologous graft, but not catheter), and by the presence or absence of S. aureus nasal carriage, defined by the recovery of S. aureus in at least one of 2 consecutive nasal swab cultures obtained at least 4 days apart.
Vaccine
The investigational bivalent S. aureus T5 and T8 capsular polysaccharide conjugate vaccine contained 100μg of each capsular polysaccharide conjugated to a total of 200μg recombinant non-toxic variant of Pseudomonas aeruginosa exotoxin A (rEPA). Vaccine and placebo were administered intramuscularly into the deltoid.
Assessment of efficacy
All episodes of bacteremia were recorded. An episode of bacteremia was considered new when at least one blood culture was positive for a bacterium and if the species had not been isolated from blood within the previous 10 days; if the isolate was obtained after antibiotic therapy had been terminated; or if a previously recovered species was detected after at least 10 days of systemic antibiotic therapy (to which the original isolate was sensitive), and for which at least 2 blood cultures obtained at least 24 hours apart had been negative for that same organism.
S. aureus isolates were to be sent to Nabi Biopharmaceuticals, Rockville, USA for serotyping.
Assessment of immunogenicity
Blood samples were tested for S. aureus serology on the day of each vaccination (day 0 and 245) and 7, 14, 21, 42, 84 126, 168 days after each dose. An additional blood sample was taken on day 210 after dose 1. Anti- T5 and anti-T8 were measured by ELISA (lower limit of detection is 0.1 μg/ml) .Citation35,36
Post-hoc assessments of immunogenicity
Additional assessments of immunogenicity were done after unblinding to investigate possible reasons for failure to demonstrate efficacy.
OPK antibodies from serum samples from study subjects who had participated in the present study (1371) and the previous study (1356) reported by Shinefield et al,Citation18 were determined using an HL60 cell line and guinea pig complement as previously described.Citation35,37 The OPK titer was determined as the reciprocal of the highest dilution yielding 50% killing of bacteria. OPK assay was done on a subset of equally representative day 42 samples from the whole population that were randomly chosen to include high, medium and low responders.
Antibody affinity was measured by an ELISA method in which sodium thiocyanate (NaSCN) was used to dissociate immune complexes, assuming that the higher the affinity, the more NaSCN is needed to dissociate the antibody-antigen complex.Citation38 Affinity assays were done on subsets of Day 42 samples from subjects in both studies (1356 and 1371), selected to equally represent the 4 quartiles of IgG levels. Pre-vaccination sera from study 1371 and sera from unvaccinated healthy adults were also assessed for T8 antibody affinity at baseline.
A murine lethal challenge model assessed passive and active protection against lethal S. aureus infection. Passive protection was assessed from purified and quantified IgG obtained from serum samples from study subjects who had participated in the present study (1371) and the previous study reported by Shinefield et al,Citation18 (study 1356). Balb/c mice received 400μg of capsular polysaccharide specific IgG 48 hours prior to challenge. Controls received AltaStaph™ (hyperimmune IgG prepared from plasma donors who had received the vaccine) and MEP-IVIG (IgG prepared from plasma donors immunized with P. aeruginosa mucoexopolysaccharide). A separate control group consisted of mice who received PBS. Protective efficacy of active vaccination was assessed from lots used in study 1356 and 1371 (or placebo). Mice received 3 vaccine doses (subcutaneously) at 2 week intervals. Seven days after the last vaccination mice received a standardized aliquot (1-2.5x10Citation5 CFU/ml administered intraperitoneally), containing S. aureus T5, (ST021) or T8 (K17654) (strains isolated from clinical trial participants). Post-challenge morbidity and survival was recorded until study termination on day 5-7 post-challenge.
Finally, to assess the possible role of poor antibody affinity on lack of protection, we re-formulated the 1371 vaccine in alum and compared it to the non-adjuvanted vaccine in PBS in the mouse lethal challenge model. Affinity of antibodies generated by the 2 formulations was evaluated.
Nasopharyngeal carriage
Nasal swabs (CultureSwab, Becton Dickinson) were collected on 2 occasions during screening visits prior to vaccination for culture and identification of S. aureus. Nasal swabs were advanced upward and backward toward the vertex of the head until meeting gentle resistance. The swab was rotated 360°, removed and placed back into the sleeve containing culture medium.
Assessment of safety
Serious adverse events (SAEs) were captured for 42 days following both injections by history and physical examination. An assessment of severity and potential relationship to the study vaccine was made by the site Investigator. Deaths were captured throughout the entire study duration.
Statistical analysis and sample size
The primary cohort for the efficacy and immunogenicity analyses was the modified intent-to-treat-for-efficacy cohort, which included all injected subjects except those with known serious S. aureus infection within 3 months prior to injection, or known recurrent S. aureus infection of their current graft. VE was assessed for 3–35 weeks post-dose 1. The cumulative efficacy of 2 doses was assessed for the 3 to 60 week period post-dose 1.
The incidence rate of first-time S. aureus bacteremia (type 5, type 8 or not typed) was compared between the vaccine and placebo groups by an exact, 2-sided stratified person-time incidence calculation.Citation35,36 Four strata were defined by baseline nasal carriage status (positive/negative) and vascular access modality (graft or fistula). Person-time infection rate was equal to the number of infections divided by the accumulated person-time.
VE (with 95% CI) was computed as 1-ψ, where ψ is the common ratio of S. aureus bacteremia incidence in vaccine group relative to control group. The time-to-first S. aureus bacteremia distributions in the vaccine and placebo groups were displayed by Kaplan-Meier curves and compared by a 2-sided stratified log-rank test.
A stratified Cox model for time-to-first S. aureus bacteremia was performed as secondary analysis. This model included treatment, age, gender, diabetes and prior hemodialysis infection experience as covariates, with strata used as a stratification variable.
Anti-T5 and anti-T8 GMCs at each pre-defined time point were calculated in all patients.
Safety analyses were performed on all randomized patients who had received at least 1 dose of vaccine or placebo. A post-hoc exploratory analysis was done to compare the rate of multi-organ failures between groups by Fisher's exact test.
For all tests, differences were considered statistically significant if p-values were <0.05.
Based on data from the previous efficacy study,Citation18 3600 patients (3240 evaluable after allowing a 10% dropout rate) were considered sufficient to yield the necessary number of events to detect a difference in person-time incidence rates of S. aureus bacteremia between the 2 study groups with 80% power and an efficacy of at least 50% (2-sided α of 5%).
StaphVAX™ is a trademark of the Nabi Biopharmaceuticals.
Disclosure of Potential Conflicts of Interest
AF and KT were full-time employees of Nabi Biopharmaceuticals at time of study conduct. AF is currently employed by NanoBio Corporation. KT is currently employed by National Institute of Allergy and Infectious Diseases (NIAID). AM and JB received a study grant paid to their institution from Nabi Biopharmaceuticals for participation in this study, but declare no other conflicts of interest. SD and DB are full-time employees of GlaxoSmithKline Vaccines. DB declares ownership of GSK Vaccines stocks. DB is also an inventor of certain GSK patents.
Acknowledgments
The authors thank the volunteers who participated in the study, as well as the investigators, study nurses and other (including former) staff members from Nabi Biopharmaceuticals. The authors also thank Sophie Germain and Hugues Wallemacq from GlaxoSmithKline Vaccines R&D for their input in the manuscript, Dr Joanne Wolter (independent) for writing the first draft of the manuscript, Dr Wouter Houthoofd (XPE Pharma & Science) for publication coordination on behalf of GlaxoSmithKline Vaccines.
Funding
Nabi Biopharmaceuticals was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and the corresponding author was responsible for submission of the publication.
References
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 2013; 34:1-14; PMID:23221186; http://dx.doi.org/10.1086/668770
- Vandecasteele SJ, Boelaert JR, De Vriese AS. Staphylococcus aureus infections in hemodialysis: what a nephrologist should know. Clin J Am Soc Nephrol 2009; 4:1388-400; PMID:19590063; http://dx.doi.org/10.2215/CJN.01590309
- Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008; 3:1526-33; PMID:18701615; http://dx.doi.org/10.2215/CJN.00950208
- Chan KE, Warren HS, Thadhani RI, Steele DJR, Hymes JL, Maddux FW, Hakim RM. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol 2012; 23:1551-9; PMID:22904350; http://dx.doi.org/10.1681/ASN.2012010050
- Li Y, Friedman JY, O’Neal BF, Hohenboken MJ, Griffiths RI, Stryjewski ME, Middleton JP, Schulman KA, Inrig JK, Fowler VG Jr, et al. Outcomes of Staphylococcus aureus infection in hemodialysis-dependent patients. Clin J Am Soc Nephrol 2009; 4:428-34; PMID:19118117; http://dx.doi.org/10.2215/CJN03760708.
- Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, Ray SM, Harrison LH, Lynfield R, Dumyati G, et al. Health care-associated invasive MRSA infections, 2005-2008. JAMA 2010; 304:641-8; PMID:20699455.
- Fitzgerald SF, O’Gorman J, Morris-Downes MM, Crowley RK, Donlon S, Bajwa R, Smyth EG, Fitzpatrick F, Conlon PJ, Humphreys H. A 12-year review of Staphylococcus aureus bloodstream infections in haemodialysis patients: more work to be done. J Hosp Infect 2011; 79:218-21; PMID:21856042; http://dx.doi.org/10.1016/j.jhin.2011.06.015
- Moist LM, Trpeski L, Na Y, Lok CE. Increased hemodialysis catheter use in Canada and associated mortality risk: data from the Canadian Organ Replacement Registry 2001-2004. Clin J Am Soc Nephrol 2008; 3:1726-32; PMID:18922993; http://dx.doi.org/10.2215/CJN.01240308
- Crowley L, Wilson J, Guy R, Pitcher D, Fluck R. Chapter 12 Epidemiology of Staphylococcus aureus bacteraemia amongst patients receiving dialysis for established renal failure in England in 2009 to 2011: a joint report from the Health Protection Agency and the UK Renal Registry. Nephron Clin Pract 2012; 120(Suppl 1):c233-45; PMID:22964570; http://dx.doi.org/10.1159/000342856
- Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol 2012; 2:12; PMID:22919604
- Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am 2009; 23:153-71; PMID:19135920; http://dx.doi.org/10.1016/j.idc.2008.10.005
- Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine 2012; 30:2921-7; PMID:22115633; http://dx.doi.org/10.1016/j.vaccine.2011.11.006
- Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 2013; 309:1368-78; PMID:23549582; http://dx.doi.org/10.1001/jama.2013.3010
- O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 2004; 17:218-34; PMID:14726462; http://dx.doi.org/10.1128/CMR.17.1.218-234.2004
- Von Eiff C, Taylor KL, Mellmann A, Fattom AI, Friedrich AW, Peters G, Becker K. Distribution of capsular and surface polysaccharide serotypes of Staphylococcus aureus. Diagn Microbiol Infect Dis 2007; 58:297-302; PMID:17376630; http://dx.doi.org/10.1016/j.diagmicrobio.2007.01.016
- Roghmann M, Taylor KL, Gupte A, Zhan M, Johnson JA, Cross A, Edelman R, Fattom AI. Epidemiology of capsular and surface polysaccharide in Staphylococcus aureus infections complicated by bacteraemia. J Hosp Infect 2005; 59:27-32; PMID:15571850; http://dx.doi.org/10.1016/j.jhin.2004.07.014
- Verdier I, Durand G, Bes M, Taylor KL, Lina G, Vandenesch F, Fattom AI, Etienne J. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J Clin Microbiol 2007; 45:725-9; PMID:17202275; http://dx.doi.org/10.1128/JCM.01572-06
- Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346:491-6; PMID:11844850; http://dx.doi.org/10.1056/NEJMoa011297
- Sutter D, Summers A, Keys C, Taylor KL, Frasch C, Braun L, Fattom A, Bash M. Capsular serotype of Staphylococcus aureus in the era of community-acquired MRSA. FEMS Immunol Med Microbiol 2011; 63:16-24; PMID:21631600; http://dx.doi.org/10.1111/j.1574-695X.2011.00822.x
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial 2007; 20:440-51; PMID:17897251; http://dx.doi.org/10.1111/j.1525-139X.2007.00283.x
- Litjens NHR, Huisman M, van den Dorpel M, Betjes MG. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol 2008; 19:1483-90; PMID:18480314; http://dx.doi.org/10.1681/ASN.2007090971
- Agrawal S, Gollapudi P, Elahimehr R, Pahl MV, Vaziri ND. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol Dial Transplant 2010; 25:737-46; PMID:19903659; http://dx.doi.org/10.1093/ndt/gfp580
- Creech CB 2nd, Johnson BG, Alsentzer AR, Hohenboken M, Edwards KM, Talbot TR 3rd. Vaccination as infection control: a pilot study to determine the impact of Staphylococcus aureus vaccination on nasal carriage. Vaccine 2009; 28:256-60; PMID:19799842; http://dx.doi.org/10.1016/j.vaccine.2009.09.088
- Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun 2005; 73:3502-11; PMID:15908379; http://dx.doi.org/10.1128/IAI.73.6.3502-3511.2005
- Lu T, Porter AR, Kennedy AD, Kobayashi SD, Deleo FR. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. Infection and Immunity 2014; PMID:24713863.
- Cheung GYC, Otto M. The potential use of toxin antibodies as a strategy for controlling acute Staphylococcus aureus infections. Expert Opin Ther Targets 2012; 16:601-12; PMID:22530584; http://dx.doi.org/10.1517/14728222.2012.682573
- Ardura MI, Banchereau R, Mejias A, Di Pucchio T, Glaser C, Allantaz F, Pascual V, Banchereau J, Chaussabel D, Ramilo O. Enhanced monocyte response and decreased central memory T cells in children with invasive Staphylococcus aureus infections. PLoS ONE 2009; 4:e5446; PMID:19424507; http://dx.doi.org/10.1371/journal.pone.0005446
- Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:560-7; PMID:22186773; http://dx.doi.org/10.1093/cid/cir828
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009; 5:e1000703; PMID:20041174; http://dx.doi.org/10.1371/journal.ppat.1000703
- Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, Avanesian V, Bayer AS, Filler SG, Lipke P, Otoo H, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 2008; 76:4574-80; PMID:18644876; http://dx.doi.org/10.1128/IAI.00700-08
- Wang J, Roderiquez G, Norcross MA. Control of adaptive immune responses by Staphylococcus aureus through IL-10, PD-L1, and TLR2. Sci Rep 2012; 2:606; PMID:22930672
- Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 2012; 15:92-9; PMID:22088393; http://dx.doi.org/10.1016/j.mib.2011.10.012
- Lalani T, Tribble D, Landrum M, Stewart S, Niknian M, Fattom A, Fraser J, Wilkins K, Kessler P, Fahim R, et al. Neutralizing activity of antibodies following vaccination with Staphylococcus aureus recombinant α-toxoid (rAT) and recombinant Panton-Valentine Leukocidin toxoid (rLukS-PV). In: ID Week. San Francisco: 2013.
- Landrum M, Lalani T, Niknian M, Maguire J, Fraser J, Wilkins K, Ellis M, Kessler P, Fahim R, Tribble D. A randomized, multi-center trial to evaluate the safety and immunogenicity of Staphylococcus aureus toxoids, rAT and rLukS-PV, in healthy volunteers. Boston, MA, USA: 2011.
- Fattom A, Schneerson R, Szu SC, Vann WF, Shiloach J, Karakawa WW, Robbins JB. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun 1990; 58:2367-74; PMID:2114365
- Fattom A, Shiloach J, Bryla D, Fitzgerald D, Pastan I, Karakawa WW, Robbins JB, Schneerson R. Comparative immunogenicity of conjugates composed of the Staphylococcus aureus type 8 capsular polysaccharide bound to carrier proteins by adipic acid dihydrazide or N-succinimidyl-3-(2-pyridyldithio)propionate. Infect Immun 1992; 60:584-9; PMID:1730492
- Fattom A, Fuller S, Propst M, Winston S, Muenz L, He D, Naso R, Horwith G. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 2004; 23:656-63; PMID:15542186; http://dx.doi.org/10.1016/j.vaccine.2004.06.043
- Granoff DM, Maslanka SE, Carlone GM, Plikaytis BD, Santos GF, Mokatrin A, Raff HV. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol 1998; 5:479-85; PMID:9665952