Abstract
Vaccination of children has had a major impact on the morbidity and mortality of many infectious diseases globally. However, with age, immune responses to vaccines can be less robust, which can be further enhanced by underlying diseases that are common in the older adult. In many countries around the globe booster vaccinations against diphtheria, tetanus, and pertussis are recommended for adults. For the older adult, vaccination against pneumococcal diseases, influenza and herpes zoster are also recommended. Despite these recommendations, the widespread use of these vaccines in the adult population clearly lags behind the vaccine uptake and successes documented for pediatric vaccination programs. Furthermore, extensive and sometimes inappropriate use of antibiotics have fostered the emergence of antibiotic-resistant bacteria (e.g., methicillin resistant Staphylococcus aureus (MRSA)) as well as increased susceptibility in the elderly to bacterial species such as Clostridium difficile. Infectious diseases remain an important unmet medical need and new concepts to successfully implement vaccination of adults are urgently needed.
Importance of Adult/Elderly Vaccination
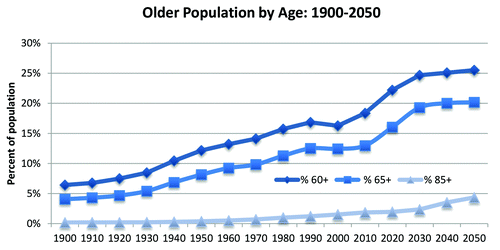
Vaccines have provided a more positive impact on public health globally than any other medical intervention. The majority of vaccines developed to date have targeted the pediatric population. Vaccine uptake for this population overall is usually well above 90% in many countries and is highly supported by vast national and international collaborative initiatives to ensure that all children have appropriate access to life-saving vaccines. Both the US-based Vaccines for Children (VFC) program and the global WHO-sponsored Global Vaccine Action Plan (GVAP) are examples of frameworks designed to prevent millions of deaths through more equitable access to existing vaccines. The CDC recently reported that for children born during 1994–2013, vaccination will prevent an estimated 21 million hospitalizations and 732 000 deaths throughout their lifetime. Together, this would result in a savings of $1.38 trillion in total costs to society.Citation1 Since life expectancy has been dramatically increased by the provision of clean water, vaccines, and other factors, the global aging population continues to grow. For example, it is expected that the number of individuals >80 y of age will triple over the next 50 yCitation2 and approximately 20% of the global population will be elderly (>65 y of age) by 2050 ().Citation3,Citation4 In this context, it is important to realize that the human immune system increasingly becomes less efficient in fighting infections or responding to vaccines. Therefore, taking advantage of existing adult vaccines earlier in adulthood and developing vaccination strategies that can overcome weakened immune systems is of fundamental importance to provide the appropriate protection for the aging adult population.
Figure 1. Projected future growth of the older population. This chart shows the percent of the population 65 and older increasing from 4 percent in 1900 to 12 percent in 2000 and projected to be 20 percent in 2050. It also shows the percent of the population 85 and older increasing from 0.2 percent in 1900 to 1.5 percent in 2000 and projected to be 4.3 percent in 2050. Graph is adapted from the Administration on Ageing, Health and Human Services.
Current Adult Vaccination Trends
Today’s vaccines recommended for adults can be divided into those for the general population and those indicated for individuals with specific risk factors such as pre-existing diseases (e.g., chronic lung or heart disease, diabetes mellitus), compromised immune system, travel, or potential occupational risks, such as exposure of healthcare workers. Age based recommendations have been developed based on periods of life in which there is increased risk of contracting infectious diseases. As an example, the majority of pneumococcal infections and invasive pneumococcal diseases (IPD) can be successfully prevented in infants and young children by use of pneumococcal conjugate vaccines in the very young.Citation1,Citation5,Citation6 Nevertheless, this leaves individuals 50 y of age and older at risk and this risk increases with ageCitation7,Citation8 (http://apps.who.int/healthinfo/statistics/mortality/whodpms, Accessed March 5, 2014). In Western countries regular booster doses against tetanus, diphtheria, and often pertussis are indicated for the general adult population.Citation9,Citation10 Influenza vaccination is currently administered annually and is recommended for all adults, though in some countries it is only indicated for adults with specified risk factors.Citation11 In addition, vaccination against pneumococcal diseases and zoster are recommended for adults at increased risk and the elderly.
Globally, the most common vaccine preventable cause of death results from infection with Streptococcus pneumoniae. According to WHO estimates, of the 1.6 million deaths due to pneumococcus annually, roughly half occur in children < 5 y and half in the elderly (WHO 2004 Global Immunization Data. http://www.who.int/immunization_monitoring/data/GlobalImmunizationData.pdf. Accessed June 7, 2009). Although the 23-valent polysaccharide pneumococcal vaccine (PPV23) is indicated for prevention of IPD there is conflicting evidence that the vaccine can prevent pneumococcal non-bacteremic pneumonia, which is the most common and life-threatening manifestation of invasive pneumococcal infection in adults.Citation12-Citation14 Moreover, effectiveness of the PPV23 vaccine against pneumococcal community acquired pneumonia (CAP) with bacteremia as well as other types of IPD are limited to an overall 24% with a duration of protection of only 3–5 y.Citation15 There is also the concern that the vaccine could possibly induce B-cell depletion and hypo-responsiveness, which may in fact increase the risk for IPD particularly in immunocompromised subjects.Citation16-Citation18 These concerns in turn have affected uptake of the vaccine in adults.Citation19
The pneumococcal polysaccharide conjugate vaccine, PCV13, recently licensed for the elderly, is a qualitatively different vaccine aimed to overcome the challenges seen with PPV23. Conjugation of the pneumococcal polysaccharides to the protein carrier CRM197 (non-toxic mutant form of diphtheria toxin) in PCV13 drives a T-cell-dependent immune response, in contrast to the T-independent response elicited by PPV23. A T-cell dependent response is particularly important in infants and older adults whose immune systems have either not fully matured or are entering immunosenescence, respectively. The biological differences with PCV13 induced by T-cells include reduction of colonization with the pneumococcus, IgG responses in infants (as compared with IgM only responses to PPV23), higher antibody responses against most vaccine-serotypes as well as induction of immunological B-cell memory, which is not seen with PPV23. Clinical studies with PCV13, including comparisons between PCV13 and PPV23, have shown the serological superiority of PCV13 for most serotypes shared by the two vaccines in more than 8000 subjects. They have also demonstrated that PCV13 responses, unlike PPV23 responses, can be boosted thus providing an important tool for continued protection against this important pathogen in the older adult.Citation20 In countries with a high PCV13 uptake for infants herd protection has been observed for the older adult populationCitation21,Citation22; however there remains sufficient disease burdenCitation23-Citation25 to drive the need to immunize not only infants but the elderly as well. For the first time ever with a pneumococcal vaccine, PCV13 has shown efficacy against non-bacteremic pneumonia in a double blind randomized clinical trial in a general population of subjects ≥ 65 y.Citation23
CAPiTA (Community-acquired Pneumonia Immunization Trial in Adults) was a Phase 4 trial to support post-approval commitments of demonstrating vaccine efficacy against all vaccine type pneumococcal community acquired pneumonia (VT-CAP) and also against non-bacteremic VT-CAP in individuals 65 y and older. The CAPiTA trial successfully demonstrated a reduction of VT-CAP cases in PCV13 vaccinated individuals and protection that lasted at least four years.Citation23 These results offer an important example of broadening the indication of a currently licensed vaccine beyond the pediatric population to provide the tools to ultimately reduce morbidity and mortality in the older adult.
Adult Vaccines: The New Horizon
As populations age, individuals have more frequent contact with the healthcare system and many live in long-term care settings. This puts older adults at increased risk of certain bacterial diseases. Weakened immunity and increases in antibiotic usage have resulted in rises of disease caused by Staphylococcus aureus and Clostridium difficile.Citation26,Citation27 No licensed vaccine currently exists to prevent infections from either of these pathogens; however several vaccines are in development.Citation28-Citation30
Both pathogens are Gram positive organisms that can cause disease with considerable consequences for the patient. S. aureus infections range from relatively mild skin infections to life-threatening invasive disease and pneumonia.Citation31 Antibiotic resistance has made S. aureus infections more difficult to treat and subjects with an infection that is resistant to antibiotics have a worse outcome and are more costly to treat.Citation32,Citation33 C. difficile is a spore-forming bacterium that resides in the gut and is the main cause of nosocomial infectious diarrhea in industrialized countries.Citation34 In the last decade, hyper virulent strains have become endemic in the hospital setting making C. difficile-associated disease (CDAD) an emerging and significant healthcare problem.Citation35 Similar to S. aureus, this is potentiated by an increased use of antibiotics, growing antibiotic resistance, and additionally due to the difficult elimination of C. difficile spores that can survive harsh environments. Normal gut flora provide natural resistance to C. difficile; however antibiotic treatment can disrupt this natural defense leading to increased infection and disease risk.Citation36 Once the organism colonizes the gut it produces toxins that cause the patient to develop severe gastroenteritis, diarrhea and can irreversibly damage the intestines. Together, S. aureus and C. difficile infections are associated with increased healthcare costs amounting to many billions of dollars even with application of good infection control practices.Citation37-Citation39 To prevent diseases caused by these organisms, distinct approaches are required.
S. aureus has a wide arsenal of virulence factors that it deploys to cause disease.Citation40,Citation41 While many pre-clinical studies were designed to identify potential vaccine targets,Citation42 only two vaccines have been tested in efficacy trials to date.Citation43,Citation44 These vaccines had different compositions, were tested in different target populations, and neither was efficacious in preventing S. aureus disease. The drawback of both vaccines was that they targeted single antigens. Now with a deeper understanding of how S. aureus causes disease,Citation41 it has become clear that a vaccine against this complex pathogen should target multiple virulence mechanisms.Citation45-Citation47 Three multi-antigen prophylactic vaccines are currently being evaluated in Phase 1/2 clinical trials. SA4Ag is a vaccine being developed by Pfizer and is composed of capsular polysaccharides (type 5 and type 8), Clumping factor A (ClfA) and Manganese transporter C (MntC).Citation41S. aureus disease-causing strains can either express type 5 or type 8 capsular polysaccharide, which forms a shield in vivo to protect the bacterium from host immune responses. Anti-capsular polysaccharide antibodies thwart this defense mechanism by enabling the bacteria to be killed by opsonophagocytosis.Citation48 ClfA is a virulence factor that facilitates the binding of S. aureus to fibrinogen early during the infectious process.Citation40 Active vaccination with ClfA elicits anti-ClfA antibodies that block the binding of S. aureus to fibrinogen.Citation49 The fourth component, MntC, is required to import manganese that is used by S. aureus to neutralize the reactive oxygen species that neutrophils produce to kill the bacterium.Citation50 The second vaccine is being developed by Novartis and is composed of a combination of proteins associated with iron acquisition,Citation51 intracellular invasionCitation52 and host binding proteins.Citation53 Glaxo Smith Kline is also developing a multi-antigen vaccine that includes capsular polysaccharide conjugates, ClfA and alpha toxin (ref. Citation64). Though there are promising early clinical results regarding how these vaccines perform in humans, controversy regarding the actual mechanism of immune protection remains and will not be resolved until efficacy is demonstrated in well controlled clinical trials.Citation29
C. difficile produces two large exotoxins, A and B (TcdA and TcdB), that are primarily responsible for causing CDAD symptoms and are therefore of considerable interest as vaccine antigens.Citation54 A prophylactic vaccine capable of eliciting toxin-neutralizing antibodies is a key approach for the C. difficile vaccines in development.Citation55 Toxins A and B were the first antigens testedCitation56 and continue to be evaluated using various approaches. Three toxin-based vaccine approaches are currently undergoing clinical testing for the primary prevention of C. difficile infection in adults and elderly. The first, developed by Sanofi Pasteur, uses purified toxins that are rendered inactive by formalin treatment.Citation28,Citation57 The second approach developed by Valneva is a recombinant vaccine that uses truncated forms of toxin A and B.Citation58 The third approach is being developed by PfizerCitation59 and utilizes ClosTron technology to stably express genetically inactivated toxoids from a toxin deficient C. difficile strain. To further control for residual toxicity, the genetically modified toxoids are subjected to a mild chemical inactivation step. This was developed to preserve determinants on the toxins that are important to elicit broad toxin neutralizing antibodies and that are destroyed by formaldehyde treatment.Citation30 Both toxoid-based and recombinant vaccines have been shown to induce toxin-neutralizing activity in healthy adults, including individuals 65 y of age or greater.Citation57,Citation58 Older adults entering long-term care facilities or individuals with chronic health problems requiring prolonged antibiotic exposure could significantly benefit from a C. difficile vaccine. The challenge will be to elicit a rapid and prolonged protective immune response in a population undergoing immunosenescence.
Challenges and Future Prospects
As evidenced by the two examples above, many important vaccine initiatives are underway to protect the growing population of older adults. However, one of the ever present challenges is that vaccine uptake in adults remains low.Citation60,Citation61 For example, just 20% of individuals > 60 y of age have received the herpes zoster (Shingles) vaccineCitation62 even though the vaccine clearly protects against severe post-herpetic neuralgia, the severe pain associated with re-activation of the virus in older individuals.Citation63,Citation64 This low vaccine uptake is encumbered in part by the current passively administered risk-based vaccination recommendation strategy, slow societal acceptance and lack of commitment from national health systems to fully implement recommended vaccines.
The current challenge that remains is to develop vaccination programs for adults that are as far reaching and effective as programs for children. To achieve this, a number of policies, public education campaigns and operational frameworks need to be put into place. This begins with clearly defined and publicly accepted vaccination goals regarding vaccine uptake and expected morbidity and mortality reduction in a defined time frame. Second, there must be a single unified plan, in contrast to what is common in most European Union (EU) countries, where plans are often designed not by country but by individual regions. Vaccines that have long been included in the immunization regimen have been found to have a more homogeneous schedule across the EU, whereas newer vaccines varied in the recommended schedule, dose, and target population.Citation65 Third, there must be a simple yet effective means to provide educational support for healthcare providers to implement such a program, including funding and advertising by unbiased local and national government agencies and health insurance organizations. This can translate into both better informed patients who may request immunization, as well as healthcare providers conducting more routine assessments of patient’s immunization status. A fourth and critical piece is to implement a process for annual monitoring of vaccine uptake and the impact of the vaccine with regard to reduced morbidity and mortality. Such programs have been extremely effective, wherever implemented for children,Citation1 and will benefit vaccine initiatives for the elderly population.
The availability of new vaccines and vaccines with new indications (e.g., PCV13, Tdap, zoster) targeted for adults and the elderly population will provide huge health benefits by protecting against many important infectious diseases. As the size of the elderly population continues to grow, the need for new vaccines to appropriately protect against prevailing infectious diseases (S. aureus, C. difficile, influenza, and respiratory syncytial virus) will remain an important need and focus for future vaccine development. Newer technologies and more potent adjuvants will need to be explored to overcome increasing immunosenescence in an aging population. In summary, based on the experience from pediatric vaccination programs, a continuing concerted effort by vaccine manufacturers, healthcare providers, and health policy makers to expand the adult vaccine repertoire and to enhance vaccine uptake will lead to improved general health and more importantly to a better quality of life for the elderly.
| Abbreviations: | ||
| PPV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| PCV13 | = | pneumococcal conjugate vaccine |
| IPD | = | invasive pneumococcal disease |
| CAPiTA | = | Community Acquired Pneumonia Immunization Trial in Adults |
| VT-CAP | = | Vaccine type-community acquired pneumonia |
| CDAD | = | Clostridium difficile-associated disease |
Disclosure of Potential Conflicts of Interest
All authors are employees of Pfizer and may own Pfizer stock.
Acknowledgements
The authors would like to thank the following employees of Pfizer for their insightful comments for this manuscript (Emilio Emini, Ingrid Scully and Paul Liberator), and for documentation assistance (Christina Regan and Jessica Brigham).
References
- WhitneyCG, ZhouF, SingletonJ, SchuchatA, Centers for Disease Control and Prevention (CDC). Benefits from immunization during the vaccines for children program era - United States, 1994-2013. MMWR Morb Mortal Wkly Rep2014; 63:352 - 5; PMID: 24759657
- The 2009 Ageing Report: economic and budgetary projections for the EU-27 Member States (2008-2060) Luxembourg: Joint Report prepared by the European Commission (DG ECFIN) and the Economic Policy Committee (AWG).. 2009
- BureauUC. . Population by Age and Sex for the United States: 1900 to 2000. PartACensus 2000 Special Reports, Series CENSR-4: US Administration on Aging. , 2002
- US Census Bureau PD. Projections of the Population by Age and Sex for the United States: 2010 to 2050. US Census Bureau, Population Division, 2008.
- FitzwaterSP, ChandranA, SantoshamM, JohnsonHL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J2012; 31:501 - 8; http://dx.doi.org/10.1097/INF.0b013e31824de9f6; PMID: 22327872
- SteensA, BergsakerMA, AabergeIS, RønningK, VestrheimDF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine2013; 31:6232 - 8; http://dx.doi.org/10.1016/j.vaccine.2013.10.032; PMID: 24176490
- KyawMH, RoseCEJr., FryAM, SingletonJA, MooreZ, ZellER, WhitneyCG, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis2005; 192:377 - 86; http://dx.doi.org/10.1086/431521; PMID: 15995950
- van HoekAJ, AndrewsN, WaightPA, StoweJ, GatesP, GeorgeR, MillerE. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect2012; 65:17 - 24; http://dx.doi.org/10.1016/j.jinf.2012.02.017; PMID: 22394683
- BridgesCB, Coyne-BeasleyT, Advisory Committee on Immunization Practices (ACIP), ACIP Adult Immunization Work Group, Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older - United States, 2014. MMWR Morb Mortal Wkly Rep2014; 63:110 - 2; PMID: 24500291
- MaltezouHC, WickerS, BorgM, HeiningerU, PuroV, TheodoridouM, PolandGA. Vaccination policies for health-care workers in acute health-care facilities in Europe. Vaccine2011; 29:9557 - 62; http://dx.doi.org/10.1016/j.vaccine.2011.09.076; PMID: 21964058
- EspositoS, MontinaroV, BosisS, TagliabueC, BaggiE, PrincipiN. Recommendations for the use of influenza vaccine in pediatrics. Hum Vaccin Immunother2012; 8:102 - 6; http://dx.doi.org/10.4161/hv.8.1.17957; PMID: 22252005
- HussA, ScottP, StuckAE, TrotterC, EggerM. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ2009; 180:48 - 58; http://dx.doi.org/10.1503/cmaj.080734; PMID: 19124790
- MoberleyS, HoldenJ, TathamDP, AndrewsRM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev2013; 1:CD000422; PMID: 23440780
- MelegaroA, EdmundsWJ. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol2004; 19:353 - 63; http://dx.doi.org/10.1023/B:EJEP.0000024701.94769.98; PMID: 15180106
- AndrewsNJ, WaightPA, GeorgeRC, SlackMP, MillerE. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine2012; 30:6802 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.09.019; PMID: 23000122
- FrenchN, NakiyingiJ, CarpenterLM, LugadaE, WateraC, MoiK, MooreM, AntvelinkD, MulderD, JanoffEN, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet2000; 355:2106 - 11; http://dx.doi.org/10.1016/S0140-6736(00)02377-1; PMID: 10902624
- ClutterbuckEA, LazarusR, YuLM, BowmanJ, BatemanEA, DiggleL, AngusB, PetoTE, BeverleyPC, MantD, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis2012; 205:1408 - 16; http://dx.doi.org/10.1093/infdis/jis212; PMID: 22457293
- TörlingJ, HedlundJ, KonradsenHB, OrtqvistA. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine2003; 22:96 - 103; http://dx.doi.org/10.1016/S0264-410X(03)00521-8; PMID: 14604576
- FedsonDS, Nicolas-SponyL, KlemetsP, van der LindenM, MarquesA, SallerasL, SamsonSI. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines2011; 10:1143 - 67; http://dx.doi.org/10.1586/erv.11.99; PMID: 21810065
- ParadisoPR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis2012; 55:259 - 64; http://dx.doi.org/10.1093/cid/cis359; PMID: 22495545
- HammittLL, BrudenDL, ButlerJC, BaggettHC, HurlburtDA, ReasonoverA, HennessyTW. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis2006; 193:1487 - 94; http://dx.doi.org/10.1086/503805; PMID: 16652275
- LexauCA, LynfieldR, DanilaR, PilishviliT, FacklamR, FarleyMM, HarrisonLH, SchaffnerW, ReingoldA, BennettNM, et al, Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA2005; 294:2043 - 51; http://dx.doi.org/10.1001/jama.294.16.2043; PMID: 16249418
- M. Bonten MB. S. Huijts, C. Webber, S. Gault, W. Gruber, D. Grobbee. Community Acquired Pneumonia Immunisation Trial in Adults (CAPITA). ISPPD. Hyperabad, India, 2014.
- SherwinRL, GrayS, AlexanderR, McGovernPC, GraepelJ, PrideMW, PurdyJ, ParadisoP, FileTMJr.. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J Infect Dis2013; 208:1813 - 20; http://dx.doi.org/10.1093/infdis/jit506; PMID: 24092845
- Grijalva CG, Wunderink RG, Williams D, Zhu Y, Balk R, Fakhran S, Courtney M, Anderson E, Qi C, Hicks L, et al. Distribution of Pneumococcal Serotypes Detected Through Urine Analysis Among US Adults Hospitalized with Pneumonia After Introduction of PCV13. ISPPD. Hyperabad, India, 2014.
- WegnerC, HübnerNO, GleichS, ThalmaierU, KrügerCM, KramerA. One-day point prevalence of emerging bacterial pathogens in a nationwide sample of 62 German hospitals in 2012 and comparison with the results of the one-day point prevalence of 2010. [8:Doc12]GMS Hyg Infect Control2013; 8:Doc12; PMID: 23967398
- KleinEY, LaxminarayanR. The potential impact of age and season on methicillin-resistant Staphylococcus aureus prevalence. Future Microbiol2013; 8:809 - 12; http://dx.doi.org/10.2217/fmb.13.65; PMID: 23841624
- FogliaG, ShahS, LuxemburgerC, PietrobonPJ. Clostridium difficile: development of a novel candidate vaccine. Vaccine2012; 30:4307 - 9; http://dx.doi.org/10.1016/j.vaccine.2012.01.056; PMID: 22682287
- JansenKU, GirgentiDQ, ScullyIL, AndersonAS. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine2013; 31:2723 - 30; http://dx.doi.org/10.1016/j.vaccine.2013.04.002; PMID: 23624095
- DonaldRG, FlintM, KalyanN, JohnsonE, WitkoSE, KotashC, ZhaoP, MegatiS, YurgelonisI, LeePK, et al. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology2013; 159:1254 - 66; http://dx.doi.org/10.1099/mic.0.066712-0; PMID: 23629868
- LowyFD. Staphylococcus aureus infections. N Engl J Med1998; 339:520 - 32; http://dx.doi.org/10.1056/NEJM199808203390806; PMID: 9709046
- KleinE, SmithDL, LaxminarayanR. Community-associated methicillin-resistant Staphylococcus aureus in outpatients, United States, 1999-2006. Emerg Infect Dis2009; 15:1925 - 30; http://dx.doi.org/10.3201/eid1512.081341; PMID: 19961671
- KleinE, SmithDL, LaxminarayanR. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis2007; 13:1840 - 6; http://dx.doi.org/10.3201/eid1312.070629; PMID: 18258033
- SimorAE, BradleySF, StrausbaughLJ, CrossleyK, NicolleLE. Committee SL-T-C. Clostridium difficile in long-term-care facilities for the elderly. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America2002; 23:696 - 703; http://dx.doi.org/10.1086/501997
- RupnikM, WilcoxMH, GerdingDN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol2009; 7:526 - 36; http://dx.doi.org/10.1038/nrmicro2164; PMID: 19528959
- SeekatzAM, YoungVB. Clostridium difficile and the microbiota. J Clin Invest2014; 1 - 8; PMID: 25036699
- GouldIM. Costs of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and its control. Int J Antimicrob Agents2006; 28:379 - 84; http://dx.doi.org/10.1016/j.ijantimicag.2006.09.001; PMID: 17045462
- DubberkeE. Clostridium difficile infection: the scope of the problem. J Hosp Med2012; 7:Suppl 3S1 - 4; http://dx.doi.org/10.1002/jhm.1916; PMID: 22407993
- CosgroveSE, QiY, KayeKS, HarbarthS, KarchmerAW, CarmeliY. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol2005; 26:166 - 74; http://dx.doi.org/10.1086/502522; PMID: 15756888
- ChengAG, DeDentAC, SchneewindO, MissiakasD. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol2011; 19:225 - 32; http://dx.doi.org/10.1016/j.tim.2011.01.007; PMID: 21353779
- AndersonAS, MillerAA, DonaldRG, ScullyIL, NanraJS, CooperD, JansenKU. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother2012; 8:1585 - 94; http://dx.doi.org/10.4161/hv.21872; PMID: 22922765
- BroughanJ, AndersonR, AndersonAS. Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Expert Rev Vaccines2011; 10:695 - 708; http://dx.doi.org/10.1586/erv.11.54; PMID: 21604989
- FowlerVG, AllenKB, MoreiraED, MoustafaM, IsgroF, BoucherHW, CoreyGR, CarmeliY, BettsR, HartzelJS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA2013; 309:1368 - 78; http://dx.doi.org/10.1001/jama.2013.3010; PMID: 23549582
- ShinefieldH, BlackS, FattomA, HorwithG, RasgonS, OrdonezJ, YeohH, LawD, RobbinsJB, SchneersonR, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med2002; 346:491 - 6; http://dx.doi.org/10.1056/NEJMoa011297; PMID: 11844850
- BradyRA, O’MayGA, LeidJG, PriorML, CostertonJW, ShirtliffME. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect Immun2011; 79:1797 - 803; http://dx.doi.org/10.1128/IAI.00451-10; PMID: 21220484
- LattarSM, Noto LlanaM, DenoëlP, GermainS, BuzzolaFR, LeeJC, SordelliDO. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun2014; 82:83 - 91; http://dx.doi.org/10.1128/IAI.01050-13; PMID: 24126523
- Stranger-JonesYK, BaeT, SchneewindO. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A2006; 103:16942 - 7; http://dx.doi.org/10.1073/pnas.0606863103; PMID: 17075065
- NanraJS, BuitragoSM, CrawfordS, NgJ, FinkPS, HawkinsJ, ScullyIL, McNeilLK, Aste-AmézagaJM, CooperD, et al. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother2013; 9:480 - 7; http://dx.doi.org/10.4161/hv.23223; PMID: 23249887
- HawkinsJ, KodaliS, MatsukaYV, McNeilLK, MininniT, ScullyIL, VernachioJH, SeverinaE, GirgentiD, JansenKU, et al. A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin Vaccine Immunol2012; 19:1641 - 50; http://dx.doi.org/10.1128/CVI.00354-12; PMID: 22896688
- HandkeLD, RogersKL, OlsonME, SomervilleGA, JerrellsTJ, RuppME, DunmanPM, FeyPD. Staphylococcus epidermidis saeR is an effector of anaerobic growth and a mediator of acute inflammation. Infect Immun2008; 76:141 - 52; http://dx.doi.org/10.1128/IAI.00556-07; PMID: 17954724
- MishraRP, MariottiP, FiaschiL, NosariS, MaccariS, LiberatoriS, FontanaMR, PezzicoliA, De FalcoMG, FalugiF, et al. Staphylococcus aureus FhuD2 is involved in the early phase of staphylococcal dissemination and generates protective immunity in mice. J Infect Dis2012; 206:1041 - 9; http://dx.doi.org/10.1093/infdis/jis463; PMID: 22829645
- KoreaCG, BalsamoG, PezzicoliA, MerakouC, TavariniS, BagnoliF, SerrutoD, UnnikrishnanM. The staphylococcal Esx proteins modulate apoptosis and release of intracellular Staphylococcus aureus during infection in epithelial cells. Infect Immun2014; http://dx.doi.org/10.1128/IAI.01576-14; PMID: 25047846
- BecherelliM, PrachiP, VicianiE, BiaginiM, FiaschiL, ChiarotE, NosariS, BrettoniC, MarchiS, BiancucciM, et al. Protective activity of the CnaBE3 domain conserved among Staphylococcus aureus Sdr proteins. PLoS One2013; 8:e74718; http://dx.doi.org/10.1371/journal.pone.0074718; PMID: 24069334
- KuehneSA, CartmanST, HeapJT, KellyML, CockayneA, MintonNP. The role of toxin A and toxin B in Clostridium difficile infection. Nature2010; 467:711 - 3; http://dx.doi.org/10.1038/nature09397; PMID: 20844489
- LeuzziR, AdamoR, ScarselliM. Vaccines against Clostridium difficile. Hum Vaccin Immunother2014; 10:1 - 12; http://dx.doi.org/10.4161/hv.28428; PMID: 24832715
- KotloffKL, WassermanSS, LosonskyGA, ThomasWJr., NicholsR, EdelmanR, BridwellM, MonathTP. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun2001; 69:988 - 95; http://dx.doi.org/10.1128/IAI.69.2.988-995.2001; PMID: 11159994
- GreenbergRN, MarburyTC, FogliaG, WarnyM. Phase I dose finding studies of an adjuvanted Clostridium difficile toxoid vaccine. Vaccine2012; 30:2245 - 9; http://dx.doi.org/10.1016/j.vaccine.2012.01.065; PMID: 22306375
- Valneva. . An Open-label Study Assessing Safety, Immunogenicity and Dose Response of IC84, NCT01296386ClinicalTrialsgov. , 2014
- Pfizer. Evaluation of a 3-dose Vaccination Regimen With One of Three Ascending Dose Levels of Clostridium Difficile Vaccine With or Without Adjuvant in Healthy Adults Aged 50 to 85 Years, NCT01706367. ClinicalTrialsgov. 2014;
- BlankPR, SchwenkglenksM, SzucsTD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect2009; 58:446 - 58; http://dx.doi.org/10.1016/j.jinf.2009.04.001; PMID: 19446340
- FioreAE, UyekiTM, BroderK, FinelliL, EulerGL, SingletonJA, IskanderJK, WortleyPM, ShayDK, BreseeJS, et al, Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep2010; 59:RR-81 - 62; PMID: 20689501
- WilliamsWW, LuPJ, O’HalloranA, BridgesCB, PilishviliT, HalesCM, MarkowitzLE, Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2012. MMWR Morb Mortal Wkly Rep2014; 63:95 - 102; PMID: 24500288
- OxmanMN, LevinMJ, JohnsonGR, SchmaderKE, StrausSE, GelbLD, ArbeitRD, SimberkoffMS, GershonAA, DavisLE, et al, Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med2005; 352:2271 - 84; http://dx.doi.org/10.1056/NEJMoa051016; PMID: 15930418
- SchmaderKE, OxmanMN, LevinMJ, JohnsonG, ZhangJH, BettsR, MorrisonVA, GelbL, GuatelliJC, HarbeckeR, et al, Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis2012; 55:1320 - 8; http://dx.doi.org/10.1093/cid/cis638; PMID: 22828595
- KanitzEE, WuLA, GiambiC, StrikasRA, Levy-BruhlD, StefanoffP, MereckieneJ, AppelgrenE, D’AnconaF, VENICE (Vaccine European New Integrated Collaboration Effort) National Gatekeepers, Contact Points. Variation in adult vaccination policies across Europe: an overview from VENICE network on vaccine recommendations, funding and coverage. Vaccine2012; 30:5222 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.06.012; PMID: 22721901
- Boutriau D. Evaluation of an investigational 4-componant Staphylococcus aureus vaccine with or without AS03B adjuvant in healthy adults: results from a phase 1 trial. International Symposium on Staphylococci and Staphylococcal Infections, Chicago, USA August 26-29 1014
