Abstract
A hepatitis A+B vaccine vaccination program of 12-year-olds was introduced in Catalonia in 1998. The aim of this study was to investigate the evolution of hepatitis A outbreaks in Catalonia and estimate the preventable fraction of cases associated with outbreaks as a measure of the impact of the vaccination program. Hepatitis A outbreaks reported to the Health Department between 1991 and 2012 were analyzed. The incidence rates of outbreaks, outbreak-associated cases and hospitalizations were calculated. The preventable fraction (PF) and 95% confidence intervals (CI) were estimated for the whole study period (pre-vaccination and post-vaccination) and the post-vaccination period.
One-hundred-eight (108) outbreaks (rate of 2.21 per 106 persons-year) were reported in the pre-vaccination period and 258 outbreaks (rate of 2.82 per 106 persons-year) in the post-vaccination period. The rate of cases associated with outbreaks was 1.52 per 105 persons-year in the pre-vaccination period and 1.28 per 105 persons-year in the post-vaccination period. Hospitalization rates were 0.08 and 0.75 per 106 persons-year, respectively. The number of person-to-person outbreaks whose index case was a school contact decreased in the post-vaccination period (aOR 2.72; 95%CI 1.35–5.48), but outbreaks whose index case was a man who has sex with men (MSM) or an immigrant increased. The PF of all outbreak-associated cases was 6.46% (95%CI 3.11–9.82) and the highest PF was in the 15–24 years age group (42.53%; 95%CI 29.30–55.75). In the 0–4 years age group, the PF was 18.35% (95%CI 9.59–27.11), suggesting a protective herd effect in unvaccinated subjects. Vaccination of immigrants traveling to endemic countries and MSM should be reinforced.
Introduction
Hepatitis A is an acute infection caused by the hepatitis A virus (HAV), an RNA virus of the Picornaviridae family. The virus can remain infectious in the environment for weeks and is transmitted person-to-person to non-immune individuals through the fecal-oral route and through ingestion of contaminated food or water. The disease has been seen as early as 15 d (days) and as late as 50 d after exposure; the mean incubation period is about a month, although this depends on the infectious dose.Citation1
HAV infection may produce a wide spectrum of outcomes, ranging from asymptomatic infection (without elevated serum aminotransferase levels) to subclinical forms (with elevated aminotransferase levels with no other clinical manifestation) or clinically evident disease.Citation2 The typical symptoms of HAV infection are indistinguishable from those caused by other forms of viral hepatitis. In older children and adults, HAV infection causes clinical disease with jaundice, but in young children jaundice is rare and most infections acquired are asymptomatic.Citation3 A cholestatic form of hepatitis A has been reported in patients with persistent jaundice and itching. Other rare atypical clinical forms include immunologic, neurologic, hematologic, pancreatic and renal extrahepatic manifestations. The most severe form of the disease (fulminant hepatitis A) is rare and is associated with older age and underlying chronic liver disease.Citation1,Citation2 In pregnant women with acute hepatitis A, premature rupture of membranes and placental separation have been reported.Citation4,Citation5
In countries with low endemicity, most cases occur in specific risk groups, such as travelers to areas of high or intermediate endemicity, injection drug users and men who have sex with men (MSM).Citation6-Citation9
In 1995, when inactivated HAV vaccines of proven immunogenicity and protective efficacy became available, a vaccination program of people belonging to risk groups was introduced in Catalonia, but the results showed that the impact of vaccination on the global incidence of the disease was small.Citation10 Therefore, at the end of 1998, a universal program of vaccination of preadolescents aged 12 y with a combined hepatitis A+B vaccine containing 360 ELISA units of HAV antigen and 10 µg of hepatitis B virus surface antigen was initiated. This, together with improvements in hygienic, sanitary and socioeconomic circumstances has led to a situation of low endemicity in which infection is usually linked to outbreaks. The nature and number of outbreak-associated cases is an important public health burden, due to the need for case investigation, surveillance and control activities.Citation11 Therefore, it is of interest to investigate whether the vaccination program has involved changes in the epidemiology of outbreaks. According to the recommendations of the STROBE statement, the impact of the intervention should be estimated using absolute impact measures rather than only association measures.Citation12
The aim of this study was to investigate the evolution of incidence rates of hepatitis A outbreaks, cases and hospitalizations for a period of 22 y, to compare the characteristics of the outbreaks in the pre- and post-vaccination periods and to estimate the preventable fraction (PF) of cases associated with outbreaks as a measure of the impact of the vaccination program.
Results
A total of 376 outbreaks [312 (83%) transmitted person to person, 36 (9.6%) transmitted through a common source, and 28 (7.5%) of unknown transmission mechanism] involving 1955 associated cases were reported between 1991 and 2012. The evolution of the incidence rates of outbreaks, associated cases and hospitalized cases is shown in . Only hospitalization rate showed a significant increase (r = 0.66; P = 0.001).
Figure 1. Incidence rate of reported hepatitis A outbreaks, associated cases, and hospitalizations. Catalonia, 1991–2012
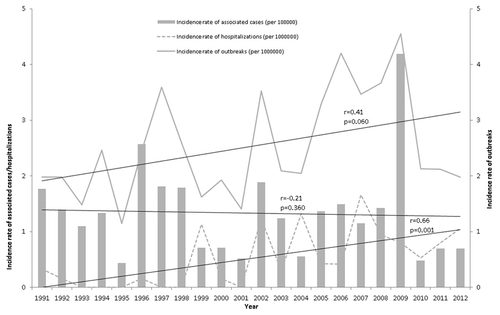
A total of 108 outbreaks (rate of 2.21 per 106 persons-year; 95%CI 1.81–2.67) were reported in the pre-vaccination period and 258 (2.82 per 106 persons-year; 95%CI 2.49–3.19) in the post-vaccination period. The number of cases associated with outbreaks in the pre-vaccination and post-vaccination periods were 746 (rate of 1.52 per 105 persons-year; 95%CI 1.42–1.64) and 1165 (1.28 per 105 persons-year; 95%CI 1.20–1.35), respectively, with the differences being statistically significant (P < 0.001).
The distribution of the number of person-to-person outbreaks according to the risk factor of the index case is shown in . In the bivariate analysis, the risk factors that fell significantly in the post-vaccination period were being a child attending a day care center (OR 3.07; 95%CI 1.20–7.84) and being a school contact of a case (OR 4.34; 95%CI 2.18–8.63), whereas outbreaks whose index case was an immigrant (OR 0.17; 95%CI 0.07–0.44) or a MSM (OR 0.04; 95%CI 0.002–0.74) increased. In the multivariate analysis, immigrant status (aOR 0.19; 95%CI 0.07–0.49), having a school contact with a case (aOR 2.72; 95%CI 1.35–5.48) and being a MSM (aOR 0.04; 95%CI 0.002–0.67) were the only statistically-significant risk factors.
Table 1. Person-to-person hepatitis A outbreaks according to the risk factor of the index case in the pre-vaccination (1991–98) and in the post-vaccination (2000–12) periods
The estimated incidence rates of outbreak-associated cases and the PF of all outbreak-associated cases in the periods considered are shown in . The PF of all cases was 6.46% (95%CI 3.11–9.82) and the highest value was in the 15–24 y age group (42.53%; 95%CI 29.30–55.75), but PF values higher than 14% were observed also in children aged < 15 y. The PF for women was 10.32% (95%CI 3.95–16.69), but no reduction was observed in men.
Table 2. Incidence rate and preventable fraction of hepatitis A cases associated with all reported outbreaks
shows the incidence rates and PF of outbreak-associated cases in the periods considered according to the risk factor of the index case. Being a school contact of a case was the risk factor with the highest PF (34.79%; 95%CI 11.29–59.30).
Table 3. Incidence rate and preventable fraction of hepatitis A cases associated with reported person-to person outbreaks according to risk factor of the index case
Discussion
Our results show a significant increase in hospitalization rates of cases of HAV infection throughout the whole study period, as found by Arteaga Rodriguez et al.Citation13 in a Spanish study using the minimum basic data set from the years 2005–2008. Studies in other countries have also found higher hospitalization rates in older people.Citation14,Citation15 The increased number of cases adults, especially those aged >45 y, due to a shift to older ages after the introduction of the vaccination program, may explain these results.Citation6
A study in the United States by Craig et al.Citation11 found a reduction in the incidence of outbreaks after the introduction of a mass vaccination program in states with high rates of HAV infection. In contrast, we found that although the incidence rates of outbreaks did not significantly change in the two periods considered, the incidence rate of cases associated with outbreaks fell in the post-vaccination period. This may reflect the fact that sensitivity to the reporting of outbreaks has increased (small size of reported outbreaks) and also that the mass vaccination of preadolescents is associated with a limited spread when outbreaks occur. Decreases in the incidence of HAV infection in the whole population (vaccinated and unvaccinated) have been reported by other authors after a mass vaccination program. Chironna et al.Citation16 reported a reduction in the incidence rate of HAV infection from 14.8 per 100 000 in 1998 to 0.8 per 100 000 in 2009, after mass vaccination in Puglia (Italy). Wasley et al.Citation17 assessed the impact of a routine vaccination strategy in some territories of the United States and found that the incidence rate fell from 12 per 100 000 in 1995 to 2.6 per 100 000 in 2003, with the reduction being observed not only in vaccinated age groups but in the whole population. In Argentina, following the introduction of a hepatitis A immunization program in 2005, a reduction of 88% with respect to the average incidence rate in the pre-vaccination period was observed.Citation18
Only person-to-person outbreaks in which the risk factor of index case was being a school contact of a case showed a reduction in associated cases in the post-vaccination period (aOR 2.72; 95%CI 1.35–5.48). The most logical explanation is that the hepatitis A+B vaccination was performed in preadolescents in schools. Other authors have also reported greater reductions in vaccinated than in unvaccinated age groups.
In contrast, person-to person outbreaks in which the risk factor of the index case was immigrant status increased in the post-vaccination period (aOR 0.19; 95% CI 0.07–0.40), in agreement with other studies that show the importance of children of immigrants returning from visiting relatives and friends in their countries of origin as a cause of outbreaks.Citation19-Citation23 Likewise, outbreaks in which the index case was a MSM increased (aOR 0.04; 95%CI 0.002–0.67) in the post-vaccination period. A large outbreak in this group, involving 189 cases, was reported by Tortajada et al.Citation24 between September 2008 and June 2009 in Barcelona. Hepatitis A vaccination has been recommended and offered free-of-charge to MSM in Catalonia since 1995, but unfortunately this strategy has not been successful, as has also occurred in other countries with low disease incidence.Citation25-Citation27 Weerakon et al.Citation28 suggested that the level of immunization required to prevent outbreaks in MSM is about 50%, a coverage not reached in MSM in Catalonia due to the difficulty of contacting these persons and actively offering vaccination.Citation29 Our results support the need to reinforce vaccination in MSM.
Although the use of measures of absolute risk has been recommended in order to determine the potential impact of preventive measures,Citation12,Citation30 not all reports use these measures to show the impact of factors associated with hepatitis A incidence rates.Citation31 In our study, the PF of cases associated with outbreaks was estimated as >40% in people aged 15–24 y, but the PF was ≥15% in younger age groups. This suggests, in agreement with the results of other studies,Citation32,Citation33 that the mass vaccination program has produced a protective herd effect, meaning that the disease incidence has decreased in an unimmunized segment of the population due to immunization of a proportion of the population,Citation34 such as the vaccination program of preadolescents in Catalonia. However, the results of studies in the United States,Citation33 Israel,Citation35 and ArgentinaCitation18 with universal immunization programs in the second year of life suggest that the benefits for the whole population could be still higher in Catalonia if the age of universal vaccination were advanced to the second year of life, as infections in infants are frequently asymptomatic, and unvaccinated infants can act as a source of infection of their parents, caregivers and relatives.Citation36 The previously-mentioned increase in cases associated with MSM may explain why we observed a PF > 10% in women but no reduction in men.
According to the risk factor of the index case, the highest PF (34.79%; 95%CI 11.29–59.30) was found in outbreaks in which the index case was a school contact of a case. This seems logical because vaccination was administered in schools, and the coverage was >90%.Citation37 Some benefit was also observed in the prevention of cases associated with outbreaks whose index case was a day-care contact, reinforcing the previously-mentioned protective herd effect, although the lower CI of the PF was <0.
Our work has strengths and limitations. A main strength is that the study was based-population, with all reported outbreaks of hepatitis A in Catalonia during the study period being included, and the same definitions of cases and outbreaks was used. Another strength is the large number of years analyzed (8 y in the pre-vaccination period and 13 y in the post-vaccination period), which minimized the possible influence of disease cycles.
The limitations of the study include the fact that it is difficult to determine whether the decrease in incidence rates in the post-vaccination period was due only to the vaccination program or whether there are other environmental and hygienic factors that could contribute to this reduction. Therefore, the possibility that factors other than vaccination could explain some of the differences observed in the post vaccination period cannot be ruled out. Another possible limitation is underreporting during the study period. However, as the incidence rates of associated cases show, the size of outbreaks fell during the last years of the study and this suggests greater sensitivity in detecting hepatitis A cases and outbreaks in the post-vaccination period. Consequently, the decreases observed and the estimated PF are probably lower than the true figures.
In conclusion, our results show that the incidence rate of cases of HAV infection associated with outbreaks has decreased in the post-vaccination period in Catalonia, mainly due to a reduction in person-to-person outbreaks whose index case was a school contact. The preventable fractions attributed to the vaccination program show that the highest value was in the 15–24-y-age-group, which includes the cohorts vaccinated in schools, but a protective herd effect was also observed in children under this age. The benefit of universal vaccination would probably be greater if the age of administration of the vaccine was advanced to the second year of life. In addition to universal vaccination, our results show the need to reinforce the vaccination of immigrants who travel to endemic areas and of men who have sex with men.
Material and Methods
The study was performed in Catalonia, a region with 7.5 million inhabitants situated in the northeast of Spain.
Cases of hepatitis A must be statutorily reported to the Health Department in Catalonia. A clinical case of hepatitis A infection was defined as an acute illness with discrete onset of symptoms (malaise, nausea, anorexia, fever, malaise, or abdominal pain) and jaundice, dark urine or elevated aminotransferase levels. A hepatitis A outbreak was defined as ≥2 epidemiologically-linked cases with at least one laboratory-confirmed case (detection of immunoglobulin antibody to HAV). Outbreaks were classified as due to a common source or transmitted person to person.
For each reported hepatitis A outbreak to the Health Department between 1991 and 2012, the following variables were collected: year, number of cases associated with outbreaks, number of hospitalizations (cases associated with outbreaks that required hospitalization), and risk factor of the index case identified as the origin of the outbreak. Risk factors were defined as: living in the household of a case, being a school contact of a case, day-care attendance, day-care worker, immigrant status, travel to endemic areas, and being a MSM. When the index case had travelled to their or their parents’ native country and was also an immigrant, immigrant status was the risk factor considered. An immigrant was defined as a person who moved to Spain from another country and children born in Spain to non-Spanish nationals. The rate of outbreaks (per 106 inhabitants), the rate of cases associated with outbreaks (per 105 inhabitants) and the rate of hospitalizations (per 106 inhabitants) and their 95% confidence intervals (CI) were calculated. The yearly population of Catalonia estimated by the National Institute of Statistics was used to calculate incidence rates.Citation38
Spearman’s correlation coefficients were calculated to evaluate the trend of rates. Statistical significance was established assuming an α error of 0.05. Crude odds ratios (OR), and their 95%CI were calculated by bivariate analysis comparing each category of risk factor with all the others. Adjusted odds ratios (aOR) were calculated by multiple logistic regression. We excluded the year 1999 from the analysis, as this was the year when preadolescents completed vaccination. The statistical analyses were made using the Statistical Package for Social Sciences (SPSS 19.0 for Windows) and R 3.0.3 (R Development Core Team 2014). To avoid the problem of quasi-complete separation, the method proposed by FirthCitation39 was used and implemented in R.Citation40 In order to determine the proportion of the disease that would be prevented if the vaccination program was implemented during the whole period and not only since 1999, we calculated the PF, which was estimated using the formula (Ip-Iv)/Ip,Citation30 where Ip was the incidence rate in the whole period considered (before the introduction of the vaccination program [1991–1998] and after the vaccination program [2000–2012]), and Iv the incidence in the period after the introduction of the vaccination program (2000–2012); the 95% CI of the PF were estimated using the method proposed by Szklo and Nieto.Citation41
| Abbreviations: | ||
| aOR | = | adjusted odds ratio |
| CI | = | confidence interval |
| d | = | days |
| HAV | = | hepatitis A virus |
| ELISA | = | enzyme-linked immunosorbent assay |
| MSM | = | men who have sex with men |
| OR | = | odds ratio |
| PF | = | preventable fraction |
| y | = | years |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
This work was partially funded by CIBER Epidemiología y Salud Pública (CIBERESP), Spain and by AGAUR (expedient number 2009/SGR 42).
We thank the reporting Physicians and the technicians of the Epidemiological Surveillance Units of the Public Health Agency of Catalonia and of the Epidemiology Service of the Public Health Agency of Barcelona.
The other members of the Working Group for the Study of Hepatitis A in Catalonia
M Alsedà, C Arias, I Barrabeig, N Camps, M Carol, M Company, J Ferràs, G Ferrús, I Parrón, A Rovira, R Torras (Public Health Agency of Catalonia); P Olalla, and J Caylà (Public Health Agency of Barcelona and CIBER Epidemiología y Salud Pública).
References
- Murphy TV, Feinstone SM, Bell BP. Hepatitis A vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia: Elsevier 2013: 183-204.
- Ciocca M. Clinical course and consequences of hepatitis A infection. Vaccine 2000; 18 (Supl 1): S71-4.
- Blechová Z, Trojánek M, Kynčl J, Cástková J, John J, Malý M, Herrmannová K, Marešová V. Clinical and laboratory features of viral hepatitis A in children. Wien Klin Wochenschr 2013; 125:83 - 90; http://dx.doi.org/10.1007/s00508-012-0316-9; PMID: 23358879
- Motte A, Blanc J, Minodier P, Colson P. Acute hepatitis A in a pregnant woman at delivery. Int J Infect Dis 2009; 13:e49 - 51; http://dx.doi.org/10.1016/j.ijid.2008.06.009; PMID: 18774327
- Elinav E, Ben-Dov IZ, Shapira Y, Daudi N, Adler R, Shouval D, Ackerman Z. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology 2006; 130:1129 - 34; http://dx.doi.org/10.1053/j.gastro.2006.01.007; PMID: 16618407
- Centers for Disease Control and Prevention. Viral hepatitis surveillance, United States 2010. Available at: http://www.cdc.gov/hepatitis/Statistics/2010Surveillance/PDFs/2010HepSurveillanceRpt.pdf.
- Termorshuizen F, Dorigo-Zetsma JW, de Melker HE, van den Hof S, Conyn-Van Spaendonck MA. The prevalence of antibodies to hepatitis A virus and its determinants in The Netherlands: a population-based survey. Epidemiol Infect 2000; 124:459 - 66; http://dx.doi.org/10.1017/S0950268899003842; PMID: 10982070
- Broman M, Jokinen S, Kuusi M, Lappalainen M, Roivainen M, Liitsola K, Davidkin I. Epidemiology of hepatitis A in Finland in 1990-2007. J Med Virol 2010; 82:934 - 41; http://dx.doi.org/10.1002/jmv.21759; PMID: 20419806
- Freeman E, Torvaldsen S, Tobin S, Lawrence G, MacIntyre CR. Trends and risk factors for hepatitis A in NSW, 2000-2009: the trouble with travel. N S W Public Health Bull 2012; 23:153 - 7; http://dx.doi.org/10.1071/NB11036; PMID: 23043748
- Domínguez A, Salleras L, Carmona G, Batalla J. Effectiveness of a mass hepatitis A vaccination program in preadolescents. Vaccine 2003; 21:698 - 701; http://dx.doi.org/10.1016/S0264-410X(02)00583-2; PMID: 12531343
- Craig AS, Watson B, Zink TK, Davis JP, Yu C, Schaffner W. Hepatitis A outbreak activity in the United States: responding to a vaccine-preventable disease. Am J Med Sci 2007; 334:180 - 3; http://dx.doi.org/10.1097/MAJ.0b013e3181425411; PMID: 17873531
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344 - 9; http://dx.doi.org/10.1016/j.jclinepi.2007.11.008; PMID: 18313558
- Arteaga-Rodríguez A, Carrasco-Garrido P, de Andrés AL, de Miguel AG, Santos J, Jiménez-García R. Changes in the epidemiology of hepatitis A in Spain (2005–2008): trends of acute hepatitis A hospitalizations, comorbidities, and costs associated with the hospitalization. Eur J Gastroenterol Hepatol 2010; 22:1284 - 9; http://dx.doi.org/10.1097/MEG.0b013e32833bce39; PMID: 20964258
- Erhart LM, Ernst KC. The changing epidemiology of hepatitis A in Arizona following intensive immunization programs (1988-2007). Vaccine 2012; 30:6103 - 10; http://dx.doi.org/10.1016/j.vaccine.2012.07.029; PMID: 22835739
- Zakaria S, Fouad R, Shaker O, Zaki S, Hashem A, El-Kamary SS, Esmat G, Zakaria S. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis 2007; 44:e30 - 6; http://dx.doi.org/10.1086/511074; PMID: 17243045
- Chironna M, Prato R, Sallustio A, Martinelli D, Tafuri S, Quarto M, Germinario C. Hepatitis A in Puglia (South Italy) after 10 years of universal vaccination: need for strict monitoring and catch-up vaccination. BMC Infect Dis 2012; 12:271; http://dx.doi.org/10.1186/1471-2334-12-271; PMID: 23098290
- Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA 2005; 294:194 - 201; http://dx.doi.org/10.1001/jama.294.2.194; PMID: 16014593
- Vacchino MN. Incidence of hepatitis A in Argentina after vaccination. J Viral Hepat 2008; 15:Suppl 2 47 - 50; http://dx.doi.org/10.1111/j.1365-2893.2008.01029.x; PMID: 18837834
- Gervelmeyer A, Nielsen MS, Frey LC, Sckerl H, Damberg E, Mølbak K. An outbreak of hepatitis A among children and adults in Denmark, August 2002 to February 2003. Epidemiol Infect 2006; 134:485 - 91; http://dx.doi.org/10.1017/S0950268805005200; PMID: 16194292
- Faber MS, Stark K, Behnke SC, Schreier E, Frank C. Epidemiology of hepatitis A virus infections, Germany, 2007-2008. Emerg Infect Dis 2009; 15:1760 - 8; http://dx.doi.org/10.3201/eid1511.090214; PMID: 19891863
- Matheson K, Halperin B, McNeil S, Langley JM, Mackinnon-Cameron D, Halperin SA. Hepatitis A and travel amongst Nova Scotia postsecondary students: evidence for a targeted vs. universal immunization strategy. Vaccine 2010; 28:8105 - 11; http://dx.doi.org/10.1016/j.vaccine.2010.09.107; PMID: 20955828
- Nielsen US, Larsen CS, Howitz M, Petersen E. Hepatitis A among Danish travellers 1980-2007. J Infect 2009; 58:47 - 52; http://dx.doi.org/10.1016/j.jinf.2008.10.010; PMID: 19059649
- Faillon S, Martinot A, Hau I, Puget A, Moulin F, Noel G, Guen CG, Lorrot M, Callamand P, Hue V, et al. Impact of travel on the seroprevalence of hepatitis A in children. J Clin Virol 2013; 56:46 - 51; http://dx.doi.org/10.1016/j.jcv.2012.10.004; PMID: 23127561
- Tortajada C, de Olalla PG, Diez E, Pinto RM, Bosch A, Perez U, Sanz M, Caylà JA, Saunas Working Group. Hepatitis A among men who have sex with men in Barcelona, 1989-2010: insufficient control and need for new approaches. BMC Infect Dis 2012; 12:11; http://dx.doi.org/10.1186/1471-2334-12-11; PMID: 22264382
- Sfetcu O, Irvine N, Ngui SL, Emerson C, McCaughey C, Donaghy P. Hepatitis A outbreak predominantly affecting men who have sex with men in Northern Ireland, October 2008 to July 2009. Euro Surveill 2011; 16:19808; PMID: 21392487
- Pini A, Tomasoni LR, Cristini G, Puoti M, Matteelli A, Carosi G, Castelli F. Hepatitis A in men having sex with men (MSMs) in northern Italy. Infez Med 2010; 18:113 - 4; PMID: 20610934
- Mazick A, Howitz M, Rex S, Jensen IP, Weis N, Katzenstein TL, Haff J, Molbak K. Hepatitis A outbreak among MSM linked to casual sex and gay saunas in Copenhagen, Denmark. Euro Surveill 2005; 10:111 - 4; PMID: 16077208
- Weerakoon AP, Chen MY, Read TRH, Bradshaw C, Fairley CK. Immunity to hepatitis A when outbreaks of infection in men who have sex with men (MSM) are rare. Vaccine 2012; 30:3430 - 4; http://dx.doi.org/10.1016/j.vaccine.2012.03.024; PMID: 22449421
- Tortajada C, de Olalla PG, Pintó RM, Bosch A, Caylà J. Outbreak of hepatitis A among men who have sex with men in Barcelona, Spain, September 2008-March 2009. Euro Surveill 2009; 14:11 - 19175; PMID: 19371516
- Spasoff RA. Epidemiologic methods for health policy. New York: Oxford University Press, 1999: 49-54.
- Tosti ME, Spada E, Romanò L, Zanetti A, Mele A, SEIEVA collaborating group. Acute hepatitis A in Italy: incidence, risk factors and preventive measures. J Viral Hepat 2008; 15:Suppl 2 26 - 32; http://dx.doi.org/10.1111/j.1365-2893.2008.01025.x; PMID: 18837830
- Lopalco PL, Prato R, Chironna M, Germinario C, Quarto M. Control of hepatitis A by universal vaccination of adolescents, Puglia, Italy. Emerg Infect Dis 2008; 14:526 - 8; http://dx.doi.org/10.3201/eid1403.070900; PMID: 18325288
- Averhoff F, Shapiro CN, Bell BP, Hyams I, Burd L, Deladisma A, Simard EP, Nalin D, Kuter B, Ward C, et al. Control of hepatitis A through routine vaccination of children. JAMA 2001; 286:2968 - 73; http://dx.doi.org/10.1001/jama.286.23.2968; PMID: 11743837
- John TJ, Samuel R. Herd immunity and herd effect: new insights and definitions. Eur J Epidemiol 2000; 16:601 - 6; http://dx.doi.org/10.1023/A:1007626510002; PMID: 11078115
- Belmaker I, Dukhan L, Yosef Y, Leventhal A, Dagan R. Elimination of hepatitis A infection outbreaks in day care and school settings in southern Israel after introduction of the national universal toddler hepatitis A immunization program. Pediatr Infect Dis J 2007; 26:36 - 40; http://dx.doi.org/10.1097/01.inf.0000247105.45185.13; PMID: 17195703
- Tsou TP, Liu CC, Huang JJ, Tsai KJ, Chang HF. Change in hepatitis A epidemiology after vaccinating high risk children in Taiwan, 1995-2008. Vaccine 2011; 29:2956 - 61; http://dx.doi.org/10.1016/j.vaccine.2011.02.001; PMID: 21329774
- Domínguez A, Oviedo M, Carmona G, Batalla J, Bruguera M, Salleras L, Plasència A. Impact and effectiveness of a mass hepatitis A vaccination programme of preadolescents seven years after introduction. Vaccine 2008; 26:1737 - 41; http://dx.doi.org/10.1016/j.vaccine.2008.01.048; PMID: 18325642
- Institut d'Estadística de Catalunya. (IDESCAT). Municipal Population Register. Idescat 2014. Available from: http://www.idescat.cat/en/poblacio/padro.html.
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80:27 - 38; http://dx.doi.org/10.1093/biomet/80.1.27
- Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002; 21:2409 - 19; http://dx.doi.org/10.1002/sim.1047; PMID: 12210625
- Szklo M. Nieto J. Epidemiology: beyond the basics. 2nd ed. Boston: Mass, Jones & Barlet Publishers, 2007.
