Abstract
Measles and rubella are infectious diseases and humans are the only reservoir of these infections. Effective vaccines are available with the potential for measles (MV) and rubella (RuV) virus eradication. According to the World Health Organisation guidelines, a national plan was approved in Italy in 2013 to achieve the MV/RuV elimination by 2015, and active MV/RuV integrated surveillance initiated. Towards this purpose, a regional laboratory centre was set up on 1 September 2013 in Lombardy, Northern Italy. This paper aimed at: (1) evaluating measles-mumps-rubella (MMR) vaccine coverage and MV/RuV notified cases retrospectively; and (2) presenting the results of MV/RuV integrated surveillance (laboratory confirmed and viral genetic profiles).
The 95% target for MMR vaccine coverage was achieved in 2001, and coverage increased until 2007 (96.6%), but then a decreasing trend was observed. Since 2000 to 2014, 3026 rubella cases were notified, with nearly 58% of them in the 2002 epidemic. From 2009, less than 45 RuV cases per year were reported. From 2000 to 2014, 5024 measles cases were notified. Since 2008, three large outbreaks (in 2008, 2011, and 2013) were observed. From data obtained during our surveillance activity, there were no rubella cases, and 57.5% (46/80) collected samples were MV-positive by real-time RT-PCR. A fragment of the MV N gene was sequenced from 37 MV-positive samples; D8, D9, and B3 genotypes were detected.
Data obtained retrospectively and from active surveillance underline the necessity to achieve and maintain high vaccination coverage and to improve surveillance and the effectiveness of healthcare actions.
Introduction
Measles and rubella are highly contagious diseases caused by measles virus (MV) and rubella virus (RuV), respectively. Effective vaccines containing measles, mumps, rubella (MMR) or measles and rubella (MR) are available and their use can completely eradicate these viruses in the human population.Citation1 Vaccination campaigns and effective surveillance have stopped their endemic circulation in some countries, such as the Americas. However, in the World Health Organization (WHO) European Region, although the MMR vaccine was introduced in national vaccination schedules 20 years ago, several measles outbreaks have occurred because of the underutilization of the vaccine.Citation2,Citation3 In fact, during the first three months of 2014, 1197 measles cases were notified in Europe.Citation4 In the same period, 795 suspected cases of measles were reported in Italy,Citation5 which resulted to be the country with the highest number of suspected cases of measles in Europe (60.1% of all cases in Europe). Since the beginning of 2014, 2350 rubella cases were notified in Europe, mostly in Poland.Citation4 Considering these data, the WHO European Region goals to eliminate endemic cases of measles and rubella and to reduce the incidence of congenital rubella syndrome (CRS) to less than 1 case per every 100 000 live births will be not achieved by 2015,Citation6 as opposed to what reported in the WHO “Global Measles and Rubella Strategic Plan 2012–2020”.Citation1
In Italy, measles has been a notifiable disease since 1934, and an improved surveillance system was introduced in 2007.Citation7 Moreover, in March 2011, the Italian Ministry of Health approved a plan to eliminate measles, rubella and CRS.Citation8 The Italian national health system is decentralized and every region has to introduce a healthcare plan, in accordance with the national authorities' guidelines. Therefore, in 2013, an Italian network for the integrated surveillance of measles, rubella and CRS was set up.Citation9 In the Lombardy region (Northern Italy), integrated surveillance of measles and rubella started on 1 September 2013.Citation10 As the regional centre (RC), our commitments are to improve epidemiological and virological surveillance and to genotype circulating MV/RuV. The aim of this paper is to describe the epidemiological trends of MV and RuV, as well as the MMR vaccination coverage in Lombardy from 2000 to 2011. Moreover, data on virological surveillance and on the genotypes circulating in Lombardy are presented.
Results
Epidemiological profiles of measles and rubella from 2000 to 2014
Vaccination coverage
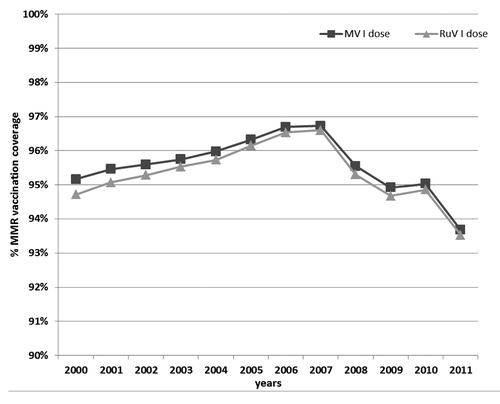
In Lombardy, since 1999, 12–15-mo-old babies are routinely immunized with MMR. In 2001, the 95% target for vaccination coverage with the first dose of the MMR vaccine was achieved. Vaccination coverage increased until 2007 when the highest percentage was observed (MV: 96.7%; RuV: 96.6%). Then, a gradual decrease was observed below the 95% target level (with the lowest level in 2011: MV: 93.7%; RuV: 93.5%) ().
Notified cases of measles and rubella
From the year 2000 to date, 3026 rubella cases were reported. The largest rubella outbreak occurred in 2002 when 1749 cases were notified with an incidence rate of 5.9 per 100 000 inhabitants. From 2003 to date, the number of notified cases was less than 100 per year, with the exception of 2008 when 292 cases were reported ().
Figure 2: Notified cases of (A) rubella and (B) measles per year from 2000 to 30 May 2014 and MMR-vaccine coverage trend from 2000 to 2011. *Notified cases in Lombardy from 1 January to 30 May 2014.
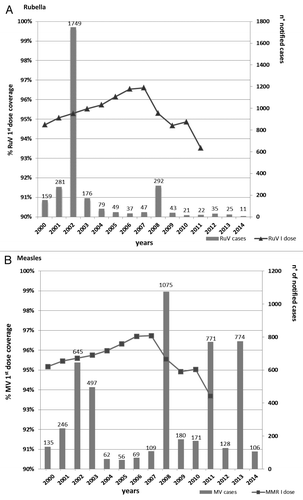
In the same period, 5024 measles cases were notified in Lombardy. shows the number of measles cases notified per year and the trend in vaccination coverage. The 95% target was achieved in 2001. Nevertheless, in 2002 an outbreak occurred with 645 notified cases. From 2004 to 2007, the vaccination coverage target was maintained and exceeded, and, at the same time, a decrease in the number of cases (overall 296) was observed. From 2008 to 2011, a descending trend in the vaccination coverage rate was reported. From 2008 to the present, 3205 measles cases were notified, accounting for 63.8% of the total notified cases of measles in Lombardy since 2000.
Focus on measles cases from 2008 to 2013
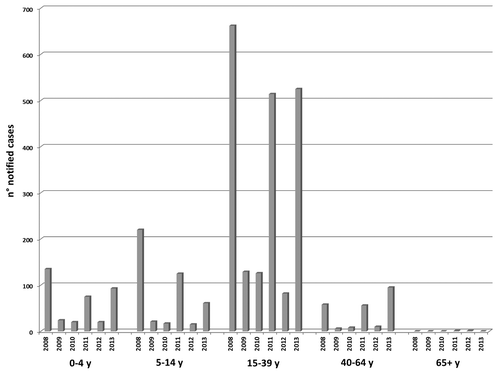
From 2008 to 2013, 3099 measles cases were notified (females 47.7%). The age group more affected was the 15–39-y-olds and the mean age of measles cases changed from 22.4 y (median age: 23 y) in 2008 to 25.2 y (median age: 27 y) in 2013. Moreover, in 2013, the notified cases increased in 40–64-y-old (12.6% of total cases reported) (). Of these cases, 1721 (55.5%) were laboratory-confirmed measles infections, and 137 (4.4%) were probable cases. Overall, 689 measles cases (22.1%) were hospitalized. An 18-y-old female who developed laboratory-confirmed measles in late 2011 died about two months after the rash onset. This patient was affected by immunodeficiency and the cause of death was pneumonia with respiratory failure.
From 2008 to 2013, three large outbreaks of measles were observed. The largest outbreak occurred in 2008 with 1075 notified cases and an incidence rate of 2 per 100 000 inhabitants. A short inter-epidemic period (2009–2010) followed. During this period, the number of notified cases per year was more than two-fold higher than that reported in the previous inter-epidemic period (mean number of notified cases of measles per year: 74 in 2004–2007 vs. 175.5 in 2009–2010). A second milder outbreak was observed in 2011 (771 cases, mean age: 22 y, median age: 23 y) (). A third outbreak occurred in 2013 (774 cases) after a very short inter-epidemic period in 2012 (128 measles cases; mean age: 23.3 y, median age: 26 y). The number of cases during the 2012 inter-epidemic period was almost double the number of cases per year reported in the 2004–2007 inter-epidemic period (). In 2013 a 23-y-old female developed a laboratory-confirmed measles encephalitis. The patient recovered after weeks at the Intensive Care Unit and several months rehabilitation. This particular patient worked in a kindergarten at the time of infection and had not been vaccinated during her infancy because the personal beliefs of her parents.
Overall, from 2008 to 2013, 2889 of the 3099 notified measles cases (93.2%) were unvaccinated. Of the remaining 210 (6.8%) vaccinated cases, 158 (75.2%) had received only 1 dose of measles-containing vaccine, and 53 (25.2%) were vaccinated with 2 doses. In 11 vaccinated cases, the onset of clinical symptoms occurred within one month of immunization. No data were available regarding the genetic characteristics of the infecting strains.
The percentage of vaccinated cases with respect of all notified cases ranged between 4.7% and 11.7%, and it was higher during the inter-epidemic periods ().
Table 1. Number of measles notified cases and percentage of vaccinated cases during outbreaks (2008–2011–2013) and inter-epidemic periods (2009/2010–2012)
Measles and rubella integrated surveillance from 1 September 2013 to 30 May 2014
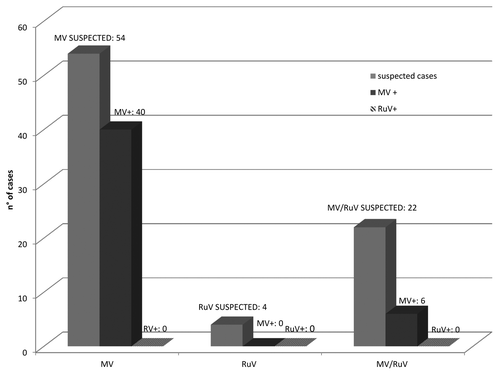
From September 2013, biological samples were collected from 80 (46.3% females) suspected cases of MV/RuV for virological analysis. Overall, the mean age of the suspected cases was 22.6 y, with a median age of 24 y (range: 3 mo to 65 y). Half of the cases were aged 15–39 y (mean age: 26.4 y), followed by 18 (22.5%) ≤5-y-of-age (mean age: 2.1 y), 11 (13.7%) aged 40-64 y (mean age: 48.5 y), 8 (10%) aged 40–64 y (mean age: 48.5 y), and 1 (1.1%) aged 65 y.
Forty-one (51.3%) cases were not vaccinated. Seven (8.7%) individuals were vaccinated with the first MMR dose and 5 (6.3%) cases were vaccinated with two doses. Information on vaccination status was not available for 27 (33.7%) of the suspected measles cases.
Laboratory analysis of samples collected from these 80 suspected cases revealed that 57.5% (46/80) were MV-positive by real-time reverse transcription polymerase chain reaction (RT-PCR). All negative samples for MV were tested for RuV and no RuV-positive samples were detected (). Of the laboratory-confirmed measles cases, 54.3% (25/46) were females and the mean age was 26 y (range: 3 mo to 61 y). Most (28/46: 60.9%) MV-positive cases were unvaccinated. In 14 cases (30.4%) the vaccine status was not reported, in 1 (2.1%) case the vaccination status was unknown, 2 individuals (4.3%) were vaccinated with 1 dose, and 1 (2.1%) subject received 2 vaccine doses.
Molecular characterization
From 1 September 2013 to 30 May 2014, 37 sequences of the N gene of MV, which was circulating in Lombardy, were analysed and compared with Italian (2013–2014) and reference sequences present in the GenBank database.Citation11 The sequences belonged to the genotypes D8, D9, and B3.
The D8 genotype was the most frequently detected (31/37: 83.8%). The sequences of the D8 genotype showed 95.5% to 97.7% similarity with the reference strain Manchester.UNK/30.94[D8]. These D8 sequences clustered in five genetic groups. In particular, 15/31 of the D8 sequences (48.4%) fell in the same group (100% identity) and shared 97.5% similarity with the reference strain. One-third of the sequences were obtained from samples collected in 2013 and the remaining two-thirds were obtained from samples collected in 2014.
Two identical sequences analyzed in 2013 and three sequences with 100% identity detected in 2014 clustered into two distinct genetic groups with 96.1% and 95.5% similarity with the reference strain, respectively. One sequence (MVs/Milan.ITA/8.14[D8]) segregated in a separate genetic group with an identity of 97.7% with the reference strain Manchester.UNK/30.94[D8].
The sequences of the D9 genotype clustered into two genetic groups: 1 group consisted of 2 identical sequences and the other group consisted of 1 sequence. The D9 sequences identified in 2013 had similarities ranging between 95.3% and 97.2% with the reference strain Victoria.AUS/12.99[D9]. In 2014, D9 sequences were not detected in Lombardy.
During spring 2014, three B3 sequences were detected (100% identity), which were identical to another Italian sequence (MVs/Rome.ITA/12.14/BuMaBa[B3]) detected in the same period. The similarity with the reference strain MVi/Ibadan.NIE/97/1[B3] was 97.7%.
Conclusion
Measles and rubella are acute viral illness with the potential for severe and life-threatening complications. Measles is a highly contagious disease and it can lead to ear infection, pneumonia, encephalitis, subacute sclerosing panencephalitis, blindness, and death.Citation12,Citation13 Rubella causes a relatively mild disease in children compared to the measles, but when the infection occurs during pregnancy, CRS may be observed.Citation14 Moreover, rare complications, such as skin bleeding, encephalitis, neuritis, and epididymo-orchitis, have been reported in adults.Citation15
MV and RuV meet all eradication criteria and a large vaccination campaign was initiated in all WHO regions to achieve the goal of elimination by 2015.Citation1 As with the previous failure to reach this goal in 2010, this objective will not be reached by 2015 in the WHO European Region. The principal problem is the decline in immunization rates in some countries, such as Italy.Citation16,Citation17 The distrust of the population, especially in developed countries, may be attributed to a rise in scepticism about vaccines in general, and, in particular, about the MMR vaccine.Citation18,Citation19 Moreover, the influence of migration, population movement, religion and anti-vaccine activists may contribute to this increased worries, particularly in disadvantaged communities. In addition, the differences observed in vaccination coverage across Europe also occur at the country level. A recent Italian report showed different vaccination coverage rates across Italy with percentages ranging between 72.4% (in Friuli Venezia Giulia) and 95.8% (in Sardinia).Citation17
In Lombardy, the 95% target for vaccination coverage with the first dose of the MMR vaccine was achieved in 2001. Vaccination coverage increased until 2007 when the rate reached 96.6%. Then, during the last 6 y, the vaccination coverage rate gradually decreased to levels under the 95% target. During this period, Lombardy experienced a severe measles burden with a cumulative number of cases that was approximately 2-fold higher than that reported in the previous 9-y-period.
In this study we focused on the epidemiological profile of measles from 2008 to 2013. Overall, 3099 measles cases were reported, of which 22.1% cases were hospitalized. Approximately 50% of cases were laboratory-confirmed measles infections. During the same period, three large outbreaks were observed. The largest outbreak occurred in 2008 whereas two milder outbreaks were observed in 2011 and in 2013. The three outbreaks were interspersed with short inter-epidemic periods (2009–2010 and 2012), but the number of notified cases per year during these periods was more than two-fold greater than that in the previous inter-epidemic period (2004–2007). During the outbreaks, the most affected age group was the 15–39 year olds. Moreover, in 2013, the number of notified cases among 40–64 year olds (12.6% of total cases reported) increased 1.6-fold compared to the number of cases in the same age group during the previous two outbreaks. As expected, most reported cases (93%) were unvaccinated. These data indicate that there are large groups of susceptible individuals, especially among adolescents and young adults. Therefore, efforts to prevent measles must be aimed at improving MMR vaccine uptake not only in small children but also in older age groups.
Moreover, to achieve the goal of elimination it is important to improve measles and rubella surveillance. MV/RuV integrated surveillance is an adequate tool to guarantee sensitive virological surveillance. In fact, measles and rubella affect the same age groups and do not present clear clinical symptoms, so the diagnosis of these two diseases can be difficult. Measles and rubella can be confused with other fever and rash diseases (e.g., parvovirus B19, enterovirus, human herpesvirus-6).Citation20,Citation21 In Lombardy, MV/RuV integrated surveillance started on 1 September 2013Citation10 and, since then, 46 laboratory-confirmed measles cases were detected, mostly among unvaccinated subjects or individuals vaccinated with only one dose of the vaccine. No RuV cases were detected.
Molecular characterisation of MV in Lombardy revealed that there were three circulating genotypes: D8, D9, and B3. D8 was the predominant genotype that circulated in 2013 and 2014. The D8 sequences clustered in 5 different genetic groups, and 4 of these groups included closely related sequences. In late 2013 a MV with the D8 genotype was identified for the first time in Lombardy. MVs with the D9 genotype circulated only in the latter part of 2013. In spring 2014, MV strains with the B3 genotype were detected in a limited geographic area of Lombardy. The B3 sequences were closely related to another sequence detected in the same period in Rome, Italy. The molecular surveillance of wild-type viruses in Lombardy showed the circulation of a limited number of MV genotypes, similar to what has been observed in other Italian regions. This scenario is peculiar to countries with endemic transmission of MV, as opposed to countries approaching to elimination, where co-circulation of multiple imported genotypes generally occurs.Citation22
The data presented here highlight the relevant burden of measles in Lombardy and the importance of achieving high vaccination coverage not only in young children but also in adolescents and young adults. Moreover, the genetic analysis of the circulating strains enriches epidemiological investigation and surveillance activity, which are both essential to control the transmission of the measles virus.
Materials and Methods
Epidemiological surveillance and vaccination coverage
According to the national surveillance guidelines, the Lombardy regional surveillance system requires, that physicians report all suspected measles, rubella and CRS cases to the local health units (LHUs) within 12 hours of the onset of symptoms. The LHUs are in charge of carrying out all epidemiological investigations and collecting specimens from each suspected case. Notified cases are reported systematically to the regional data warehouse. In this study, we analyzed data aggregated by year from 2000 to 2014, and we focused on data obtained from 2008 to 2013 for which information (such as vaccination status, age, gender, serological confirmation results, and hospitalization data) on notified measles cases was available. According to the European Decision 2002/253/ECCitation23 and WHO guidelines,Citation24 measles and rubella cases are classified as “possible” if the case meets the clinical criterion only, “probable” if the case meets the clinical criterion plus an epidemiological link or “confirmed” if the case meets the clinical and laboratory criteria.
In Italy, the first dose of the MMR vaccine is recommended for children aged 12–15 mo, and since 2002, a second dose is recommended for 5–6-y-old children. The data on vaccination coverage were retrieved from the regional data warehouse and analyzed starting in 2000. Vaccine coverage is assessed in 2-y-old children each year. Vaccination coverage (first dose) data were available from 2000 to 2011.
Virological surveillance
Sample collection and preparation
The LHUs collect whole blood, dried blood spot (DBS), urine and saliva/throat swab from all suspected cases not more than 10 days after the onset of rash. The regional laboratory centre (RLC) confirms the cases and sends the laboratory results to the Italian Ministry of Health and to the Directorate General for Health, Regione Lombardia. DBS are stored at room temperature, and the urine samples and swabs are transported in a cool box at 4 °C.
RNA was extracted from blood, DBS, urine and swabs with a commercial kit (Invisorb® Spin Virus RNA Mini kit, Stratec Molecular, Germany, cat. number: 1040300300). The RNA samples were then stored at −80°C. DBS were pre-treated by rehydration with molecular grade water for 2 h at 37°C on a thermo-vortex.
One-step real-time RT -PCR assay
Two, in-house, one-step, singleplex real-time RT -PCR assays were performed. Five µl of extracted RNA were added to each one-step real-time RT-PCR mixture, which were prepared and adjusted following the instructions for the AgPath-ID One-Step RT-PCR kit (Ambion, cat. number: AM1005). Each singleplex real-time RT -PCR was performed and adapted using primers and probes designed by Hübschen et al.Citation25 to amplify the MV N gene (nucleotides, nt: 584-697) and RuV p150 gene (nt: 195-323). Amplification was carried out on a 7300 Real-time PCR System (Applied Biosystem) following the same thermal profile (45 °C ×10'; 95 °C×10' and 50 cycles at 95 °C ×15" + 60 °C ×1’).
Genotyping assays
Two, one-step, nested, RT-PCR assays were performed to amplify and sequence the N gene of MV and the E1 gene of RuV, according to WHO indication. The measles primers were designed by the RLC, and the rubella primers were previously described by Tran et al.Citation26
A nested RT-PCR was performed using primers to amplify a 450-nt fragment encoding the C-terminal end of the nucleoprotein (N). The following primers were used for the amplification: MV-F1 5’-ggattgcyga aatgatatgt racattgaya c-3’ and MV-R1 5’-ccgccttcag ttgatccaat tgctg-3’ in the first round of RT-PCR, and MV-F2 5’-aaggtcagtt ccacattggc a-3’ and MV-R2 5’-gcatgrtttg ctgagacccg aa-3’ in the nested PCR.
Specific complementary DNA (cDNA) of the N gene was synthesized by reverse transcription at 55 °C ×30’, immediately followed by PCR amplification in the same tube, using the Transcriptor One-Step RT-PCR kit (Roche; cat. number: 0465588500) according to the manufacturer's instructions. The PCR cycling program consisted of a denaturation step at 94 °C for 7’ followed by 45 cycles of 10’’ at 94 °C, 30’’ at 58 °C, and 1’30’’ at 68 °C with a final extension step at 68 °C for 5’.
The nPCR was carried out using AmpliTaq Gold polymerase (Applied Biosystems, cat. number: N8080240) according to the manufacturer's instructions. The cycling program consisted of a denaturation step for 10 min at 94 °C followed by 35 cycles of 20’’ at 95 °C, 30’’ at 58 °C, and 1’ at 72 °C with a final extension step at 72 °C for 5’.
A RT-nested PCR was performed to amplify a 880-nt fragment that covers the 739-nt region of the E1 RuV gene, as recommended for identification by the WHO. Briefly, in the first step, reverse transcription was performed at 42 °C for 60’ using the SuperScript III one-step RT-PCR kit (Invitrogen, cat. number: 12574-035). The reaction (45 µl) was prepared using 5 µl of extracted RNA, 25 µl of supplied buffer, 2 µl of SuperScript III/Platinum Taq polymerase, 2 µl of each outer primer (25 µM; GRUB739F1 and GRUBR1)Citation26 and 14 µl of molecular grade water (thermal amplification profile: 95 °C×2’; [95 °C×1’; 58 °C×1’; 68 °C ×90”]× 40 cycles; 68 °C×7’). Five µl of the product from the first step were added as the template in 40 µl of the second round-mix, which was prepared using 0.5 µl of GoTaq (5 U/µl) polymerase (Promega), 10 µl of coloured GoTaq reaction buffer, 2 µl of deoxynucleotide triphosphates (dNTPs, 10 mM, Promega), 1 µl of each inner primer (25 µM; GRUB739F2 and GRUB765)Citation22 and 30.5 µl of molecular grade water (thermal profile: 94 °C×3’, [95 °C ×1’-58 °C ×1’-72 °C ×90’’] ×40 cycles, 72 °C ×7’). The amplification was carried out on a ABI 9700 (Applied Biosystems) thermal cycler, and the products were visualized on a 2% agarose gel stained with 5 µl of SYBR Green (Atlas ClearSight DNA Stain, BioAtlas, cat. number: BH40501).
RT-PCR products were purified with Wizard® SV Gel and PCR Clean-Up System (Promega Corporation; cat. number: A9282) and sequenced with a BigDye Terminator Cycle-Sequencing Kit (Applied Biosystems; cat. number: 4337450). The nucleotide sequences were obtained by automated DNA sequencing based on fluorescent dye terminator on the genetic analyser ABI PRISM 3100 Genetic Analyser (Applied Biosystems). Multiple sequence alignment was conducted using ClustalW version 2.0.10.
| Abbreviations: | ||
| CRS | = | Congenital Rubella Syndrome |
| LHU | = | Local Health Unit |
| MV | = | Measles Virus |
| PCR | = | Polymerase Chain Reaction |
| RC | = | Regional Centre |
| RLC | = | Regional Laboratory Centre |
| RT | = | Retro Transcription |
| RuV | = | Rubella Virus |
| WHO | = | World Health Organization |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- World Health Organization (WHO). Global measles and rubella strategic plan: 2012-2020; WHO Library Cataloguing-in-Publication Data, 2012. Available from: http://www.who.int/immunization/newsroom/Measles_Rubella_StrategicPlan_2012_2020.pdf (Last access: 06/26/2014).
- World Health Organization (WHO). Vaccine-preventable disease monitoring system, 2010 global summary. Available from: http://whqlibdoc.who.int/hq/2010/WHO_IVB_2010_eng.pdf (Last access: 06/26/2014).
- Williams JR, Manfredi P, Butler A, Ciofi degli Atti ML, Salmaso S. Heterogeneity in regional notification patterns and its impact on aggregate national case notification data: the example of measles in Italy. BMC Public Health 2003; 18:3 - 23; http://dx.doi.org/10.2466/pms.1963.17.1.3; PMID: 14045763
- European Center for Disease Control (ECDC). Latest surveillance data; http://www.ecdc.europa.eu/en/healthtopics/measles/epidemiological_data/Pages/measles_past12months.aspx, update 03-28-2014 (Last access: 06/26/2014).
- Istituto superiore di Sanità (ISS) and CNESPS. Morbillo&rosolia news; monthly report n°4; April 2014, https://www.iss.it/site/rmi/morbillo/ (Last access: 06/26/2014).
- Muscat M, Shefer A, Ben Mamou M, Spataru R, Jankovic D, Deshevoy S, Butler R, Pfeifer D. The state of measles and rubella in the WHO European Region, 2013. Clin Microbiol Infect 2014; 20:Suppl 5 12 - 8; http://dx.doi.org/10.1111/1469-0691.12584; PMID: 24520948
- Italian Ministry of Health. Lettera circolare del 20 aprile 2007. Piano nazionale di eliminazione del morbillo e della rosolia congenita: istituzione di un sistema di sorveglianza speciale per morbillo Available from: http://www.epicentro.iss.it/focus/morbillo/pdf/sorveglianza-speciale_morbillo.pdf (Last access: 06/26/2014).
- Italian Ministry of Health. Piano nazionale per l’eliminazione del morbillo e della rosolia congenita (PNEMoRc) 2010-2015. 2011, Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1519_allegato.pdf (Last access: 06/26/2014)
- Italian Ministry of Health. Istituzione di un Sistema integrato per il morbillo e la rosolia alla luce del nuovo Piano Nazionale di Eliminazione del morbillo e della rosolia congenita 2010-2015. Available from: http://www.fimproma.org/index.php?option=com_k2&view=item&id=147:ministero-della-salute-istituzione-di-un-sistema-di-sorveglianza-integrato-per-il-morbillo-e-la-rosolia-alla-luce-del-nuovo-piano-nazionale-di-eliminazione-del-morbillo-e-della-rosolia-congenita-2010-2015&Itemid=174 2013. (Last access: 06/26/2014).
- Directorate General for Health. Regione Lombardia. Istituzione di un sistema di sorveglianza integrato per il morbillo e la rosolia alla luce del nuovo Piano Nazionale di Eliminazione del morbillo e della rosolia congenita 2010-2015. Indicazioni operative; Available from: www.regione.lombardia.it, Milan, 2013 (Last access: 06/26/2014).
- GenBank. http://www.ncbi.nlm.nih.gov/genbank/ (Last access: 06/26/2014).
- Semba RD, Bloem MW. Measles blindness. Surv Ophthalmol 2004; 49:243 - 55; http://dx.doi.org/10.1016/j.survophthal.2003.12.005; PMID: 14998696
- Center for Disease Control (CDC). Epidemiology and prevention of vaccine-preventable disease, The Pink book: course textbook, chapter 12, 12th edition, second printing, 2012, CDC
- Center for Disease Control (CDC). Congenital rubella syndrome; manual for the Surveillance of Vaccine-Preventable Diseases, chapter 15, 5th edition, 2012, CDC
- Center for Disease Control (CDC). Epidemiology and prevention of vaccine-preventable disease, The Pink book: course textbook, chapter 19, 12th edition, second printing, 2012, CDC
- European Center for Disease Control (ECDC) technical report: Review of outbreaks and barriers to MMR vaccination coverage among hard-to reach populations in Europe; Venice II Consortium – September 2012
- Filia A, Bella A, Rota MC, Tavilla A, Magurano F, Baggieri M, et al. Analysis of national measles surveillance data in Italy from October 2010 to December 2011 and priorities for reaching the 2015 measles elimination goal; Euro Surveill. 2013;18 (20): pii=20480. Available on line: http://www.eurosurveillance.org/viewArticle.aspx?Articleld=20480 (Last access: 06/26/2014)
- Nicholson MS, Leask J. Lessons from an online debate about measles-mumps-rubella (MMR) immunization. Vaccine 2012; 30:3806 - 12; http://dx.doi.org/10.1016/j.vaccine.2011.10.072; PMID: 22063388
- Uno Y, Uchiyama T, Kurosawa M, Aleksic B, Ozaki N. The combined measles, mumps, and rubella vaccines and the total number of vaccines are not associated with development of autism spectrum disorder: the first case-control study in Asia. Vaccine 2012; 30:4292 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.01.093; PMID: 22521285
- Oliveira SA, Siqueira MM, Camacho LAB, Nogueira RM, Spinetti CCJ, Cubel Garcia RCN, Knowles W, Brown DW. The aetiology of maculopapular rash diseases in Niterói, State of Rio de Janeiro, Brazil: implications for measles surveillance. Epidemiol Infect 2001; 127:509 - 16; http://dx.doi.org/10.1017/S0950268801005908; PMID: 11811885
- Ramsay M, Reacher M, O’Flynn C, Buttery R, Hadden F, Cohen B, Knowles W, Wreghitt T, Brown D. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child 2002; 87:202 - 6; http://dx.doi.org/10.1136/adc.87.3.202; PMID: 12193426
- Rota JS, Rota PA, Redd SB, Redd SC, Pattamadilok S, Bellini WJ. Genetic analysis of measles viruses isolated in the United States, 1995-1996. J Infect Dis 1998; 177:204 - 8; http://dx.doi.org/10.1086/513825; PMID: 9419189
- Official Journal of the European (ECDC) Communities. Commission Decision of 19 March 2002 laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:086:0044:0062:EN:PDF (Last access: 06/26/2014)
- World Health Organization. Manual for the laboratory diagnosis of measles and rubella virus infection, second edition, WHO/IVB/07.01, 2007. Available from: http://www.who.int/ihr/elibrary/manual_diagn_lab_mea_rub_en.pdf (Last access: 06/26/2014)
- Hübschen JM, Kremer JR, De Landtsheer S, Muller CP. A multiplex TaqMan PCR assay for the detection of measles and rubella virus. J Virol Methods 2008; 149:246 - 50; http://dx.doi.org/10.1016/j.jviromet.2008.01.032; PMID: 18353451
- Tran DN, Pham NTK, Tran TTT, Khamrin P, Thongprachum A, Komase K, Hayakawa S, Mizuguchi M, Ushijima H. Phylogenetic analysis of rubella viruses in Vietnam during 2009-2010. J Med Virol 2012; 84:705 - 10; http://dx.doi.org/10.1002/jmv.23199; PMID: 22337313