Abstract
Influenza vaccination aims at reducing the incidence of serious disease, complications and death among those with the most risk of severe influenza disease. Influenza vaccine effectiveness (VE) through sentinel surveillance data from the PIDIRAC program (Daily Acute Respiratory Infection Surveillance of Catalonia) during 2010–2011, 2011–2012, and 2012–2013 influenza seasons, with three different predominant circulating influenza virus (IV) types [A(H1N1)pdm09, A(H3N2) and B, respectively] was assessed. The total number of sentinel samples with known vaccination background collected during the study period was 3173, 14.7% of which had received the corresponding seasonal influenza vaccine. 1117 samples (35.2%) were positive for IV. A retrospective negative case control design was used to assess vaccine effectiveness (VE) for the entire period and for each epidemic influenza season. An overall VE of 58.1% (95% CI:46.8–67) was obtained. Differences in VE according to epidemic season were observed, being highest for the 2012–2013 season with predominance of IV type B (69.7% ;95% CI:51.5–81) and for the 2010–2011 season, with predominance of the A(H1N1)pdm09 influenza virus strain (67.2% ;95%CI:49.5–78.8) and lowest for the 2011–2012 season with A(H3N2) subtype predominance (34.2% ;95%CI:4.5–54.6).
Influenza vaccination prevents a substantial number of influenza-associated illnesses. Although vaccines with increased effectiveness are needed and the search for a universal vaccine that is not subject to genetic modifications might increase VE, nowadays only the efforts to increase vaccination rates of high-risk population and healthcare personnel let reduce the burden of influenza and its complications.
Introduction
Influenza vaccination aims at reducing the incidence of serious disease, complications and death among those with high risk of severe influenza disease. Every winter there are sharp rises in medical visits, hospitalizations and deaths from acute respiratory illness worldwide. Influenza is an important cause of these and is the only common viral respiratory pathogen with licensed vaccines available that are safe and effective in preventing disease. In Catalonia, as in over 50 countries which have national vaccination programs focusing on the elderly population and those at high risk,Citation1 every season a vaccination campaign is set forth to immunize targeted population.Citation2 But there remains a need for further improvement in vaccine effectiveness, vaccine administration and compliance.
Influenza vaccine composition is reviewed each year, and often changed, in an effort to maintain their effectiveness against drifted influenza viruses. Estimates of vaccine effectiveness can help decide the changes to be made in future seasons regarding target groups to be addressed and risk management.Citation3,Citation4 Four major factors affect most epidemiological studies of vaccine efficacy: case definition, case ascertainment detecting cases among both vaccinated and unvaccinated populations, vaccination status ascertainment accurately based on a recorded date of vaccination and comparability of exposure to infectious agent for both vaccinees and non-vaccinees.
The ideal vaccine efficacy study is a clinical trial starting with persons susceptible to disease and the vaccine effectiveness (VE) can be determined by a variety of means including screening, outbreak investigations, secondary attack rates in clusters, vaccine coverage assessments, and case-control studies.Citation5 Mathematical models of disease transmission and vaccination typically assume that protective vaccine efficacy (i.e., the relative reduction in the transmission rate among vaccinated individuals) is equivalent to direct effectiveness of vaccine.Citation4 Vaccine efficacy measures the protective effects of vaccination by the reduction in the infection risk of a vaccinated individual relative to that of a susceptible, unvaccinated individual in ideal conditions. In contrast, vaccine effectiveness is defined as the reduction in the transmission rate for an average individual in a population with a vaccination program at a given level of coverage compared with an average individual in a comparable population with no vaccination program.Citation6 It is possible to use a negative case control method to estimate vaccine effectiveness from sentinel surveillance data when all patients in a surveillance system are tested for influenza and their vaccination status is known.Citation7 Influenza sentinel surveillance data collection relies on morbidity and virological indicators from primary care reporting of ILI (Influenza-like Illness) cases by the sentinel surveillance physicians’ network. In Catalonia influenza surveillance is based on well-established network of sentinel practitioners (PIDIRAC: Daily Acute Respiratory Infection Surveillance of Catalonia)Citation8 that includes general practitioners (GPs) and pediatricians who report cases of acute respiratory infections (ARI) or influenza like illness to the Public Health Agency of Catalonia coordinating centre. Physicians take nose and/or throat swabs from a sample of cases and send the specimens to the reference centre where they are tested for influenza and other respiratory viruses.Citation9,Citation10
The PIDIRAC network was established to provide timely epidemiological and virological information on influenza activity in Catalonia. In addition, it also participates in the Spanish and the European Influenza Surveillance Network (EISN). Since the 2009–2010 pandemic season, the PIDIRAC has been enhanced by including severe influenza cases which require hospitalization and collecting information on the presence of chronic conditions and risk factors. This approach has positively impacted by improving the quality and accuracy of surveillance information.
Influenza surveillance data have been used in Australia, USA, UK, Canada, and SpainCitation7,Citation11-Citation14 to monitor influenza VE using the test-negative control approach for a rapid estimation of VE.Citation3 Yet there is no regular review of influenza vaccine effectiveness at the end of influenza seasons in Catalonia. The aim of this study was to assess influenza vaccine effectiveness through sentinel surveillance data from the PIDIRAC program during 2010–2013 seasons which had three different predominant circulating influenza virus (IV) types: A(H1N1)pdm09, A(H3N2), and B, respectively.
Results
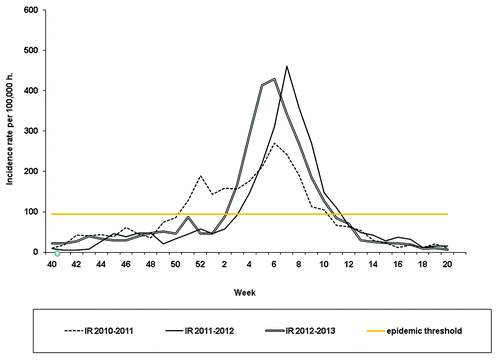
The total number of sentinel samples collected during the three seasons was 3609, of which, those with known vaccination background collected during the study period were 3173. Registers with unknown immunization status (436) were not included in the analysis. Statistically significant difference was observed as to the number of samples with available information on immunization status, with a lower compliance in the 2011–2012 season (10.3% of samples with unknown vaccination information). The overall percentage of vaccinated ILI cases was 14.7% (464/3173). No differences between seasons were observed as to percentage of vaccinated ILI cases (). Positivity rate to IV was 35.2% (1117/3173). Evolution of ILI activity for the three influenza seasons included in the study is shown in .
Table 1. Distribution according to influenza season of number of samples with available information and percentage of vaccinated cases. Catalonia, 2010–2013
Figure 1. Evolution of ILI activity for the three influenza seasons 2010-2011, 2011-2012 and 2012-2013. Catalonia, Spain
Distribution by age group of vaccinated samples was highest for the >60 y (65.5%) followed by the 15–59 y group (8.2%) and the 0–14 y (7.1%). No differences in percentage of vaccinated confirmed IV patients by age group was observed, being highest for the 10–14 y group (22.7%) followed by the 15–59 y (20%) and for the >60 y (19.3%) (). A subsample of 463 patients corresponding to cases who presented at least one risk factor for complications due to influenza was analyzed. Of these cases, information as to vaccination status was available for 445 (96%), and 223 (50.1%) were vaccinated for seasonal influenza. Distribution of vaccinated cases fulfilling recommendation criteria for vaccination for underlying chronic diseases is shown on according to age group. Statistically significant differences were found in the <14 y age group (48.8% vs. 32.5%) and older than 60 y (72.8% vs. 52.8%) in the influenza vaccine coverage observed in sentinel practitioners and in the SISAP register.
Table 2. Distribution according to influenza season of samples with available information and percentage of vaccinated and unvaccinated patients. Catalonia, 2010–2013
Table 3. Distribution of vaccination coverage in two different sources of information according to age group in ILI cases sampled with at least one risk factor for complications. Catalonia, 2010–2013
VE estimates were calculated for the 3173 patients for whom age, laboratory results, and vaccination status data were available. The estimate of VE for each of the three seasons ranged from 34.2% to 69.7%. Combined VE for the three seasons was estimated at 58.1% (95% CI:46.8–67). Differences in VE according to epidemic season were observed, being highest for the 2012–2013 season with predominance of IV type B [69.7% (95% CI:51.5–81)], for the 2010–2011 season with predominance of IV type A(H1N1)pdm09 being 67.2% (95%CI:49.5–78.8) and lowest for the 2011–2012 season with IV A(H3N2) predominance [34.2% (95%CI:4.5–54.6)] ().
Table 4. Vaccine effectiveness for influenza vaccines in three epidemic seasons. Catalonia, 2010–2013
Discussion
The total number of sentinel samples collected during the three seasons was high and, although quality of data upon collection could be improved, the overall availability of data was suitable to allow for seasonal characterization. In our study, the overall percentage of vaccinated ILI cases was 14.7%, being highest in the >60 y old with a 65.5%. The vaccination coverage required to establish herd immunity for influenza ranges from 13–30% depending on the circulating seasonal epidemic virusCitation15 which would set our findings within the lower range. Although the set objectives of vaccination coverage proposed in Europe are 75% in elderly and high-risk personsCitation16 we observed a 65.5% of sampled population 60 y and over showing that vaccine coverage even in the elderly must be improved. The higher vaccine coverage for targeted at risk groups in our results from sentinel physicians with respect to the overall targeted at risk population reflects the positive advocacy effect for vaccination by those healthcare professionals who are closely engaged in preventive and public health collaborative tasks.
Lower VE in the 2011/12 influenza season could be explained by its late presentation () and because of mismatching of strain contained in the vaccine with the circulating strain as a result of viral drift.Citation17 As herd immunity increases during the epidemic season, we should expect to see more antigenic drift; however, if immunity is high enough to prevent the population-wide spread of the pathogen, the epidemic cannot take off and the virus does not evolve. Thus, an intermediate amount of population-wide immunity results in the most antigenic drift.Citation18 In our study, the only season with a slight mismatch was the 2012–2013 season with IV type B predominance; no mismatch was observed in the other two seasons. The 2011–12 influenza season was a late season, thus patients presenting with influenza had a long delay between onset of symptoms and the vaccination because campaigns were performed in the autumn of 2011. The observed fall in VE may also be due in part to waning of the immunity induced by the vaccine.Citation19,Citation20 Besides waning, other circumstances could eventually affect VE, such as manufacturing and processing alterations or cold chain disruption; therefore understanding suboptimal VE requires broad consideration of complex factors within the full epidemiologic triad of agent, host, and environment interactions. Differentiating their separate effects and varying contributions from year to year will require in-depth and adequately powered immunoepidemiologic investigation across multiple seasons.Citation21,Citation22
Our results are in accordance with the moderate protective effect of the trivalent seasonal vaccine against influenza A(H1N1)pdm09 virus and a low effect against A(H3N2) virus found in other studies such as in the UK (51% for the 2012–2013 season), the USA (60%),Citation23 but slightly higher than the VE obtained in Navarre (Spain) which was 31%.Citation24
Designing better influenza vaccinesCitation25 to improve the selection of strains contained in the vaccine should be a priority for future vaccines, yet even in seasons in which the effectiveness of influenza vaccine is low, vaccination may appreciably reduce the number of cases and hospitalizations in high-risk persons.Citation26-Citation29 Furthermore, the fact that our results from sentinel surveillance practitioners showed higher vaccine coverage also reflect the effect of attitude towards vaccination, showing higher advocacy for those professionals who are motivated for disease prevention and public health.
There are some limitations to the study that must be pointed out. Because of the observational nature of this study, we cannot exclude biases. We used a test-negative design which is subject to the usual selection biases particularly for the control group.Citation30 The test-negative design is a commonly used, but not validated study design.Citation31 Using test-negative controls is considered to adjust for healthcare-seeking behavior more so than if community controls were selected, as vaccination coverage varies by healthcare seeking behavior.Citation32 In our study, participants were selected according to a systematic sampling procedure by practitioners, who are blinded to the case and control status of the patients, so this should minimize selection bias. Another limitation is that our study focus on VE assessment conducted within sentinel practitioner networks and therefore it only addresses issues arising when measuring VE against outcomes that are observed in primary care settings. Hospital based studies can also estimate VE against severe outcomes like all hospitalizations, hospitalizations for respiratory or cardiovascular diseases or for severe acute respiratory infections confirmed as influenza.Citation33 In addition, early detection and investigation of influenza clusters (e.g., schools, work place) could also provide prompt VE estimates.Citation3
In conclusion, influenza vaccination prevents a substantial number of influenza-associated illnesses. Efforts to increase vaccination rates of population at risk of complications and healthcare personnel will further reduce the burden of influenza. Sentinel data reflect the greater awareness of influenza complications in at risk population by physicians engaged in public health surveillance.
Although vaccines with increased effectiveness are needed towards the search for a universal vaccine that is not subject to genetic modifications, in the meantime improving vaccine recommendation practices in all primary care facilities should be advocated. Further virological studies are needed on an annual basis quantifying drift over time and production of an improved seasonal influenza vaccine with greater effectiveness should be given a high priority.
Methods
Practitioners used standardized questionnaires to collect information on ILI signs and symptoms, gender, age, seasonal influenza vaccination in the corresponding season, pregnancy and chronic conditions (including obesity). Using systematic sampling, practitioners swabbed ILI/ARI patients within seven days of symptom onset. Among ILI patients fulfilling the inclusion criteria, we defined an influenza case as a study participant whose swab tested positive for influenza virus by reverse-transcriptase polymerase chain reaction (RT-PCR). Swabs’ testing for influenza and genetic characterization was performed at the Influenza Sentinel Surveillance System Reference Laboratory of Catalonia.
A retrospective case negative control design was used to assessCitation7,Citation34 seasonal influenza VE estimates against laboratory-confirmed infections for the each of the predominant influenza viruses A(H1N1)pdm09, A(H3N2), and B for the 2010–2011, 2011–2012, and 2012–2013 seasons, respectively. We included in the analyses only those ILI patients with available information on vaccination status. Influenza VE was computed counting all patients whose swabs were positive for influenza virus RNA as cases and all other patients whose swabs were negative or positive for another respiratory virus as controls.
Database was compiled with information from three post-pandemic seasons’ feedback from the PIDIRAC network reference laboratory. We compared influenza-positive to influenza laboratory-negative patients among those meeting the EU ILI case definition.Citation35
We defined cases and controls as vaccinated if they had received at least one dose of corresponding seasonal influenza vaccine more than 14 d prior to ILI/ARI symptom onset. All others were classified as unvaccinated. We performed an analysis restricted to the influenza seasonal surveillance period. Analyses for vaccine information availability of each of the three influenza seasons and vaccine coverage for the sentinel population included in the study were performed. Coverage of target groups for vaccinationCitation2 among the sentinel population stratified by age groups (0–14, 15–59, and >60 y) were compared with the mean coverage data given by the SISAP (Sistemes d’Informació dels Serveis d’Atenció Primària del’ICS) of the Catalan Health Institute according to three age groups.
We estimated vaccine effectiveness as a percentage: VE = (1-OR)x100, where OR was the odds of being a vaccinated case divided by the odds of being a vaccinated control. The baseline characteristics of cases and controls were compared using Chi-square or Fisher’s exact tests, as appropriate. The Chi-square test was used to compare proportions and P < 0.05 was considered to be statistically significant. Odds ratios (OR) and their corresponding 95% confidence intervals (95%CI) were obtained. Data was analyzed on SPSS® 18 (IBM Statistical Package Inc. Chicago, USA).
| Abbreviations: | ||
| ARI | = | Acute Respiratory Infection |
| CI | = | Confidence Interval |
| ICS | = | Catalan Institute of Health |
| PIDIRAC | = | Surveillance of Catalonia |
| EISN | = | European Influenza Surveillance Network |
| EU | = | European Union |
| GP | = | General Practitioner |
| ILI | = | Influenza-Like Illness |
| IV | = | Influenza Virus |
| VE | = | Vaccine Effectiveness |
| SISAP | = | Information System for Primary Health Care Centers |
| IR | = | Incidence Rate |
| RT-PCR | = | Reverse-transcription Polymerase Chain Reaction |
| RF | = | Risk factor |
| UK | = | United Kingdom |
| USA | = | United States of America |
| VE | = | Vaccine Effectiveness |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank all physicians members of the influenza sentinel surveillance network of Catalonia (PIDIRAC): Aizpurua, P; Alonso, J; Azemar, J; Basas, D; Besora, R ; Callado, M; Casanovas JM; Cid, A; Ciurana, E; Cots JM; De la Rica, D; Elizalde del Rio, G; Estabanell, A; Fau, E; Fernandez, O; Ferrer, C; Forcada, A; Forga, FX; Fos,E; Gadea, G; Garcia, J; Garrido, P; Gatius, C; Grivé, M; Guzman, MC; Hernandez, R; Juscafresa,D; Leon, I; Macia, E; Mainou, A; Marco, E; Martinez, M; Martinez, E; Molinero, C; Moncosi, X; Lopez-Mompó, C; Naranjo, MA; Navarro, D; Ortola, ME; Pérez MC; Perez, MM; Prat, M; Pujol, R; Pujol, J; Ribatallada, A; Sánchez, R; Sarra, N; Teixidor, A; Valen, E; Valencia, I; Van Esso, D; Vila, C; Zabala, E; Zurilla, E.
Financial Disclosure
This work was partially supported by the Agency for the Management of Grants for University Research (AGAUR Grant number 2009 SGR 42) and CIBER Epidemiology and Public Health (CIBERESP).
References
- HandaR, TeoS, BooyR. Influenza: current evidence and informed predictions. Expert Rev Vaccines2004; 3:443 - 51; http://dx.doi.org/10.1586/14760584.3.4.443; PMID: 15270649
- Public Health Agency of Catalonia, Immunization Program. Technical guidelines for seasonal influenza immunization.[cited 2014 Mar 15] Available from. URL: http://www20.gencat.cat/portal/site/canalsalut
- ValencianoM, KisslingE, CiancioBC, MorenA. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine2010; 28:7381 - 8; http://dx.doi.org/10.1016/j.vaccine.2010.09.010; PMID: 20851086
- ShimE, GalvaniAP. Distinguishing vaccine efficacy and effectiveness. Vaccine2012; 30:6700 - 5; http://dx.doi.org/10.1016/j.vaccine.2012.08.045; PMID: 22944629
- OrensteinWA, BernierRH, DonderoTJ, HinmanAR, MarksJS, BartKJ, SirotkinB. Field evaluation of vaccine efficacy. Bull World Health Organ1985; 63:1055 - 68; PMID: 3879673
- HalloranME. Overview of vaccine field studies: types of effects and designs. J Biopharm Stat2006; 16:415 - 27; http://dx.doi.org/10.1080/10543400600719236; PMID: 16892904
- KellyH, CarvilleK, GrantK, JacobyP, TranT, BarrI. Estimation of influenza vaccine effectiveness from routine surveillance data. PLoS One2009; 4:e5079; http://dx.doi.org/10.1371/journal.pone.0005079; PMID: 19333374
- Surveillance and Response to Emergencies in Public Health, Public Health Agencyu of Catalonia. Pla d'informació de les infeccions respiratòries agudes a Catalunya PIDIRAC.[cited 2014 Apr 1]; Available from. URL: http://www20.gencat.cat/portal/site/canalsalut
- TornerN, BaricotM, MartínezA, ToledoD, GodoyP, DominguezÁ, Influenza Sentinel Surveillance Primary care physicians’ Network of Catalonia (PIDIRAC). Influenza sentinel surveillance network: a public health-primary care collaborative action to assess influenza A(H1N1)pmd09 in Catalonia, Spain. Hum Vaccin Immunother2013; 9:671 - 4; http://dx.doi.org/10.4161/hv.23264; PMID: 23396181
- TornerN, MorteruelM, MartinezA, BasileL, IsantaR, PumarolaT. Differences in sentinel influenza confirmed incidence rates and clinical presentation of influenza virus: 2008-09 seasonal vs 2009-10 pandemic influenza. Hum Vaccin2011; 7:Suppl230 - 3; http://dx.doi.org/10.4161/hv.7.0.14612; PMID: 21285535
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness - United States, February 2013. MMWR Morb Mortal Wkly Rep2013; 62:119 - 23; PMID: 23425960
- McMenaminJ, AndrewsN, RobertsonC, FlemingD, DurnallH, von WissmannB, EllisJ, LackenbyA, CottrellS, SmythB, et al. Effectiveness of seasonal 2012/13 vaccine in preventing laboratory-confirmed influenza infection in primary care in the United Kingdom: mid-season analysis 2012/13. Euro Surveill2013; 18:18; PMID: 23399421
- JanjuaNZ, SkowronskiDM, De SerresG, DickinsonJ, CrowcroftNS, TaylorM, WinterAL, HottesTS, FonsecaK, CharestH, et al. Estimates of influenza vaccine effectiveness for 2007-2008 from Canada’s sentinel surveillance system: cross-protection against major and minor variants. J Infect Dis2012; 205:1858 - 68; http://dx.doi.org/10.1093/infdis/jis283; PMID: 22492921
- Jimenez-JorgeS, PozoF, de MateoS, Delgado-SanzC, CasasI, Garcia-CenozM, CastillaJ, SanchoR, Etxebarriarteun-AranzabalL, QuinonesC, et al, Spanish Influenza Sentinel Surveillance System (SISS). Influenza vaccine effectiveness in Spain 2013/14: subtype-specific early estimates using the cycEVA study. Euro Surveill2014; 19:19; http://dx.doi.org/10.2807/1560-7917.ES2014.19.9.20727; PMID: 24626206
- Plans-RubióP. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med2012; l55:72 - 7; http://dx.doi.org/10.1016/j.ypmed.2012.02.015; PMID: 22414740
- World Health Organization (WHO). Prevention and control of influenza pandemics and annual epidemics.2003. Report No.: WHA56.19.
- CarratF, FlahaultA. Influenza vaccine: the challenge of antigenic drift. Vaccine2007; 25:6852 - 62; http://dx.doi.org/10.1016/j.vaccine.2007.07.027; PMID: 17719149
- BoniMF. Vaccination and antigenic drift in influenza. Vaccine2008; 26:Suppl 3C8 - 14; http://dx.doi.org/10.1016/j.vaccine.2008.04.011; PMID: 18773534
- OsterholmMT, KelleyNS, SommerA, BelongiaEA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis2012; 12:36 - 44; http://dx.doi.org/10.1016/S1473-3099(11)70295-X; PMID: 22032844
- CastillaJ, Martínez-BazI, NavascuésA, Fernandez-AlonsoM, ReinaG, GuevaraM, ChamorroJ, OrtegaMT, AlbénizE, PozoF, et al, Primary Health Care Sentinel Network, Network for Influenza Surveillance in Hospitals of Navarre. Vaccine effectiveness in preventing laboratory-confirmed influenza in Navarre, Spain: 2013/14 mid-season analysis. Euro Surveill2014; 19:19; http://dx.doi.org/10.2807/1560-7917.ES2014.19.6.20700; PMID: 24556347
- SkowronskiDM, JanjuaNZ, De SerresG, SabaiducS, EshaghiA, DickinsonJA, FonsecaK, WinterAL, GubbayJB, KrajdenM, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One2014; 9:e92153; http://dx.doi.org/10.1371/journal.pone.0092153; PMID: 24667168
- SkowronskiDM, JanjuaNZ, De SerresG. Understanding suboptimal influenza vaccine effectiveness within the agent, host, and environment paradigm. Clin Infect Dis2013; 57:476 - 7; http://dx.doi.org/10.1093/cid/cit256; PMID: 23619812
- TreanorJJ, TalbotHK, OhmitSE, ColemanLA, ThompsonMG, ChengPY, PetrieJG, LofthusG, MeeceJK, WilliamsJV, et al, US Flu-VE Network. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis2012; 55:951 - 9; http://dx.doi.org/10.1093/cid/cis574; PMID: 22843783
- CastillaJ, Martínez-BazI, Martínez-ArtolaV, ReinaG, PozoF, García CenozM, GuevaraM, MoránJ, IrisarriF, ArriazuM, et al, Primary Health Care Sentinel Network, Network for Influenza Surveillance in Hospitals of Navarre. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill2013; 18:18; PMID: 23399423
- LambertLC, FauciAS. Influenza vaccines for the future. N Engl J Med2010; 363:2036 - 44; http://dx.doi.org/10.1056/NEJMra1002842; PMID: 21083388
- BaxterR, RayGT, FiremanBH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine2010; 28:7267 - 72; http://dx.doi.org/10.1016/j.vaccine.2010.08.088; PMID: 20832494
- NicholKL. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine2009; 27:6305 - 11; http://dx.doi.org/10.1016/j.vaccine.2009.07.006; PMID: 19840665
- CastillaJ, GodoyP, DomínguezA, Martínez-BazI, AstrayJ, MartínV, Delgado-RodríguezM, BaricotM, SoldevilaN, MayoralJM, et al, CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis2013; 57:167 - 75; http://dx.doi.org/10.1093/cid/cit194; PMID: 23532475
- RidenhourBJ, CampitelliMA, KwongJC, RosellaLC, ArmstrongBG, MangtaniP, CalzavaraAJ, ShayDK. Effectiveness of inactivated influenza vaccines in preventing influenza-associated deaths and hospitalizations among Ontario residents aged ≥ 65 years: estimates with generalized linear models accounting for healthy vaccinee effects. PLoS One2013; 8:e76318; http://dx.doi.org/10.1371/journal.pone.0076318; PMID: 24146855
- ValencianoM, KisslingE, I-MOVE Case-Control Study Team. Early estimates of seasonal influenza vaccine effectiveness in Europe: results from the I-MOVE multicentre case-control study, 2012/13. Euro Surveill2013; 18:3; PMID: 23449183
- KisslingE, ValencianoM, LarrauriA, OrosziB, CohenJM, NunesB, PitigoiD, RizzoC, RebolledoJ, Paradowska-StankiewiczI, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro Surveill2013; 18:18; PMID: 23399425
- BelongiaEA, KiekeBA, DonahueJG, GreenleeRT, BalishA, FoustA, LindstromS, ShayDK, Marshfield Influenza Study Group. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis2009; 199:159 - 67; http://dx.doi.org/10.1086/595861; PMID: 19086915
- Puig-BarberàJ, Díez-DomingoJ, VareaAB, ChavarriGS, RodrigoJA, HoyosSP, VidalDG. Effectiveness of MF59-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. Vaccine2007; 25:7313 - 21; http://dx.doi.org/10.1016/j.vaccine.2007.08.039; PMID: 17889411
- OrensteinEW, De SerresG, HaberMJ, ShayDK, BridgesCB, GargiulloP, OrensteinWA. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol2007; 36:623 - 31; http://dx.doi.org/10.1093/ije/dym021; PMID: 17403908
- European Centers for Disease Control. Influenza case definition. [cited 2014 Jun 1]; Available from: URL: http://ecdc.europa.eu/en/activities/surveillance/eisn/surveillance/pages/influenza_case_definitions.aspx
