Abstract
Infections caused by Chlamydia trachomatis (Ct) and human papillomavirus (HPV) are the two main sexually transmitted infections; however, epidemiological data on Ct prevalence and Ct/HPV co-infection in Italy are scant. This study aimed at estimating the prevalence of Ct infection and Ct/HPV co-infection in young HPV-unvaccinated females with normal cytology, and placed particular attention on the possible association between Ct-DNA positivity and different HPV infecting genotypes. Five hundred 66 healthy females aged 16–26 years without cervical lesions, previously assessed for HPV infection (HPV-DNA prevalence: 18.2%), were tested for Ct-DNA. The overall prevalence of Ct was 5.8% (95% CI: 4.2–8.1), while Ct/HPV co-infection was recorded in 2.7% (95% CI: 1.6–4.3) of subjects. Compared with HPV-DNA-negative females, HPV-DNA positive subjects had significantly (P < 0.001) higher odds of being infected with Ct (odds ratio of 4.20, 95% CI: 2.01–8.71). Both Ct and Ct/HPV infections were much more prevalent in under 18-year-olds than in older women. Subjects positive for single high-risk HPV genotypes and various multiple HPV infections had higher odds of being Ct-DNA positive. Our findings confirm that HPV and Ct infections are very common among asymptomatic young Italian females. This underlines the urgent need for nationwide Ct screening programs and reinforcement of sexual health education, which would be the most important public health strategies, since no Ct vaccines are currently available.
Introduction
Sexually transmitted infections (STIs) are a worldwide public health challenge, with more than a million new cases occurring every day; these are caused by more than 30 different bacteria, viruses and parasites.Citation1 Among these, human papillomavirus (HPV) and Chlamydia trachomatis (Ct) are the two main sexually transmitted pathogens.Citation2,Citation3
It is now universally recognized that several oncogenic HPV genotypes are the necessary, but not sufficient, cause of cervical cancer (CC); most women infected with HPV do not develop CC.Citation4,Citation5 Various endogenous and exogenous co-factors, including other STIs, that influence the risk of progression from HPV infection to invasive CC exist and have been extensively studied.Citation5 Among these potential co-factors, urogenital Ct infection is of particular concern owing to its wide diffusion and heavy public health burden. Indeed, genital Ct infection is the most prevalent bacterial STI, especially among adolescents and young women.Citation6,Citation7 Although often asymptomatic, genital Ct infection in women may lead to numerous serious and costly complications, such as pelvic inflammatory disease, tubal factor infertility, ectopic pregnancy and chronic pelvic pain.Citation8,Citation9 In Europe, the prevalence of Ct in unscreened asymptomatic women varies significantly among countries, ranging from 1.7 to 17%.Citation6 In Italy, no nationwide population-based studies on the prevalence of Ct infection have yet been performed;Citation10 Ct case notification is not mandatory and no screening policy exists.Citation11 A few ad hoc studies conducted in ItalyCitation11-Citation17 have found Ct prevalence rates ranging from 1.8–10.4% among sexually active women, depending on the study population and geographic area.
A specific role of Ct infection in the pathogenesis of cervical intraepithelial neoplasia (CIN) and subsequent CC is biologically plausible, although as yet there is no univocal and well-established evidence on this issue.Citation18,Citation19 Earlier studies have found a significantly higher prevalence of Ct-DNA among HPV-positive women than HPV-negative onesCitation2,Citation20-Citation22 as well as evidence that Ct is an HPV co-factor in the etiology of displastic and neoplastic cervical abnormalities and CC.Citation23-Citation25 Cervical Ct infection has been clinically documented to be associated with cytological atypia and cervical metaplasia, thus damaging epithelial integrity, allowing the HPV access to the basal epithelium and increasing viral load.Citation19,Citation24 Ct may be responsible for increasing the persistence of HPV infection by affecting viral clearance. Furthermore, the natural history of HPV infection may be influenced by Ct-induced chronic cervical inflammation, a reduction in antigen-presenting cells and inhibition of cell-mediated immunity. A local deficit of immunity during the reparative phase of the epithelial surface under inflammatory conditions may make cervical epithelial cells more susceptible to other pathogens.Citation18,Citation19
On the other hand, it has been also suggested that the association between Ct and HPV lies only in their shared route of transmission, as both pathogens are markers of sexual risk behaviors.Citation25
Our previous studyCitation26 revealed an overall HPV prevalence of 18.2% among unvaccinated young females with normal cytological findings and a particularly high prevalence (10.1%) of high-risk HPV genotypes, underlining the need to optimize preventive measures against STIs among healthy young females. Owing to the absence of a national surveillance system and few epidemiological data available, we conducted a study to estimate the prevalence of Ct infection and Ct/HPV co-infection in young asymptomatic women without cervical lesions. Our second goal was to assess the possible association between Ct-DNA positivity and various HPV infecting genotypes.
Results
In our previous studyCitation26 566 cervical samples were studied to determine the prevalence of different genotypes of HPV in 16–26-y-old Italian females with normal cytology; the results are reported elsewhere.Citation26 Briefly, 103 HPV-DNA-positive and 463 HPV-DNA-negative samples were identified; in the present study, these samples were analyzed for Ct. HPV-DNA-positive and -negative groups were comparable in terms of age [19.8 (SD 2.5) and 19.7 (SD 2.4) years, respectively; t(564) = 0.19, P = 0.85].
Prevalence of Chlamydia trachomatis infection
Overall, 33 out of 566 young women were positive for Ct: a prevalence of 5.8% (95% CI: 4.2–8.1%). A higher prevalence of Ct infection was found in Genoa [7.7% (95% CI: 4.5–13.0%)] than in Milan [6.4% (95% CI: 4.1–9.9%)] and Turin [2.3% (95% CI: 0.8–6.6%)]. This difference was not, however, statistically significant (χ2(2) = 4.04, P = 0.13).
Ct positivity among 16–17-y-olds (n = 27) was at least 3.5-fold higher [18.5% (95% CI: 8.2–36.7%)] than in older age-classes [18–20 y (n = 376): 5.3% (95% CI: 3.5–8.1%); 21–23 y (n = 105): 4.8% (95% CI: 2.1–10.7%); 24–26 y (n = 58): 5.2% (95% CI: 1.8–14.1%)] with a borderline significance level (Fisher exact test: P = 0.075).
Chlamydia trachomatis/HPV co-infection
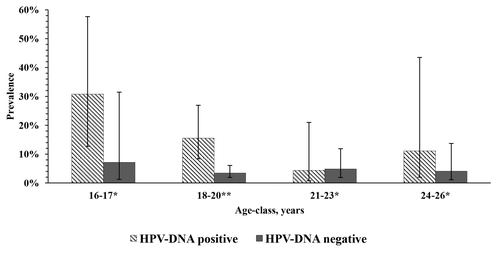
Ct/HPV co-infection was found in 15 subjects [2.7% (95% CI: 1.6–4.3%)]. The prevalence of Ct-DNA positivity was about 3.7-fold higher among HPV-DNA-positive subjects than among HPV-DNA-negative subjects [14.6% (95% CI: 9.0–22.6%) vs. 3.9% (95% CI: 2.5–6.1%); χ2(1) = 17.49, P < 0.001] with an odds ratio (OR) of 4.20 (95% CI: 2.01–8.71). The Ct-DNA prevalence was highest among adolescents 16–17 y old and tended to be higher among HPV-DNA-positive subjects than HPV-DNA-negative ones across almost all age-classes, although a statistically significant difference was observed only in the 18–20 age-class (). Indeed, within the HPV-DNA-positive group, the mean age of females with Ct/HPV co-infection was significantly lower (t(101) = 2.05, P = 0.043) than that of Ct-DNA-negative females [18.6 (SD 2.1) vs. 20.0 (SD 2.5) years]. The effect size was medium (Cohen’s d = 0.61). By contrast, no difference emerged between the mean ages of Ct-DNA-positive and Ct-DNA-negative subjects within the HPV-DNA-negative group [19.8 (SD 2.7) and 19.7 (SD 2.3) years, respectively; t(461) = 0.18, P = 0.86].
Figure 1. Prevalence of Chlamydia trachomatis DNA positivity by age-class and HPV positivity status. *Not significant; **Fisher exact test P = 0.002.
Among Ct/HPV co-infected females, 12 out of 15 [80.0% (95% CI: 54.8–92.3%)] were positive to at least one high-risk (HR) or possible/probable-risk (PR) HPV. The most frequent HPV genotype was HPV-16, which was detected in 4 subjects. By contrast, low-risk (LR) HPV genotypes were much less frequent [13.3% (95% CI: 3.7–37.9%)] ().
Table 1. HPV genotypes found among Chlamydia trachomatis DNA-positive subjects, by urban area and age
Logistic regression revealed that females infected with single PR [OR = 6.51 (95% CI: 1.60–26.47) P = 0.009], double LR+PR [OR = 27.35 (95% CI: 1.47–508.16) P = 0.026] and triple HR+HR+LR [OR = 22.90 (95% CI: 1.36–385.80) P = 0.030] HPV infections had significantly higher odds of being Ct positive. Although females infected with HR or HR+HR HPV genotypes did not reach statistical significance at the P < 0.05 level, the effect sizes were large [OR = 2.98 (95% CI: 0.94–9.44) P = 0.064 and OR = 9.84 (95% CI: 0.98–98.84) P = 0.052, respectively]. Other types of single or multiple HPV infections (LR, LR+LR, LR+PR, PR+PR, LR+HR, HR+PR and HR+HR+PR) were not statistically significant.
Discussion
This study is among the first to explore some epidemiological features of Ct/HPV co-infection among Italian female adolescents and young women with normal cytology. These results potentially have several implications for future health policy planning. Indeed, knowledge of the prevalence of and risk factors for these two most common STIs is a prerequisite for undertaking effective preventive and control measures.
Most of the few studies on Ct prevalence conducted in Italy have involved women with abnormal cytological findings or symptoms of STIs.Citation14,Citation15,Citation17 This makes direct comparison difficult, since our study population was made up of asymptomatic female adolescents and young women with normal cytology. Our Ct prevalence estimate is in line with those of several European population-based studies among sexually active female adolescents and young women, which have reported prevalence rates ranging from 3.6 to 8.0%.Citation27-Citation30 Notably, the majority of the women in our sample accessed gynecology centers seeking the prescription of oral contraceptives (results not shown), and the mode of Ct prevalence among European women seeking contraception is estimated, as in our study, to be about 6%.Citation6 In a recently performed meta-analysis, Ct prevalence in high-income countries was estimated to be 4.32% among sexually active females aged ≤ 26 y.Citation10 By contrast, in a study conducted in Brescia (Italy),Citation12 an unexpectedly low Ct prevalence of 1.9% among sexually experienced healthy female students was documented. This inconsistency with our results could be explained in several ways. First, it could be due to the difference in study locations, as Ct prevalence varies geographically.Citation6 Indeed, we observed a 3.3-fold difference in Ct prevalence between Genoa and Turin; thus, the estimate from Brescia is almost consistent with the figure we recorded in Turin. Second, our study population also included female adolescents aged 16–17 y—the age-class which displayed the highest Ct prevalence—while the study by Matteelli et al.Citation12 enrolled only women aged ≥18 y. Finally, the difference could be ascribed to the recruitment process; the participants in our study were enrolled at the gynecology centers of local health units (LHUs), while those in Brescia were recruited at high schools.
Our results confirm previous findings of significantly higher Ct prevalence among HPV-positive than HPV-negative women.Citation2,Citation20-Citation22 A systematic review by Silva et al.Citation19 reported Ct-DNA positivity prevalence rates of 4–59% among HPV-positive women living in different continents; however, when only estimates from Europe were considered, this range narrowed to 10–24%. By contrast, Ct-DNA positivity among HPV-negative controls reported in their paper was substantially lower, rarely exceeding 10%.Citation19 Research conducted in Italy by Verteramo et al.Citation17 found a Ct frequency of 13.9% among HPV-positive women, as against 5.4% among HPV-negative women; these figures are similarly to our estimates. However, in a recent paper by Sammarco et al.,Citation13 Ct prevalence in HPV-positive women aged 18–63 y without cervical lesions was estimated to be as low as 6.3%, which is about half our figure. Again, the difference observed is very probably due to the different age profiles of the study populations.
In our study, both Ct-DNA positivity and HPV/Ct co-infection displayed age disparities, with a much higher prevalence among female adolescents under 18 y old; this is in line with the well-established evidence that sexually active adolescent females are at higher risk of genital Ct infection than older females.Citation31-Citation34 Indeed, a meta-analysis by Adams et al.Citation35 revealed that the OR of being infected with Ct diminished as age increased. Similarly, in a large Italian study,Citation16 female adolescents between 15 and 19 y had the highest Ct prevalence. Another interesting finding concerns the association between the lower age of Ct-infected subjects than of Ct-DNA-negative ones within the HPV-DNA-positive group. That patients with concurrent STIs tend to be younger has already been suggested by Griffiths et al.Citation36 However, Tamim et al.Citation2 did not find any association between age and Ct positivity within the HPV-positive group; this was probably the result of the lower sample size in their study. By contrast, our finding not only reached statistical significance at α level <0.05, this between-group age difference of about 1.5 y is also of practical significance, as demonstrated by the effect size. This suggests the need to strengthen health education, counseling and other preventive measures before sexual debut, which has been estimated to be around 15 y old.Citation37
An association between Ct and HR HPV genotypes has been described previously.Citation19 As expected, the most frequently detected HPV genotype among our subjects co-infected with Ct was HPV-16, and most of co-infections involved at least one HPV genotype belonging to groups 1, 2A or 2B of the International Agency for Research on Cancer (IARC).Citation38 This finding is not surprising, since HPV-16 is the most prevalent genotype in ItalyCitation39,Citation40 and in many other geographical areas.Citation41 Analogous results have been reported by Tamim et al.Citation2 who found HPV-16 to be the most frequent genotype in HPV/Ct co-infections. The results of our regression analysis showed that women infected with single HR/PR and different combinations of multiple HPV genotypes with at least one HR/PR HPV type had significantly higher odds of being co-infected with Ct. This finding is of particular importance for the primary prevention of CC, since concurrent Ct infection has been found to be associated with the persistence of HR and multiple HPV genotypes in female adolescents.Citation42 In turn, the persistence of HR HPV genotypes among cytologically normal women leads to a greatly increased risk of CC.Citation43
It is noteworthy that the findings from our cross-sectional study did not allow us to determine which infection, HPV or Ct, occurred first. However, our main aim was to determine Ct and Ct/HPV infection prevalence among HPV-unvaccinated young women, and not to establish any causality. Another limitation of this explorative study is its relatively small sample size; this explains the large confidence intervals of the coefficients obtained from the logistic regression model. At the same time, the sample size was sufficient to identify large effect sizes associated with some specific HPV genotypes and their combinations.
In conclusion, the results of the present study highlight the fact that genital Ct infection is common among young HPV-unvaccinated females with normal cytology in three large metropolitan areas in northern Italy. Approximately one fifth of asymptomatic females in our sample tested positive for HPV and/or Ct, suggesting that the epidemiological features of STIs in Italy may fit into the “silent epidemic” context.Citation44 Indeed, the study population referred no symptoms of the STIs confirmed by subsequent gynecological examination. This observation stresses the need for nationwide Ct screening programs, which would be the most important viable and potentially cost-effective public health strategy in the absence of a Ct vaccine.Citation45 With regard to Ct vaccines, several candidates are under preclinical development, and a safe and effective vaccine is seen as the ultimate goal in the control of Ct;Citation45 indeed, mathematical modeling has shown that fully protective vaccination would eliminate Ct epidemics in about two decades.Citation46
Finally, in Italy public health policies towards these two most common STIs display several critical features, including far from optimal HPV vaccinationCitation47 and cervical screening coverage,Citation48 and inadequate Ct case notification and Ct screening;Citation11 these issues must be addressed as public health priorities.
Materials and Methods
The study was approved by the Ethics Committee of the LHU in Genoa, Italy.
Study population
In this study, cervical samples which had already been tested for HPV-DNA were examined to detect Ct-DNA.Citation16 Briefly, subjects aged 16–26 y were recruited in three cities in northern Italy (Turin, Milan and Genoa). All consecutive eligible young women who spontaneously accessed the gynecology centers of LHUs for medical consultations from 1st January to 30th June 2010 were enrolled.
All participants gave written informed consent. The inclusion criteria were: being sexually active, not being pregnant, not being symptomatic for any STI, not having been vaccinated against HPV, and no previous history of cervical abnormalities. All participants underwent a Pap smear and only those with normal cytology were studied.
Sample collection
Cervical smear samples were collected by means of a spatula, immersed and rinsed in a vial containing 20 ml of PreservCyt® solution (ThinPrep Pap Test, Hologic; cod.70136-001) and stored until processing at room temperature. The samples were analyzed in the molecular laboratory of the Department of Biomedical Sciences for Health of Milan University.
PreservCyt® solution (10 ml for each sample) containing cervical cells was centrifuged at 3800 g for 10 min at room temperature. After centrifugation, pellets were resuspended in 1 ml of phosphate buffered saline, transferred into a new 1.5 ml collection test-tube and stored at −20 °C until nucleic acid extraction.
Nucleic acid extraction
The NucliSENS® EasyMAG® (bioMérieux; cod. 200111) commercial kit was used for DNA extraction following the manufacturer’s instructions. A Thermo Scientific NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc) was used to ascertain the concentration and purity of the DNA extracted, while DNA integrity was evaluated by amplification of a 268 base pair (bp) fragment in the ubiquitous β-globin gene by using the primer pair PCO4 and GH20.Citation49
HPV DNA detection and Restriction Fragment Length Polymorphism genotype analysis
HPV DNA was detected by PCR amplification of a 450-bp segment of ORF L1, as previously described.Citation50,Citation51 Briefly, 50–100 ng of DNA was added to the PCR master mix containing an equimolar mixture of each primer ELSI-f and ELSI-r (30 pmol/μl), and the GoTaq® DNA Polymerase (Promega; cod. M3175). Amplification reaction conditions were 5 min denaturation at 94 °C, followed by 40 cycles of amplification consisting of a denaturation step at 94 °C for 30 s, an annealing step at 55 °C for 30 s and an elongation step at 72 °C for 30 s. The last cycle was followed by a 7 min elongation step at 72 °C.
Positive (DNA extracted from HPV 16-positive cells, Caski) and negative (water) control samples were inserted in each PCR run. The amplified products were revealed by electrophoresis analysis of 2% agarose gels containing ethidium bromide. The amplification product bands were then compared with molecular weight standards (DNA Molecular Weight, Marker 100, Sigma-Aldrich; cod. G8291).
All amplified fragments were genotyped by means of Restriction Fragment Length Polymorphism (RFLP) capable of identifying all genotypes, including HR, PR and LR genotypes, according to the latest IARC classification.Citation38
One μg of each amplified product was added to three different digestion solutions, each containing 1U of either RsaI, DdeI or HaeIII (New England BioLabs; cod. R0167L; cod. R0175L; cod. R0108L) restriction enzymes diluted in their respective buffers for 1 h at 37 °C. The digestion products were determined following separation in 3% agarose gels, and restriction patterns were compared with appropriate standards (DNA Molecular Weight, Marker 100+20, Sigma-Aldrich; cod. D7808). The pattern of fragments generated by the three restriction enzymes allowed genotypes to be identified.Citation52,Citation53
Each sample that displayed a complex/undetermined pattern on RFLP was retested. Samples that could not be assigned to an HPV genotype after 2 consecutive analyses were defined as “untypeable” (NT).
Chlamydia trachomatis
DNA detection. A nested PCR assay targeting a 150 bp segment of the cryptic plasmid of Ct was performed. The two primer pairs of primers used in the two PCR runs have previously been described by Jalal et al.Citation54 Briefly, 50–100 ng of DNA was added to the PCR master mix containing an equimolar mixture of each primer (25 pmol) and the GoTaq® DNA Polymerase (Promega; cod. M3175). Each run included positive controls (DNA extracted from human Ct-positive cervical cells) and negative (water) controls. Amplification reaction conditions were 5 min of denaturation at 94 °C, followed by 30 cycles (first run) and 25 cycles (second run). Each cycle consisted of a denaturation step at 94 °C for 30 s, an annealing step at 50 °C for 30 s, and an elongation step at 72 °C for 30 s. The last cycle was followed by a 7 min elongation step at 72 °C. The amplification products were revealed by means of electrophoresis analysis of 2% agarose gels containing ethidium bromide. Amplified product bands were compared with molecular weight standards (DNA Molecular Weight, Marker 100, Sigma-Aldrich; cod. G8291).
Statistical analysis
The normally distributed continuous variable “age” was expressed as mean and standard deviation (SD) and the two-sample Student’s t test was used to evaluate between-group difference in age. As the measure of effect size for the t test, Cohen’s d was used. Cohen’s d was interpreted as follows: small (d = 0.2), medium (d = 0.5) and large (d = 0.8).Citation55 The prevalence of Ct-DNA positivity with 95% confidence intervals (CIs), estimated by using Wilson’s score, was calculated both for the overall study population and by age-class (broken down into 4 age-classes: 16–17, 18–20, 21–23, and 24–26 y old on the basis of previous researchCitation26), province of residence and HPV-DNA positivity status. To compare frequency distributions, Fisher’s exact test was performed if ≥20% of expected frequencies were <5; otherwise, the chi-square test was applied. To detect a possible association between Ct-DNA positivity (dependent variable) and specific HPV genotypes (dummy-coded independent variable with the reference category of HPV-DNA-negative observations), a multivariable logistic regression analysis was performed; all ORs were adjusted for age and province of residence. Categories of single HPV infections were dummied in accordance with the latest IARC classificationCitation38 and presented as HR, PR and LR genotypes. Double and triple infections were dummied on the basis of our previously reportedCitation26 different HPV combinations: LR+LR, LR+PR, PR+PR, LR+HR, HR+PR, HR+HR, HR+HR+LR and HR+HR+PR. A two-sided p-value < 0.05 was considered statistically significant. All analyses were performed by means of the R stats package, version 3.0.1.Citation56
| Abbreviations: | ||
| CC | = | cervical cancer |
| CI | = | confidence interval |
| CIN | = | cervical intraepithelial neoplasia |
| Ct | = | Chlamydia trachomatis |
| DNA | = | deoxyribonucleic acid |
| HR | = | high-risk |
| HPV | = | human papillomavirus |
| IARC | = | International Agency for Research on Cancer |
| LHU | = | local health unit |
| LR | = | low-risk |
| NT | = | untypeable |
| OR | = | odds ratio |
| ORF | = | open reading frame |
| PCR | = | polymerase chain reaction |
| PR | = | possible/probable-risk |
| RFLP | = | restriction fragment length polymorphism |
| SD | = | standard deviation |
| STI | = | sexually transmitted infection |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
The study was financed by the Italian Ministry of University and Research (MIUR, project PRIN 2009; Grant number: 2009ZPM4X4). The authors thank: Dr Angela Lidia Grondona, Dr Elisa Di Capua, Dr Paolo Cristoforoni, Dr Roberto Ferraro, Dr Massimo Benzi, Dr Paola Voltolina, and Dr Albina Godani for their support in sample collection. They also thank Dr Klodiana Sulaj, Dr Morena Martinese, Dr Miriam Divita, and Dr Graziella Romani for their support in data collection and quality control.
The authors thank Dr Bernard Patrick for revising the manuscript.
References
- World Health Organization. Sexually transmitted infections (STIs). Fact sheet N° 110. Available at: http://www.who.int/mediacentre/factsheets/fs110/en/. Accessed: July 2, 2014.
- TamimH, FinanRR, SharidaHE, RashidM, AlmawiWY. Cervicovaginal coinfections with human papillomavirus and Chlamydia trachomatis. Diagn Microbiol Infect Dis2002; 43:277 - 81; http://dx.doi.org/10.1016/S0732-8893(02)00403-0; PMID: 12151187
- TáboraN, ZelayaA, BakkersJ, MelchersWJ, FerreraA. Chlamydia trachomatis and genital human papillomavirus infections in female university students in Honduras. Am J Trop Med Hyg2005; 73:50 - 3; PMID: 16014831
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(Suppl 3):S3/1–10.
- CastellsaguéX, MuñozN. Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr2003; 31:20 - 8; http://dx.doi.org/10.1093/oxfordjournals.jncimonographs.a003477; PMID: 12807941
- WilsonJS, HoneyE, TempletonA, PaavonenJ, MårdhPA, Stray-PedersenB, EU Biomed Concerted Action Group. A systematic review of the prevalence of Chlamydia trachomatis among European women. Hum Reprod Update2002; 8:385 - 94; http://dx.doi.org/10.1093/humupd/8.4.385; PMID: 12206472
- BursteinGR, GaydosCA, Diener-WestM, HowellMR, ZenilmanJM, QuinnTC. Incident Chlamydia trachomatis infections among inner-city adolescent females. JAMA1998; 280:521 - 6; http://dx.doi.org/10.1001/jama.280.6.521; PMID: 9707141
- Fernández-BenítezC, Mejuto-LópezP, Otero-GuerraL, Margolles-MartinsMJ, Suárez-LeivaP, VazquezF, Chlamydial Primary Care Group. Prevalence of genital Chlamydia trachomatis infection among young men and women in Spain. BMC Infect Dis2013; 13:388; http://dx.doi.org/10.1186/1471-2334-13-388; PMID: 23968487
- LewisD, NewtonDC, GuyRJ, AliH, ChenMY, FairleyCK, HockingJS. The prevalence of Chlamydia trachomatis infection in Australia: a systematic review and meta-analysis. BMC Infect Dis2012; 12:113; http://dx.doi.org/10.1186/1471-2334-12-113; PMID: 22583480
- European Centre for Disease Prevention and Control. Chlamydia control in Europe: literature review. Stockholm: ECDC; 2014.
- SalfaMC, LatinoMA, RegineV, De MariaD, De IntinisG, CamoniL, RaimondoM, SuligoiB. Prevalence and determinants of Chlamydia trachomatis infection among sexually active women in Turin, Italy. IJPH2011; 8:295 - 301
- MatteelliA, SulisG, CapelliM, ApostoliA, ToninelliG, Bernoni D’AversaF, GargiuloF, SalinaroF, Castel liF, DonatoF. Prevalence of genital Chlamydia trachomatis and Neisseria gonorrhoeae infections among adolescents in Northern Italy. Sex Transm Infect2013; 89:A154; http://dx.doi.org/10.1136/sextrans-2013-051184.0480
- SammarcoML, Del RiccioI, TamburroM, GrassoGM, RipabelliG. Type-specific persistence and associated risk factors of human papillomavirus infections in women living in central Italy. Eur J Obstet Gynecol Reprod Biol2013; 168:222 - 6; http://dx.doi.org/10.1016/j.ejogrb.2013.01.012; PMID: 23395560
- LatinoMA, CaneparoA, RossoC, De MariaD, De IntinisG, IntorciaP, PetrincoM. Prevalence and risk factors for Chlamydia trachomatis infection in young women in north-west of Italy. Minerva Ginecol2008; 60:29 - 37; PMID: 18277350
- ZanettiS, UsaiD, MolicottiP, DeriuA, SechiLA. Presence of Chlamydia trachomatis in young women in Northern Sardinia. New Microbiol2007; 30:63 - 4; PMID: 17319603
- MarconeV, RecineN, GallinelliC, NicosiaR, LichtnerM, DegenerAM, ChiariniF, CalzolariE, VulloV. Epidemiology of Chlamydia trachomatis endocervical infection in a previously unscreened population in Rome, Italy, 2000 to 2009. Euro Surveill2012; 17:pii: 20203; PMID: 22748006
- VerteramoR, PierangeliA, ManciniE, CalzolariE, BucciM, OsbornJ, NicosiaR, ChiariniF, AntonelliG, DegenerAM. Human Papillomaviruses and genital co-infections in gynaecological outpatients. BMC Infect Dis2009; 9:16; http://dx.doi.org/10.1186/1471-2334-9-16; PMID: 19216747
- BoyleDC, SmithJR. Infection and cervical intraepithelial neoplasia. Int J Gynecol Cancer1999; 9:177 - 86; http://dx.doi.org/10.1046/j.1525-1438.1999.99007.x; PMID: 11240764
- SilvaJ, CerqueiraF, MedeirosR. Chlamydia trachomatis infection: implications for HPV status and cervical cancer. Arch Gynecol Obstet2014; 289:715 - 23; http://dx.doi.org/10.1007/s00404-013-3122-3; PMID: 24346121
- GolijowCD, AbbaMC, MourónSA, LaguensRM, DuloutFN, SmithJS. Chlamydia trachomatis and Human papillomavirus infections in cervical disease in Argentine women. Gynecol Oncol2005; 96:181 - 6; http://dx.doi.org/10.1016/j.ygyno.2004.09.037; PMID: 15589598
- FinanRR, TamimH, AlmawiWY. Identification of Chlamydia trachomatis DNA in human papillomavirus (HPV) positive women with normal and abnormal cytology. Arch Gynecol Obstet2002; 266:168 - 71; http://dx.doi.org/10.1007/s00404-001-0261-8; PMID: 12197559
- LehmannM, GrohA, RödelJ, NindlI, StraubeE. Detection of Chlamydia trachomatis DNA in cervical samples with regard to infection by human papillomavirus. J Infect1999; 38:12 - 7; http://dx.doi.org/10.1016/S0163-4453(99)90021-X; PMID: 10090499
- SmithJS, MuñozN, HerreroR, Eluf-NetoJ, NgelangelC, FranceschiS, BoschFX, WalboomersJM, PeelingRW. Evidence for Chlamydia trachomatis as a human papillomavirus cofactor in the etiology of invasive cervical cancer in Brazil and the Philippines. J Infect Dis2002; 185:324 - 31; http://dx.doi.org/10.1086/338569; PMID: 11807714
- SmithJS, BosettiC, MuñozN, HerreroR, BoschFX, Eluf-NetoJ, MeijerCJ, Van Den BruleAJ, FranceschiS, PeelingRW, IARC multicentric case-control study. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer2004; 111:431 - 9; http://dx.doi.org/10.1002/ijc.20257; PMID: 15221973
- VerhoevenV, BaayM, WeylerJ, AvontsD, LardonF, Van RoyenP, VermorkenJB. Concomitant Chlamydia trachomatis and human papilloma virus infection cannot be attributed solely to sexual behaviour. Eur J Clin Microbiol Infect Dis2004; 23:735 - 7; http://dx.doi.org/10.1007/s10096-004-1194-5; PMID: 15322933
- PanattoD, AmiciziaD, TanziE, BianchiS, FratiER, ZottiCM, LaiPL, BechiniA, RossiS, GaspariniR. Prevalence of human papillomavirus in young Italian women with normal cytology: how should we adapt the national vaccination policy?. BMC Infect Dis2013; 13:575; http://dx.doi.org/10.1186/1471-2334-13-575; PMID: 24313984
- van den BroekIV, van BergenJE, BrouwersEE, FennemaJS, GötzHM, HoebeCJ, KoekenbierRH, KretzschmarM, OverEA, SchmidBV, et al. Effectiveness of yearly, register based screening for chlamydia in the Netherlands: controlled trial with randomised stepped wedge implementation. BMJ2012; 345:e4316; http://dx.doi.org/10.1136/bmj.e4316; PMID: 22767614
- AndersenB, OlesenF, MøllerJK, ØstergaardL. Population-based strategies for outreach screening of urogenital Chlamydia trachomatis infections: a randomized, controlled trial. J Infect Dis2002; 185:252 - 8; http://dx.doi.org/10.1086/338268; PMID: 11807700
- KlavsI, RodriguesLC, WellingsK, KeseD, HayesR. Prevalence of genital Chlamydia trachomatis infection in the general population of Slovenia: serious gaps in control. Sex Transm Infect2004; 80:121 - 3; http://dx.doi.org/10.1136/sti.2003.005900; PMID: 15054174
- GouletV, de BarbeyracB, RaherisonS, PrudhommeM, SemailleC, WarszawskiJ, CSF group. Prevalence of Chlamydia trachomatis: results from the first national population-based survey in France. Sex Transm Infect2010; 86:263 - 70; http://dx.doi.org/10.1136/sti.2009.038752; PMID: 20660590
- OliveiraFA, PflegerV, LangK, HeukelbachJ, MirallesI, FragaF, SousaAQ, Stoffler-MeilickeM, IgnatiusR, KerrLF, et al. Sexually transmitted infections, bacterial vaginosis, and candidiasis in women of reproductive age in rural Northeast Brazil: a population-based study. Mem Inst Oswaldo Cruz2007; 102:751 - 6; http://dx.doi.org/10.1590/S0074-02762007000600015; PMID: 17924006
- BeydounHA, DailJ, TamimH, UgwuB, BeydounMA. Gender and age disparities in the prevalence of Chlamydia infection among sexually active adults in the United States. J Womens Health (Larchmt)2010; 19:2183 - 90; http://dx.doi.org/10.1089/jwh.2010.1975; PMID: 20950136
- MagderLS, HarrisonHR, EhretJM, AndersonTS, JudsonFN. Factors related to genital Chlamydia trachomatis and its diagnosis by culture in a sexually transmitted disease clinic. Am J Epidemiol1988; 128:298 - 308; PMID: 3394697
- Istituto Superiore di Sanità. Le infezioni sessualmente trasmesse: I dati di due Sistemi di sorveglianza sentinella attivi in Italia. Available at: http://www.iss.it/binary/ccoa/cont/Notiziario_ISS_vol_27_n_4_Aprile_2014.pdf Accessed July 3, 2014.
- AdamsEJ, CharlettA, EdmundsWJ, HughesG. Chlamydia trachomatis in the United Kingdom: a systematic review and analysis of prevalence studies. Sex Transm Infect2004; 80:354 - 62; http://dx.doi.org/10.1136/sti.2003.005454; PMID: 15459402
- GriffithsV, CheungWH, CarlinEM, Ahmed-JushufI. Incidence of concurrent sexually transmitted infections in patients with genital warts. Int J STD AIDS2006; 17:413 - 4; http://dx.doi.org/10.1258/095646206777323328; PMID: 16734966
- PanattoD, AmiciziaD, TrucchiC, CasabonaF, LaiPL, BonanniP, BoccaliniS, BechiniA, TiscioneE, ZottiCM, et al. Sexual behaviour and risk factors for the acquisition of human papillomavirus infections in young people in Italy: suggestions for future vaccination policies. BMC Public Health2012; 12:623; http://dx.doi.org/10.1186/1471-2458-12-623; PMID: 22871132
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans: a review of human carcinogens: part B. Biological agents. 100th edition. Lyon, France: IARC; 2011.
- RoncoG, GhisettiV, SegnanN, SnijdersPJ, Gillio-TosA, MeijerCJ, MerlettiF, FranceschiS. Prevalence of human papillomavirus infection in women in Turin, Italy. Eur J Cancer2005; 41:297 - 305; http://dx.doi.org/10.1016/j.ejca.2004.07.005; PMID: 15661556
- BianchiS, FratiER, PanattoD, MartinelliM, AmiciziaD, ZottiCM, MartineseM, BonanniP, BoccaliniS, CoppolaRC, et al. Detection and genotyping of human papillomavirus in urine samples from unvaccinated male and female adolescents in Italy. PLoS One2013; 8:e79719; http://dx.doi.org/10.1371/journal.pone.0079719; PMID: 24255711
- de SanjoséS, DiazM, CastellsaguéX, CliffordG, BruniL, MuñozN, BoschFX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis2007; 7:453 - 9; http://dx.doi.org/10.1016/S1473-3099(07)70158-5; PMID: 17597569
- SamoffE, KoumansEH, MarkowitzLE, SternbergM, SawyerMK, SwanD, PappJR, BlackCM, UngerER. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol2005; 162:668 - 75; http://dx.doi.org/10.1093/aje/kwi262; PMID: 16120706
- ChenHC, SchiffmanM, LinCY, PanMH, YouSL, ChuangLC, HsiehCY, LiawKL, HsingAW, ChenCJ, CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst2011; 103:1387 - 96; http://dx.doi.org/10.1093/jnci/djr283; PMID: 21900119
- WalshC, AndersonLA, IrwinK. The silent epidemic of Chlamydia trachomatis: the urgent need for detection and treatment in women. J Womens Health Gend Based Med2000; 9:339 - 43; http://dx.doi.org/10.1089/15246090050020637; PMID: 10868604
- HafnerLM, WilsonDP, TimmsP. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine2014; 32:1563 - 71; http://dx.doi.org/10.1016/j.vaccine.2013.08.020; PMID: 23973245
- HowieSE, HornerPJ, HorneAW, EntricanG. Immunity and vaccines against sexually transmitted Chlamydia trachomatis infection. Curr Opin Infect Dis2011; 24:56 - 61; http://dx.doi.org/10.1097/QCO.0b013e3283421081; PMID: 21124214
- European Centre for Disease Prevention and Control. Introduction of HPV vaccines in EU countries – an update. Stockholm: ECDC; 2012.
- La sorveglianza Passi (The Italian behavioral risk factor surveillance system). Rapporto nazionale Passi 2011: screening cervicale. Available at: http://www.epicentro.iss.it/passi/rapporto2011/ScreeningCervicale.asp Accessed July 3, 2014.
- PuranenM, SaarikoskiS, SyrjänenK, SyrjänenS. Polymerase chain reaction amplification of human papillomavirus DNA from archival, Papanicolaou-stained cervical smears. Acta Cytol1996; 40:391 - 5; http://dx.doi.org/10.1159/000333842; PMID: 8669167
- TanziE, BianchiS, FasoloMM, FratiER, MazzaF, MartinelliM, ColzaniD, BerettaR, ZappaA, OrlandoG. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J Med Virol2013; 85:91 - 8; http://dx.doi.org/10.1002/jmv.23434; PMID: 23097252
- OrlandoG, TanziE, ChatenoudL, GramegnaM, RizzardiniG, VALHIDATE Study Group. Rationale and design of a multicenter prospective cohort study for the eVALuation and monitoring of HPV infections and relATEd cervical diseases in high-risk women (VALHIDATE study). BMC Cancer2012; 12:204; http://dx.doi.org/10.1186/1471-2407-12-204; PMID: 22646512
- BernardHU, ChanSY, ManosMM, OngCK, VillaLL, DeliusH, PeytonCL, BauerHM, WheelerCM. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis1994; 170:1077 - 85; http://dx.doi.org/10.1093/infdis/170.5.1077; PMID: 7963696
- GarbugliaAR, PiselliP, LapaD, SiasC, Del NonnoF, BaiocchiniA, CimagliaC, AgrestaA, CapobianchiMR. Frequency and multiplicity of human papillomavirus infection in HIV-1 positive women in Italy. J Clin Virol2012; 54:141 - 6; http://dx.doi.org/10.1016/j.jcv.2012.02.013; PMID: 22437054
- JalalH, StephenH, Al-SuwaineA, SonnexC, CarneC. The superiority of polymerase chain reaction over an amplified enzyme immunoassay for the detection of genital chlamydial infections. Sex Transm Infect2006; 82:37 - 40; http://dx.doi.org/10.1136/sti.2005.015362; PMID: 16461600
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013.
