Abstract
A randomized, double-blind, study was conducted to evaluate the safety, tolerability and immunogenicity of a live attenuated Japanese encephalitis chimeric virus vaccine (JE-CV) co-administered with live attenuated yellow fever vaccine (YF-17D strain; Stamaril®, Sanofi Pasteur) or administered successively. Participants (n = 108) were randomized to receive: YF followed by JE-CV 30 days later, JE followed by YF 30 days later, or the co-administration of JE and YF followed or preceded by placebo 30 days later or earlier. Placebo was used in a double-dummy fashion to ensure masking. Neutralizing antibody titers against JE-CV, YF-17D and selected wild-type JE strains was determined using a 50% serum-dilution plaque reduction neutralization test. Seroconversion was defined as the appearance of a neutralizing antibody titer above the assay cut-off post-immunization when not present pre-injection at day 0, or a least a four-fold rise in neutralizing antibody titer measured before the pre-injection day 0 and later post vaccination samples. There were no serious adverse events. Most adverse events (AEs) after JE vaccination were mild to moderate in intensity, and similar to those reported following YF vaccination. Seroconversion to JE-CV was 100% and 91% in the JE/YF and YF/JE sequential vaccination groups, respectively, compared with 96% in the co-administration group. All participants seroconverted to YF vaccine and retained neutralizing titers above the assay cut-off at month six. Neutralizing antibodies against JE vaccine were detected in 82-100% of participants at month six. These results suggest that both vaccines may be successfully co-administered simultaneously or 30 days apart.
Introduction
Japanese encephalitis virus (JEV) is a mosquitoborne member of the Flaviviridae family and is a leading cause of viral encephalitis in Asia. The first generation of prophylactic vaccines against JEV employed virus derived from the brains of infected mice and inactivated with formalin.Citation1,Citation2 These vaccines provide very effective short term immunityCitation3–Citation5 however associated rare adverse eventsCitation1,Citation6–Citation8 and uncertainty as to the duration of protection against infectionCitation9 are matters of concern. To overcome these concerns a live attenuated Japanese encephalitis chimeric virus vaccine (JE-CV) also known as Acambis's ChimeriVax®-JE has been developed by removing pre-membrane and envelope coding sequences from the yellow fever vaccine virus (strain 17D) and replacing them with the corresponding sequences from the attenuated JEV strain, SA14-14-2.Citation10,Citation11
There is a possibility YF-17D vaccine and JE-CV could interact as they share antigenic determinants and prior immunization with one might boost or suppress immune responses to the other. Furthermore, JE-CV as a chimeric vaccine shares non-structural coding sequences from the YF-17D vaccine so prior vaccination with YF vaccines could similarly boost or suppress immune responses and visa versa. Monath et al.Citation11 found no significant difference between the anti-JE-CV neutralizing antibody responses in JE-CV vaccinated participants with and without a history of YF immunization. However a subsequent small-scale study suggested that immunization with JE-CV 30 days prior to YF vaccine resulted in a reduced rate of yellow fever virus (YFV) seroconversion (64% compared with 91%) and lower titers.Citation12 Neither of these observed differences was statistically significant (p > 0.05) but warranted further clinical investigation given the possibility that a military or travelling population would use both vaccines concurrently or in close proximity.
We report a study designed to further assess the safety profile of JE-CV administered concomitantly or one month before or after YF vaccine, and to assess the immune response elicited by concomitant administration of JE and YF vaccines. We also assessed the persistence of neutralizing antibody responses six months after vaccination. Finally we explored whether JE-CV vaccine-induced antibodies are able to neutralize a panel of wild-type JEV strains, representative of the four main genotypes.
Results
Study population.
Of the 137 screened volunteers, 108 participants were enrolled, and randomized per protocol, 106 of whom completed the study to day 60 (). Mean participant age was 26 years (range 18 to 53 years) with a 57/43% male to female ratio. Mean weight was 72 kg, (range 42–117 kg) and 92% of participants were Caucasian. More than a third (34/108) of participants was excluded retrospectively from the per-protocol (PP) population because neutralizing flavivirus antibody titers to one or more flaviviruses were detected in their baseline serum sample by 50% serum-dilution plaque reduction neutralization test. These flaviviruses included Alfuy virus (n = three), dengue virus serotype one, two and four (n = 21, nine and two, respectively), JEV (n = three), Murray valley encephalitis virus (n = three) and YFV (n = four). Despite the high prevalence of flaviviruses antibodies across the study population; the six treatment groups were well matched with respect to demographic characteristics (data not shown).
Reactogenicity and safety.
There were no deaths or serious AEs during the study and no participant withdrew from the study because of an AE. Systemic AEs occurring 30 days after the first immunization on day 0 for each treatment group was similar (97–100% of subjects experienced a systemic AE). The incidence of AEs was higher after the first immunization than the second (92% vs. 79%) for all participants. Fatigue and malaise were the most commonly reported treatment related, systemic AEs, and were typically mild to moderate in intensity (). There were four severe systemic AEs reported by three participants; three of these occurred in two participants following immunization with YF vaccine and the last in a placebo recipient. One participant in the YF/JE group reported severe fatigue and headache that developed five and seven days, respectively following YF vaccination. Both events resolved within three days and were deemed probably related to treatment.
Local reactogenicity.
Within 30 days after the first injection, pain and erythema were the most commonly reported treatment related, local AEs and occurred at comparable rates in all groups, i.e., were comparable when either vaccine or placebo was administered separately, or when vaccines were co-administered (). Four participants had local adverse events of moderate severity, while all other participants described local reactions of mild severity. There were no severe local adverse events. Observations after the second injection were comparable with those after the first, but with lower reaction rates (data not shown).
Laboratory parameters.
Mean values for blood biochemistry and haematology variables were similar in each treatment group at screening and there were no apparent changes in any of the mean variables from screening to days 15 and 30, or from day 30 (prior to immunization with the second vaccine) to days 45 and 60 [data not shown]. There was no significant difference between treatment groups in the incidence or magnitude of out of range hematology, biochemistry or urinalysis results. The most commonly observed haematology shifts were increases or decreases in neutrophils and eosinophils.
Forty one participants had creatinine phosphokinase (CPK) values that met protocol defined mild toxicity grading during the follow-up (>237 units Unit(s) [U]/L for females and >255 U/L for males). Creatinine phosphokinase was monitored during the study as part of the chemistry panel but these findings prompted further investigation to determine whether they were treatment related. No concomitant abnormal elevation for the cardiac CPK isoenzyme was observed in these participants. Prior to any immunization two and nine participants reached moderate (≥400 to <1,000 U/L) and mild (255 to <400 U/L males and 255 to <400 U/L females) CPK levels, respectively, at screening. CPK monitoring continued throughout the treatment phase at day 15 (seven participants), day 30 (10 participants), day 45 (four participants) and at day 60 (eight participants) reached mild to moderate CPK levels. The single elevated CPK value that reached severe toxicity at day 60 was in a participant in the YF/JE group, 30 days after immunization with JE-CV. The parameter resolved to mild toxicity, unrelated to immunization, four days later. Retrospective questioning of the participant revealed that he had undertaken mountain climbing before testing at day 60. For the remaining participants, all other reported mild to moderate CPK values resolved spontaneously.
Immunogenicity.
All participants in the JE/YF group and 91% of participants in the YF/JE group seroconverted to JE-CV, 30 days after JE vaccination. When co-administered with YF vaccine, 96% of participants seroconverted to JE-CV within 30 days of vaccination. Two participants in the YF/JE group had detectable neutralizing antibodies (PRNT50 >1:20) to JE-CV prior to JE vaccination, and did not demonstrate a four-fold rise in titer post immunization. One participant in the co-administration group did not seroconvert to JE-CV ().
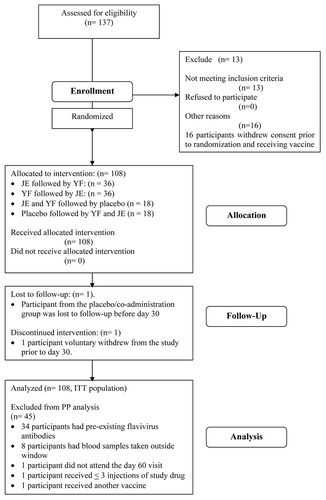
The neutralizing geometric mean titers (GMTs) for anti-JECV antibodies are shown in . The anti-JE-CV GMT for the JE/YF group was significantly higher than that for the YF/JE group (ratio of means 0.3, 95% CI 0.1, 0.9). When JE and YF vaccines were co-administered, the anti-JE-CV titer at day 30 also was significantly lower than that for the JE/YF participants (ratio of means 0.2, 95% CI 0.1, 0.7).
Fewer participants seroconverted to the wild-type strains than to the homologous JE-CV vaccine strain with the exception of genotype I strain 1991 TVP-8236. The lowest seroconversion rates were against virus strain JKT 9092/TVP-6265 particularly by participants in co-administration/placebo group ().
All participants seroconverted to YF vaccine and retained neutralizing titers (PRNT50 =1:20) at month six ( and ). One participant in the co-administration/placebo group showed evidence of neutralizing antibodies to YFV before receiving YF vaccine at day 30 and demonstrated a four-rise in titer following immunization.
The neutralizing GMTs for anti-YFV antibody are shown in . There was no significant difference between anti-YFV antibody GMTs when YF vaccine was administered 30 days before or after JE vaccine (ratio of means 1.1, 95% CI 0.5, 2.6) or when co-administered with JE vaccine (ratio of means 0.6, 95% CI 0.1, 0.7).
Discussion
We found no evidence that immunization with YF vaccine together with, prior to or after immunization with JE vaccine resulted in any increase in the rate of AEs compared to that associated with JE-CV alone. Adverse events were similar to those experienced in previous JE-CV trials.Citation11,Citation12 Fatigue and malaise were the most commonly reported treatment-related systemic AEs while injection site pain and erythema were the most commonly observed, local AEs.
The most commonly reported laboratory abnormality was elevated levels of CPK which may have been due to physical exercise-induced muscle injury.Citation13 The study participants were predominately physically active adults and there was no requirement for participants to reduce levels of exercise prior to immunization or follow-up blood sampling. These abnormal CPK values were detected at screening, were transient and self-limiting, and were not associated with any particular treatment group or local AE. It is unlikely, therefore, that these elevations in CPK were related to immunization. Other haematology, urinalysis and liver enzyme values outside the normal range were minor and unrelated to clinical syndromes. The tolerability and safety of JE-CV therefore is consistent with the available safety data for JE-CV and the safety profile of YF-17D.
Monath et al.Citation12 previously reported a reduction in the number of participants seroconverting to YF vaccine (64%) when participants were immunized with JE-CV 30 days prior to YF vaccine. In contrast, 91% of JE-CV naïve participants in this study seroconverted to YF vaccine. The magnitude of the anti-YFV neutralizing response (log neutralization index) was also lower in participants who had received JE-CV 30 days prior (1.59, standard deviation [SD] = 1.47) compared to JE-CV naïve subjects (2.29, SD = 1.03). In our study, all participants seroconverted to YFV and there were no statistically significant differences in anti-YFV GMTs between any of the treatment groups. The differing results for our study and those of Monath et al.Citation11,Citation12 are difficult to assess due to the small sample sizes. YF-VAX® has approximately 5 log10 PFU of YF-17D virus and is stabilized with sorbitol and hydrolyzed porcine gelatin, while Stamaril® contains approximately 3–4 log10 PFU of YF-17D virus and has no animal derived stabilizers. The formulations of YF vaccines used and the handling after reconstitution as well as YF vaccination histories of participants enrolled in each study may account for these differences.
Anti-JE-CV antibody titers were higher in participants who were immunized first with JE vaccine than in those who received YF vaccine first or when the vaccines were co-administered. Monath et al.Citation11,Citation12 previously showed that immunization with YF vaccine at least nine months before inoculation with JE-CV did not interfere with viremia or antibody responses. JE-CV shares the non-structural genes of YF-17D virus so it is possible that recently acquired cytotoxic immunity to the shared non-structural proteins of the YFV (anti-viral vector immunity) inhibited the replication of the JE-CV virus and the development of anti-JEV antibody titers following JE vaccine administration 30 days after YF vaccine. Although replication kinetics were not specifically assessed in this study, a reduced antigenic mass of replicating JE-CV compared to YF-17D may have reduced the anti-JE-CV GMTs in the co-administration group. JE-CV has been reported to cause lower levels of viremia in primates and humansCitation11,Citation14 than YF vaccine, hence JE-CV may have been “out-competed” by the faster replication of YF vaccine when both vaccines were administered together.
Antibody responses against the JE vaccine strain were higher than the wild type strains (with the exception of JEV strain 1991 TVP-8236). Reduced in vitro cross-neutralizing responses to heterologous JEV genotypes have been observed with inactivated mouse-brain derived JE vaccines and was attributed to local strain variation and to original antigenic sin.Citation15 Despite these laboratory observations, vaccines derived from a limited number of JEV strains (Beijing, Nakayama and SA14) appear to protect against clinical infections with other strains of JEV.Citation6,Citation8,Citation16 JE-CV is derived from the SA14-14-2 vaccineCitation10 which has been shown to be protective in pre-licensing studies in the Peoples Republic of ChinaCitation17 and immunization of children with a single dose of SA14-14-2 is associated with long-term protection.Citation18–Citation20 Recently, Beasley et al.Citation21 also demonstrated passive cross-protection by anti-JE-CV antibodies against a panel of wild-type strains from the four JEV genotypes in an in vivo mouse model.
Participants and Methods
The study was conducted at a single site (Q-Pharm) in Queensland, Australia from July 2004 to April 2005. After screening, participants were randomized by the unblinded study pharmacist to six treatment groups according to a randomization schedule with a block size of six prepared by an independent statistician. To assess specific local reactions, JE and YF vaccines were administered in separate arms by the blinded Investigators. No participant was made aware of their treatment but blinding efficacy was not assessed. Participants returned to the clinic on days 15, 30, 45, 60 and six months after their first injection (day 0). Safety assessments were made by the Investigators and blood samples taken for antibody analysis at all visits, including day 0.
Vaccines.
JE-CV is a pilot liquid formulation prepared by culturing the JE-CV virus in Vero cells (vaccine lot number 00C02; 1.3 × 106 PFU/mL; Acambis Inc., now part of Sanofi Pasteur).Citation10,Citation11 Yellow fever vaccine (Stamaril®, batch X-5426-5 Sanofi Pasteur, Marcy L'Etoile, France) contained not less than 1,000 mouse lethal dose endpoint 50% units as a lyophilized powder. Both vaccines were diluted or re-constituted using 0.9% saline for injection and administered in a volume of 0.5 mL within 60 minutes of reconstitution. Placebo was sterile saline for injection. Participants received either 3.8 log10 PFU of JE vaccine, 1,000 mouse LD50 units of YF vaccine, or placebo as a subcutaneous injection into the upper deltoid, i.e., each participant received a total of four injections (two of placebo and one each of JE and YF vaccine), two at each visit (one in each arm). The blinded, double-dummy syringes were prepared by an unblinded study pharmacist and provided to the Investigators for injection with the syringe barrels blinded with colored tape. The study pharmacist did not reveal the blind to the Investigators and had no other role in the study.
Participants were healthy, flavivirus-naïve, volunteers, aged 18–55 years, in good general health without a clinically significant medical history, physical examination or laboratory finding at screening. Participants were required to provide written informed consent. Female participants were neither pregnant nor breast-feeding and were required to use effective contraception from at least one month prior to the study to one month after the study (day 60). Participants were excluded from the study if they had a history of flavivirus infection or immunization (based on participant responses and a review of medical records), impaired immunity, a history of serious adverse reactions to prior vaccines or chicken eggs, transfusion of blood or blood products within six months of the screening visit or during treatment, or administration of another vaccine within 28 days of study immunization. They also were excluded for fever (body temperature >38.1°C) or acute illness within three days of planned immunization. Other exclusion criteria included intention to travel out of the area during the treatment period, antibodies to hepatitis C virus and human immunodeficiency virus or hepatitis B virus surface antigen in the screening blood sample, lactation or intended pregnancy, excessive alcohol consumption, drug abuse or significant psychiatric illness or a history of damage to the blood brain barrier.
The protocol and informed consent forms were reviewed by the Australian Defence Human Research Ethics Committee (# 345/03) and the Queensland Institute of Medical Research Human Research Ethics Committee (# P753). The study was conducted in accord with Good Clinical Practice and local regulatory approvals included a clinical trial exemption (# 99/2/4014), dealing not involving release licence (071/2002) and review by the Gene and Related Therapies Advisory Panel (#04-01).
Safety evaluation.
The primary safety outcome evaluated AE incidence rates at day 30 following the first vaccine administered. Safety assessments were based on physical examination and routine laboratory testing (hematology and biochemistry) with urinalysis performed at the site by dipstick with abnormal urinalysis results confirmed at the laboratory. Adverse events were observed, reported or elicited by interview and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 7.1 (Maintenance and Support Services Organization, Chantilly, VA, USA). The incidence of all AEs and treatment emergent AEs considered related to the study drug (i.e., Investigator rated possibly, probably or definitely related) were recorded for each treatment group based on subject interview with diary cards used as an aide memoire. Severity of reactions was deemed mild (aware of sign/symptom but easily tolerated), moderate (discomfort to cause interference with usual activity) or severe (incapacitating with inability to work or perform usual activity). Local injection site reactions and rash was deemed mild (<2 cm or rash restricted to <18% body surface), moderate (>2 cm or rash equivalent to an area >18%) and severe (>6 cm or rash equivalent to an area >half the body).
Serological tests.
Serum was stored at −70 ± 5°C and all samples were tested in parallel. For the determination of PRNT, test samples were heat inactivated and three ten-fold serial dilutions were prepared in duplicate. They were mixed with a fixed amount of virus, incubated for one hour at 37°C and plated on LLC-MK2 cells in six well plates. Titers are determined as described by Russel et al.Citation22 Sera were tested by a single technician. All samples from a single time point were tested on the same day. A positive control was used throughout the tests to ensure consistency of the results. Participants who were seronegative at baseline (<1:10 titer) required a PRNT50 titer of ≥1:20 to meet the criteria for seroconversion. Any PRNT50 value reported as “<LOQ” was converted to “LOQ/2”, where LOQ was the lower limit of quantification, defined as 10 for this study. Any PRNT50 value reported as “>ULQ” was converted to “2ULQ”, where “ULQ” was the upper limit of quantification.
Immunogenicity.
The primary immunogenicity outcome was the proportion of flavivirus naïve participants that seroconverted to JE or YF vaccines after immunization. Secondary immunogenicity outcomes were GMT against JE-CV, YF-17D and wild-type JEV strains (genotype I, 1991 TVP-8236; genotype II, B1034/8; genotype III, Beijing; genotype IV, JKT 9092/TVP-6265), which were calculated by taking log10 transformation of the observed titer for each participant and calculation of the arithmetic mean.
Statistical methods.
Statistical methods examined a two sided non-directional test of the hypothesis with significance set at 5%. The study was not powered to show statistically significant treatment differences and the immunogenicity comparisons in this study are considered exploratory. A sample of 36 participants per group at 95% CI was selected to detect very common AEs (frequency ≥10%) in the safety population (using the rules of threes for calculation of sample size) with the assumption that up to 10% of participants would withdraw. The population employed to assess safety consisted of all participants who received at least one injection of vaccine or placebo (108 participants). The intent to treat (ITT) population consisted of the safety population who had provided day 0 (baseline) and post-immunization (day 30) blood samples for antibody analysis (108 participants). The PP population included the ITT population who were flavivirus naïve at day 0 (PRNT50 <1:10) and who were compliant with the protocol throughout the trial (63 participants).
Descriptive summaries were made for the proportion of participants seroconverting to each vaccine. Confidence intervals for GMTs were calculated as exponent {mean ± tdf; 0.025 se} where se is the standard error of the mean of log10 titer and tdf; 0.025 is the upper 2.5% of the t-distribution with degrees of freedom (df), n-1. Analysis of variance (treatment only) for the log10 titers of the three groups was performed with differences in means and the 95% CI calculated. Back transformation of these values gave an estimate of the ratio of mean GMTs and the associated 95% CI. The ratio of GMT was considered statistically significant if the CI did not contain the value one.
Conclusion
The results suggest that JE-CV and YF-17D vaccines are safe and immunogenic when administered sequentially, in either order, one month apart or when administered concurrently.
Conflict of Interest
Mark Reid has acted as a paid consultant to Acambis Inc., in relation to JE vaccine trials. Karen McCarthy and Niranjan Kanesa-thasan are former employees of Acambis Inc., Emmanuel Feroldi is a current employee of Sanofi Pasteur. These statements are made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of the Australian Defence Health Services or any extant policy.
Financial Disclosure
The study was supported by Acambis Inc., (Cambridge, UK).
Abbreviations
| AE | = | adverse event |
| ANOVA | = | analysis of variance |
| CI | = | confidence interval |
| CPK | = | creatinine phosphokinase |
| JE-CV | = | japanese encephalitis chimeric virus vaccine |
| df | = | degrees of freedom |
| GMT | = | geometric mean titer |
| ITT | = | intent to treat |
| JEV | = | japanese encephalitis virus |
| LOQ | = | limit of quantification |
| MedDRA | = | medical dictionary for regulatory activities |
| PFU | = | plaque forming units |
| PP | = | per-protocol |
| PRNT50 | = | plaque reduction neutralization test with a 50% endpoint |
| SD | = | standard deviation |
| se | = | standard error of the mean |
| U | = | unit(s) |
| ULQ | = | upper limit of quantification |
| YF | = | yellow fever |
| YFV | = | yellow fever virus |
| YF-17D | = | yellow fever 17D vaccine strain |
Figures and Tables
Figure 2 Geometric mean anti-JE-CV neutralizing antibody titer in the PP vaccinees following immunization with either JE, YF or both JE and YF co-administered together at day 0. Ratio of means for Group 3 (Co-ad total)/Group 1 (JE followed by YF) at day 30 = 0.2 (95% CI 0.1, 0.7). Ratio of means for Group 2 (YF followed by JE)/Group 1 (JE followed by YF) at day 30 = 0.3 (95% CI 0.1, 0.9). A significant difference between treatment groups (p < 0.05) occurs when the geometric means does not contain the value 1.
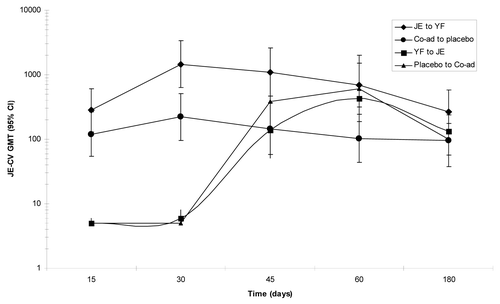
Figure 3 Percent of participants in the PP population seroconverting (PRNT50 ≥ 1:20 or 4-fold rise from baseline) to JE-CV and wild-type JEV strains, 30 days after last immunization with either JE, YF or both JE and YF co-administered together at day 0.
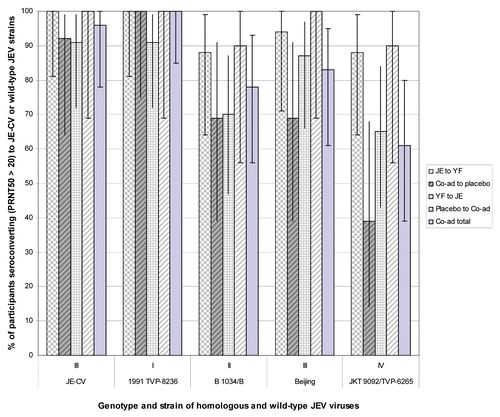
Figure 4 Geometric mean anti-YF-17D neutralizing antibody titer in the PP vaccinees following immunization with either JE, YF or both JE and YF coadministered together at day 0. Ratio of means for Group 3 (Co-ad total)/Group 1 (JE followed by YF) at day 30 = 0.6 (95% CI 0.3, 1.2). Ratio of means for Group 2 (YF followed by JE)/Group 1 (JE followed by YF) at day 30 = 1.1 (95% CI 0.5, 2.6). A significant difference between treatment groups (p < 0.05) occurs when the geometric means does not contain the value 1.
Table 1 Treatment-related adverse events reported by two or more participants during the thirty days following first administration, on Day 0 of JE-CV or YF-17D alone, or the co administration of JE-CV and YF-17D, or placebo
Table 2 Percent of participants in the PP population seroconverting (PRNT50 ≥1:20 or 4-fold rise from baseline) to JE-CV or YF-17D at day 30 or day 60 after first immunization
Acknowledgements
The authors gratefully acknowledge the assistance of Dr. Thomas Monath, Dr. Scott Kitchener and the former staff of Acambis Inc, as study Sponsors. Corporal Andrew Baron, Corporal Natalie Lehmann and staff of the Australian Army Malaria Institute for flavivirus serology. Associate Professor Sutee Yoksan and staff at the Center for Vaccine Development, Institute for Molecular Biosciences, Mahidol University at Salaya, Thailand for Japanese encephalitis virus serology. Dr. Simon Coggins and staff at Pharmaceutical Product Development Inc., for full service, contract research support as well as staff at Sanofi Pasteur for critically reviewing the manuscript.
References
- Tsai TF, Yu XY. Plotkin SA, Mortimer EA. Japanese encephalitis vaccines. Vaccines 1994; Second edition Philadelphia, PA WB Saunders 671 - 714
- Oya A. Japanese encephalitis vaccine. Act Pediatri Jpn 1998; 30:175 - 184
- Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laoraka-pongse T, Innis BL, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med 1998; 319:608 - 614
- DeFraites RF, Gambel JM, Hoke CH Jr, Sanchez SL, Withers BG, Karabatsos N, et al. Japanese encephalitis vaccine (inactivated, biken) in US soldiers: immunogenicity and safety of vaccine in two dosing regimens. Am J Trop Med Hyg 1999; 61:288 - 293
- Gambel JM, DeFraites R, Hoke C, Brown A, Sanchez J, Karabatsos N, et al. Japanese encephalitis vaccine: persistence of antibody up to 3 years after a three-dose primary series. J Infect Dis 1995; 171:1074
- Tsai TF, Chang GJ, Yu YX. Plotkin SA, Orenstein WA. Japanese encephalitis vaccines. Vaccines 1999; third edition Philadelphia, PA WB Saunders 672 - 710
- Ruff TA, Eisen D, Fuller A, Kass R. Adverse reactions to Japanese encephalitis vaccine. Lancet 1991; 338:881 - 882
- Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting Bangkok, Thailand 13–15 October 1998. Vaccine 2000; 18:1 - 25
- Nimmannitya S, Hutamai S, Kalayanarooj S, Rojanasuphot S. A field study on Nakayama and Beijing strains of Japanese encephalitis vaccines. Southeast Asian J Trop Med Public Health 1995; 26:689 - 693
- Chambers TJ, Nestorowicz A, Mason PW, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 1999; 73:3095 - 3101
- Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, et al. Clinical proof of principle for chimerivax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002; 20:1004 - 1018
- Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (chimerivax-je): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis 2003; 188:1213 - 1230
- Johnson C, Monath TP, Kanesa-thasan N, Mathis D, Miller C, Shapiro S, et al. Exercise-induced serum enzyme elevations confounding the evaluation of investigational drug toxicity. Report of two cases in a vaccine trial. Hum Vaccin 2005; 1:24 - 29
- Monath TP, Soike K, Levenbook I, Zhang ZX, Arroyo J, Delagrave S, et al. Recombinant, chimaeric live, attenuated vaccine (chimeriVax™) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever 17D-204 virus is safe, immunogenic and protective in non-human primates. Vaccine 1999; 17:1869 - 1882
- Ku CC, King CC, Lin CY, Hsu HC, Chen LY, Yueh YY, et al. Homologous and heterologous neutralization of antibody responses after immunization with Japanese encephalitis vaccine among Taiwan children. J Med Virol 1994; 44:122 - 131
- Halstead SB, Jacobson J. Plotkin S, Orenstein W, Offit P. Japanese encephalitis vaccines. Vaccines 2004; 4th edition Philadelphia, PA Saunders Elsevier 311 - 352
- Hennessy S, Zhengie L, Tsai TF, Strom BL, Chao-Min W, Hui-Lian L, et al. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case control study. Lancet 1996; 347:1583 - 1586
- Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, et al. Efficacy of a single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet 2001; 358:791 - 795
- Ohrr H, Tandan JB, Sohn YM, Shin SH, Pradhan DP, Halstead SB. Effect of single dose of SA 14-14-2 vaccine 1 year after immunisation in Nepalese children with Japanese encephalitis: a case-control study. Lancet 2005; 366:1375 - 1378
- Tandan JB, Ohrr H, Young MS, Yoksan S, Ji M, Nam CM, et al. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: A case-control study in Nepalese children 5 years after immunization. Vaccine 2007; 25:5041 - 5045
- Beasley DWC, Li L, Suderman MT, Guirakhoo F, Trent DW, Monath TP, et al. Protection against Japanese encephalitis virus strains representing four genotypes by passive transfer of sera against ChimeriVax™-JE experimental vaccine. Vaccine 2004; 22:3722 - 3726
- Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 1967; 99:285 - 290