Abstract
In a randomized, double-blind study, 202 healthy adults were randomized to receive a live, attenuated Japanese encephalitis chimeric virus vaccine (JE-CV) and placebo 28 days apart in a cross-over design. A subgroup of 98 volunteers received a JE-CV booster at month 6. Safety, immunogenicity, and persistence of antibodies to month 60 were evaluated.
There were no unexpected adverse events (AEs) and the incidence of AEs between JE-CV and placebo were similar. There were three serious adverse events (SAE) and no deaths. A moderately severe case of acute viral illness commencing 39 days after placebo administration was the only SAE considered possibly related to immunization. 99% of vaccine recipients achieved a seroprotective antibody titer ≥ 10 to JE-CV 28 days following the single dose of JE-CV, and 97% were seroprotected at month 6. Kaplan Meier analysis showed that after a single dose of JE-CV, 87% of the participants who were seroprotected at month 6 were still protected at month 60. This rate was 96% among those who received a booster immunization at month 6. 95% of subjects developed a neutralizing titer ≥ 10 against at least three of the four strains of a panel of wild-type Japanese encephalitis virus (JEV) strains on day 28 after immunization. At month 60, that proportion was 65% for participants who received a single dose of JE-CV and 75% for the booster group. These results suggest that JE-CV is safe, well tolerated and that a single dose provides long-lasting immunity to wild-type strains.
Introduction
Japanese encephalitis virus is a mosquito-borne flavivirus that causes acute neurological illness. An estimated 35,000–50,000 cases occur annually in Asia, primarily in children. The case fatality rate is high (20–30%) and the incidence of neurological or psychiatric sequelae in survivors can approach 50%.Citation1 Given the absence of specific therapy for JE, immunization is the only disease specific public health intervention available. At least three vaccines are currently licensed. These include a formalin-inactivated vaccine based on Nakayama strain of JEV grown in the brains of suckling mice,Citation2 and the SA14-14-2 strain used as a live attenuated vaccine.Citation3 The SA14-14-2 strain grown in the Vero cell line is also used as an inactivated vaccine.Citation4 The inactivated, mouse brain-derived Biken JE vaccine utilizing the Nakayama strain of JEV (JE-VAX®) and the Vero cell-derived, inactivated SA14-14-2 vaccine are both licensed outside Asia and are used to vaccinate travelers, military personnel and laboratory staff in non-JEV endemic countries. Routine immunization with Biken JE vaccine is no longer undertaken in Japan and production was discontinued in December 2005.Citation5 Japanese encephalitis chimeric virus vaccine (JE-CV) is a live, attenuated vaccine grown in Vero cells. The vaccine virus was constructed by removing pre-membrane and envelope coding sequences from the yellow fever (YF) vaccine virus (strain 17D) and replacing them with the corresponding sequences from the attenuated JEV strain, SA14-14-2.Citation6 The objectives of this study were to assess: (1) the safety profile of the vaccine; (2) participant seroconversion to JE-CV following primary immunization; (3) the effect of a booster immunization given at 6 months; (4) the persistence of the neutralizing responses to JE-CV up to 60 months (5 years) after a single dose of JE-CV followed or not by a booster 6 months later; and (5) the neutralization of a panel of four wild-type JEV isolates from different genotypes.
Results
Study population.
202 participants were enrolled and randomized into the two cross-over Groups A and B, 198 of whom (99 per group) attended the end of treatment period visit at day 56. At the month 6 follow-up visit, 55 and 43 participants from Groups A and B respectively were revaccinated with JE-CV (Group D, n = 98) and followed to year 5 while 103 participants from Groups A and B were included in Group C to be followed to year 5, without receiving any vaccination at month 6 ().
Most participants were male (86%) and Caucasian (95%) with a mean age of 27 years (range 18–55 years) and a mean weight of 80 kg (range 53–121 kg). There were no significant differences (p > 0.144, analysis of variance [ANOVA]) between the baseline demographic characteristics (gender, ethnicity, age, weight, height, body mass index and baseline flavivirus status) of the treatment groups. The study was conducted in a military population and a disproportionally higher number of males were enrolled (reflective of the overall military demography). The effect of sex on immunological and safety endpoints was not assessed as part of this study. Ten percent (6 and 15 participants from Groups A and B respectively) of the study population showed evidence (neutralizing titer of PRNT50 ≥10) of prior exposure to JE, YF or other flaviviruses, before JE-CV administration. Evidence of prior exposure to JE-serocomplex viruses or dengue virus specifically was seen in 3 and 7 participants in Groups A and B, respectively. Measurement of antibody responses to homologous JE-CV in the Intent to Treat (ITT) population with prior flavivirus exposure is presented separately in the results.
Reactogenicity and safety.
The incidence of treatment related adverse events (TRAEs) deemed possibly, probably or definitely related to immunization with either JE-CV or placebo occurring in more than one participant was 23% in both groups (). Adverse reactions were either mild (n = 79) or moderate (n = 13) during the cross-over treatment period; there were no severe TRAEs. The most commonly reported reactions reported within 28 days after immunization with JE-CV were headache, injection site pain and injection site reactions, which lasted 1–3 days in almost all cases. Adverse events by study period (day 0 to day 27 or day 28 to day 56) revealed that regardless of study treatment administered, the highest incidence of AEs was reported between day 0 and day 27. Importantly, between day 28 and day 56, when Group A subjects received placebo and Group B subjects received JE-CV there was no difference in AE incidence. Similarly, following the second JE-CV injection, the incidence and severity of reactions were lower than after the first injection ().
There were three SAEs during the study and no deaths. Two SAEs were unrelated to vaccination (gastroenteritis and post-hemorroidectomy per-rectum bleeding). A third SAE—acute viral illness resulting in hospital admission 8 days after JE-CV vaccination—was deemed possibly related to vaccination, due to the temporal association. Symptoms included malaise, diarrhea, abdominal pain, headache and dizziness.
No JE-CV viremia was detected in post-immunization samples at days 14 and 42 and there were no clinically relevant changes in mean hematology values compared to screening. Abnormal liver function test results were presented by four subjects 14 days following injection of either placebo (n = 2) or JE-CV (n = 2). All four cases were considered possibly related to vaccination, resolved spontaneously and none were associated with a clinical syndrome. The mean values of the biochemistry parameters were within normal range in the subjects who received the booster. Seven participants reported elevated body temperature of > 38.1°C for 1–7 days, two after placebo, two after the first dose of JE-CV and three after the booster. In addition, one participant had an elevated temperature of 38.7°C on admission to hospital 39 days after first immunization (this symptom was part of the SAE acute viral illness described above).
Immune response to JE-CV:
28 days after immunization with JE-CV, 99% of participants seroconverted to JE-CV with a PRNT50 ≥10 (). The geometric mean titer (GMT) of the neutralizing antibodies from these participants was 317 (95% confidence interval [CI] 260–385). At month 6, 97% of participants had seroprotective antibodies to JE-CV and 100% of participants who received a second dose of JE-CV vaccine at month 6 were seroprotected at month seven. The booster elicited a ≥4-fold titer increase in 27% of recipients, including the three subjects who had a neutralizing titer below the protective threshold at month 6. The remaining 73% showed either a moderate increase or a decrease in JE-CV titer after the booster. At month 24, seroprotective antibodies were observed in 90% of participants who received a single dose of JE-CV (Group C) and in 99% of participants who received two doses of JE-CV (Group D) ().
Long-term immune response to JE-CV (>24 months): Kaplan Meier survival analyses performed on the ITT population from month 6 provided an estimate of seroprotection at month 60. Of participants who received a booster dose, the estimate of seroprotection rate at month 60 was 96% (95% CI 89–100) compared with 87% (95% CI 78–96) of those who received a single-dose of JE-CV. The difference between the distribution of the two survival curves became statistically significant only when the month 60 time point was included in the analysis (p = 0.046, log rank test) ( and ). Geometric mean anti-JE-CV neutralizing titers reached a GMT of 317 28 days after immunization (95% CI 260–385), decreased to 151 (95% CI 125–181) at six months and then peaked 353 (95% CI 289–432) at month seven, after the booster. GMTs were significantly lower (p < 0.05, ANOVA) between month 12 and 48 months in the group that received a single dose of vaccine compared to the group that received a second dose of JE-CV at month 6 (). At month 36, anti-JE-CV titers were observed to rise in both groups receiving single or two doses of JE-CV. Seven participants from Group C who received a single JE-CV dose and eight subjects from Group D who received a booster JE-CV dose demonstrated seroconversion (≥4-fold rise) in anti-JE-CV titers between months 24 to month 60. Two participants of Group C (no booster) who were seronegative at month 24 demonstrated seroconversion (≥4-fold rise) at month 36. Eight of these subjects received non-flavivirus vaccines in preparation for military deployments in the 12 months preceding the 4-fold rise in titer. Two subjects received treatment for viral illnesses (influenza and unconfirmed viral meningitis) and three participants reported travel to tropical areas but denied febrile illness during or after travel.
Neutralization of wild-type JEV strains: 99.5% (196/197, 95% CI 97–100) of participants produced antibodies by day 28 which neutralized (PRNT50 ≥10) at least one strain from a panel of four wild-type JEV strains. 95% (187/197, 95% CI 91–98) of the participants neutralized at least three of the four strains and 85% (168/197, 95% CI 80–90) neutralized them all. The responses to each of the wild-type strains tested were lower than that against the vaccine virus with neutralization rates varying from 77 to 97% at day 28 and GMTs from 54% (95% CI 44–66) to 216 (95% CI 177–263) in previously flavivirus-negative subjects. Neutralizing antibodies persisted to month 60 with 65% (30/46, 95% CI 50–79) of the individuals who received a single dose of JE-CV still able to neutralize at least 3 of the 4 strains. In the two-dose group, the corresponding rate was 75% (27/36, 95% CI 58–88). In the single dose group, the proportion of subjects with neutralizing antibody (PRNT50 ≥10) varied between 63% (29/46, 95% CI 48–77) with a GMT of 20 (95% CI 14–30) for the Beijing strain to 78% (36/46, 95% CI 64–89) for the TVP-8236 strain (GMT = 44, 95% CI 28–70). By comparison, 43/46 (93%) of subjects who received a single immunization and who attended the month 60 visit had neutralizing antibodies to JE-CV (PRNT50 ≥10) with a GMT of 62 (95% CI 43–88). The ratio was 35/36 (97%) with a GMT of 79 (95% CI 54–115) for subjects who received a booster. The decline in antibody titers to wild-type JEV strains after month 12 was consistent with the rate of decline for neutralizing antibodies to the vaccine virus (data not shown).
Flavivirus seroimmune status at baseline had no significant effect on the neutralizing antibody response to JE-CV at day 28 (GMT of 316 [95% CI 151–663] in the baseline flavivirus seropositive subjects and of 317 [95% CI 260–385] in the baseline flavivirus naïve populations) and at month 6 (GMT of 243 (95% CI 136–432) and 142 (95% CI 117–173) respectively in baseline flavivirus seropositive and naïve populations; p > 0.05, ANOVA model). However, one month after a booster dose of JE-CV at month 6 antibody responses were significantly higher in the baseline flavivirus seropositive participants than in the baseline naïve participants, with GMTs against JE-CV of 721 (95% CI 338–1538) and 318 (95% CI 260–388), respectively (p = 0.0064, ANOVA).
Discussion
The primary objective of the first phase of the study was to document the safety of a single dose of JE-CV vaccine compared with a placebo. The reported incidence of reactions after JE-CV vaccination was found to be comparable with the incidence after placebo injection and, like in two previous JE-CV studies, no safety concerns were identified.Citation7,Citation8 There were no unexpected AEs or allergic reactions to the vaccine observed during this study. Reactions commonly reported in other vaccine trials were observed in this study with no difference between the vaccine and placebo groups during the treatment period.Citation7,Citation8 It was noted that the AE incidence declined as the study progressed, even though diary cards were used following each vaccination and this time course effect may have reduced the number of treatment related AEs reported at month 7 after the second dose of JE-CV. The participants administered the second dose of JE-CV (Group D) were selected as a convenience sample from participants randomized for initial treatment (Group A and B) based on their willingness and availability to receive a second dose of JE-CV. Better tolerance of JE-CV in Group D participants may have also reduced the reported AEs in the booster group compared to placebo during the treatment period. The safety of JE-CV was confirmed in a large-scale Phase III safety study which demonstrated a similar incidence of drug-related adverse events between JE-CV and placebo (submitted).
A neutralizing anti-JEV titer ≥10 is considered seroprotective,Citation9,Citation10 and this threshold has recently been accepted by regulatory authorities as a surrogate marker of protection for the licensure of a new inactivated JE vaccine.Citation11 This study has demonstrated that almost all participants develop seroprotective antibodies to JE-CV by day 28. This seroprotective titer is consistent with previous studies where 3.8 and 4.0 log10 plaque forming unit (PFU) doses of JE-CV in non-immune participants resulted in 91–100% seroconversion with a neutralizing GMT of 128–270 by day 31.Citation7,Citation8 Japanese encephalitis chimeric virus vaccine has also been shown to be effective in non-clinical challenge studies in monkeys and mice.Citation6,Citation12 Our study demonstrates that most persons living in non-JEV endemic area retain seroprotective titers up to at least 60 months after a single dose of JE-CV. In a similar study, 63% (44/69) of children immunized with a single dose of a live attenuated SA14-14-2 vaccine during a study of the effectiveness of SA14-14-2 vaccine in Nepal had neutralizing antibody titers to SA14-14-2 at 60 months with a GMT of 51.6 (range 11–962). However, 20.6% of these children had pre-existing anti-JEV antibodies and a sustained boosting effect from subclinical JEV infections cannot be ruled out.Citation13 A follow-up study of this Nepalese population demonstrated 96.2% (95% CI 73.1–99.9%) protection from this single-dose regimen in these children over a 60 month period.Citation14 In our study, the immunological benefit of the booster dose of JE-CV, given at 6 months after the first vaccination, was real but moderate: a 4 fold titer increase after boosting was seen in approximately one quarter of the population and the GMT increased by a factor of approximately 2, back to levels seen 28 days after the first immunization. Furthermore compared with a single dose, the booster provided only a marginally higher residual seroprotection rate at month 60. The effect of a second immunization was even lower in a previous study where a second dose of JE-CV was administered 30 days after the primary dose. It resulted in three of the 55 participants seroconverting to JE-CV (log neutralization index ≥0.7) of which one participant's seroconversion was transient.Citation8 It is explained by the neutralization or blunting of viral replication of the injected virus by the immune response elicited by the first immunization. Despite the observed differences, the long term results of the current study support the use of a single dose of JE-CV for primary immunization in an adult traveler population.
Our study has demonstrated the capacity for JE-CV to induce neutralizing antibodies against a panel of wild-type strains of JEV. All licensed JE vaccines are derived from a limited number of JEV strains and it is generally accepted that they afford protection against circulating JEV strains from the four major genotypes.Citation15–Citation17
A slight rise in anti-JE-CV titers was observed at month 36. This may be due to subclinical flavivirus infections in individuals between month seven and month 60.
Prior exposure to flaviviruses had no discernable effect on the reactogenicity or safety of JE-CV, and no significant effect on the antibody titers one and 6 months after a single vaccination with JE-CV. Interestingly, following a booster dose of JE-CV at month 6, participants with pre-existing flavivirus antibodies had statistically significantly higher anti-JE-CV GMTs at month 7 compared to participants who were flavivirus naïve at baseline. Previous studies demonstrated that JE-CV boosts the anti-YFV antibody titer in approximately half of the participants immunized previously with YF-VAX® and the titer of neutralizing antibodies to JE-CV also were higher in those immunized previously with YF-VAX®.Citation7,Citation8
Participants and Methods
The study was a randomized, double-blind, cross-over trial conducted at a single study center in the Australian Defence Force from 2003 to 2008. Participants were randomized in a 1:1 ratio by the study pharmacist according to the randomization schedule prepared by the independent statistician. Participants received either JE-CV on day 0 followed by placebo (vaccine diluent) on day 28 [Group A] or placebo on day 0 followed by JE-CV on day 28 [Group B] (). Safety assessments were made and samples collected for safety and immunological evaluations before treatment on day 0, and on days 14, 42 and 56. Viremia was assessed at 14 days after each immunization. All injections were given as 0.5 mL subcutaneously. At month 6, all participants returned to the clinic and 98 participants received a second JE-CV injection (convenience sample based on their willingness and availability and no formal randomization), with follow-up one month later. The study was extended by protocol amendment to allow a long-term follow-up phase out to 60 months. Participants enrolled in the long-term follow-up phase (Group C or Group D for subjects followed after one or two doses of JE-CV, respectively) provided additional blood samples at 12, 24, 36, 48 and 60 months for anti-JEV neutralizing antibody titration. At each yearly visit commencing at month 12, participants were questioned about their travel, immunizations, any febrile illnesses, hospitalization and confirmed flavivirus infections in the 12 months prior to the visit using a scripted questionnaire. Particular attention was given to flavivirus immunizations. This information, which is stored in a military clinical database and the service person's medical file, was checked at the study site. Any participants who received a flavivirus vaccine during the long-term follow-up period were withdrawn.
Study vaccines.
JE-CV is a live attenuated, Japanese encephalitis chimeric virus vaccine grown in Vero cells (previously referred to as ChimeriVax™-JE). The vaccine used in this study was produced at pilot scale as a liquid formulation (Acambis Inc., UK now Sanofi Pasteur, Lyon, France).Citation8 Vaccine was extemporaneously diluted in a sterile solution of vaccine diluent to give a final dose of 3.8 log10 PFU. The vaccine diluent alone was used as the placebo.
The protocol and informed consent forms were approved by the Australian Defence Human Research Ethics Committee (# 292/02). The study was conducted in accord with Good Clinical Practice guidelines and local regulatory approvals included a clinical trial exemption (#99/2/4014), dealing not involving release licence (071/2002) and review by the Gene and related Therapies Research Advisory Panel (#2002-02). The study is registered under number NCT00981175 (clinicaltrials.gov) and written informed consent was obtained from each participant at the screening visit and prior to the extension phase.
Study subjects.
Service personnel aged 18 to 55 years of age who were in good general health with no significant medical history, physical examination findings or clinically significant laboratory results were eligible for the study if they were available for the study duration. Service women of child bearing potential had to have a negative serum beta-human chorionic gonadotropin result on entry to the study and were required to use hormonal or barrier methods of birth control at least one month prior and one month following each immunization. Exclusion criteria included a history of JE-immunization, known or suspected immunodeficiency, prior adverse reactions to a vaccine characterized by urticaria or angioedema, transfusion of blood or treatment with any blood product within six months of the screening visit, administration of another vaccine 30 days preceding screening or planned during the treatment period. Service personnel were ineligible if they had a clinically significant medical condition, abnormal physical examination or abnormal laboratory parameter or; body temperature >38.1°C or acute illness within three days of inoculation (rescheduling permitted). Service personnel also were excluded if they had antibodies to hepatitis C virus, human immunodeficiency virus or hepatitis B virus surface antigen in the screening sample; if service women were lactating or intended to become pregnant during the course of the trial, if personnel had a history of excessive alcohol consumption, drug abuse, significant psychiatric illness or a known (or suspected) physiological or structural condition that compromised the integrity of the blood-brain barrier.
Safety evaluation.
The primary safety outcome evaluated AE incidence rates up to 28 days post-immunization. Safety assessments were based on AE, vital signs (including body temperature), physical examination and routine clinical laboratory evaluations. Monitoring for SAEs occurred throughout both the initial and long-term follow-up phase of the study. Daily diary cards, rulers, thermometers were issued to all participants to aid recording of AEs, new medications and oral body temperature. Adverse events were observed, reported or elicited by interview (aided by the examination of participant diary cards) and coded using the Medical Dictionary for Regulatory Activities version 6 (Maintenance and Support Services Organization, Chantilly, VA, USA). Severity was deemed mild (aware of sign/symptom but easily tolerated, able to perform duties), moderate (discomfort to cause interference with duties) or severe (incapacitating, prevention of duties). Local injection site reactions and rash was deemed mild (<2 cm or rash restricted to an area equivalent to one limb), moderate (≥2 cm or rash equivalent to an area >one limb) or severe (≥6 cm or rash equivalent to an area >half the body).
Serological tests.
Serum was stored at −70 ± 5°C from samples obtained at screening, days 14, 28, 42, 56, months 6 and 7 and annually through year 5 thereafter. For the determination of PRNT, test samples were heat inactivated and three ten-fold serial dilutions were prepared in duplicate. They were mixed with a fixed amount of virus, incubated for one hour at 37°C and plated on LLC-MK2 cells in 6-well plates. Titers are determined as described by Russell et al.Citation18 Sera were tested by a single technician. All samples from a single time point were tested on the same day. A positive control was used throughout the tests to ensure consistency of the results. Serum samples collected post-treatment were tested for neutralizing antibodies to JE-CV virus and four wild-type JEV isolates: a genotype I isolate from Korea of 1991 (1991 TVP-8236), a genotype II isolate from Thailand of 1983 (B1034/8), a genotype III isolate from China of 1949 (Beijing) and a genotype IV isolate from Indonesia of 1981 (JKT 9092/TVP-6265). Sera collected prior to immunization was tested by PRNT50 for evidence (titer ≥10) of previous exposure to flaviviruses including Alfuy, dengue virus serotypes 1, 2, 3 and 4, JEV, Murray Valley encephalitis, yellow fever or to West Nile (Kunjin strain) viruses.
Immunogenicity.
Seroconversion was defined as a change in anti-JE-CV antibody titer of <10 to ≥10 between pre- and post immunization samples or a four fold increase from baseline if pre-existing JEV antibodies existed. A seroprotective antibody titer was defined as PRNT50 ≥10 to JE-CV according to Hombach, 2005.Citation9 Any PRNT50 value reported as “LOQ“ was converted to “LOQ/2”, where LOQ was the lower limit of quantification, defined as 10 for this study. Any PRNT50 value reported as “>ULQ” was converted to “2ULQ”, where “ULQ” was the upper limit of quantification. The primary immunogenicity outcome was the proportion of participants who seroconverted (PRNT50 ≥20) to JE-CV 28 days following a single dose of vaccine. Secondary immunogenicity outcomes included the proportion of participants who seroconverted to between one and four wild-type JEV strains 28 days after vaccination and retained neutralizing antibodies (≥10) to these wild-type JEV strains during the long-term follow-up period. Neutralizing GMT for JE-CV and wild-type JEV strains was calculated by log transformation of the observed titer for each participant and calculation of the arithmetic mean. An analysis of the neutralizing GMT and seroconversion rates at interim time points and in response to a second dose of JE-CV was also undertaken.
Statistical methods.
All statistical methods examined a two-sided, non-directional test of the hypothesis with significance set at 5%. The study was not powered to show statistically significant treatment differences and the immunogenicity comparisons in this study are therefore exploratory. A sample of 100 participants per group was selected to detect AEs. A sample size of 200 participants receiving vaccine compared to their independent receipt of placebo (separated by one month) established an upper bound of 0.015 for the 95% CI for the incidence of an AE in the case that the event was not observed. Differences in baseline parameters including age, gender, ethnicity and pre-existing flavivirus antibodies between treatment groups was assessed by ANOVA. Seroconversion rates were analyzed using logistical regression methods and the persistence of the immune response to wild-type JEV serotypes was assessed from month seven to 24 using McNemar's test. For neutralizing GMTs, the geometric mean ratio after month 6 between Group C (single-dose group) and Group D (two-dose groups) was calculated by ANOVA and was considered significant if the 95% CI ratio did not contain the value one. A Kaplan-Meier survival analysis was used to estimate the proportion of participants maintaining seroconversion to JE-CV from month 6 to 60 with separate analyses undertaken to compare participants given a single dose of vaccine and those given two doses of vaccine (log rank test). Safety analyses summarized the incidence of AEs between treatment groups and were not assessed for significance. All participants who received at least one injection of vaccine or placebo (202 participants) were included in the assessment of safety. The Intent-to-treat (ITT) population consisted of those participants who had provided day 0 (baseline) and post-immunization (day 56) blood samples for antibody analysis (201 participants). The per-protocol (PP) population included the ITT population who were flavivirus naïve by PRNT50 and had no major protocol deviations.
Conclusion
The JE-CV vaccine was safe and highly immunogenic in healthy volunteers, including those with prior flavivirus exposure. The high seroconversion rate and persistence of neutralizing antibodies to the vaccine and wild-type JEV strains up to at least 60 months after single-dose primary vaccination suggests JE-CV may be able to be used in a single dose regimen in persons living in non-JEV endemic areas.
Conflict of Interest
Mark Reid has acted as a paid consultant to Acambis Inc., in relation to JE vaccine trials. Karen McCarthy and Niranjan Kanesathesan are former employees of Acambis Inc., Claude Meric is a current employee of Sanofi Pasteur. These statements are made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of the Australian Defence Health Services or any extant policy.
Financial Disclosure
The study was supported by Acambis (Cambridge, UK) now Sanofi Pasteur.
Abbreviations
| AE | = | adverse event |
| ANOVA | = | analysis of variance |
| CI | = | confidence interval |
| GMT | = | geometric mean titer(s) |
| ITT | = | intent-to-treat |
| JE-CV | = | japanese encephalitis chimeric virus vaccine |
| JE | = | japanese encephalitis |
| JEV | = | japanese encephalitis virus |
| LOQ | = | limit of quantification |
| p | = | probability |
| PFU | = | plaque forming units |
| PP | = | per-protocol |
| PRNT | = | plaque reduction neutralization test |
| SAE | = | serious adverse event |
| TRAE | = | treatment related adverse event |
| ULQ | = | upper limit of quantification |
| YF | = | yellow fever |
| YFV | = | yellow fever virus |
Figures and Tables
Figure 1 Participant disposition and reason for withdrawal at the end of the treatment phase (day 56) and at month 6, 7, 12, 24, 36, 48 and 60 for participants treated with a single 3.8 log10 dose of JE-CV and placebo with or without a booster dose of 3.8 log10 JE-CV at month 6. Treatment period withdrawals (to day 56): Group A: One Treated related adverse event (TRAE) (mild glomerulonephritis and anemia). Two military deployments/training. All three participants received JE-CV but not placebo. Group B: Two instances of protocol non-compliance. One participant with a protocol deviation received both JE-CV and placebo while one participant withdrawn for a reason in the “Other” category received only placebo. Second dose group withdrawals (to month 7): Five withdrawals—lost to follow-up. All participants received vaccine. Long term follow-up withdrawals: (1) One participant was withdrawn due to mouse brain derived, inactivated JE immunization administered between month 48 and month 60. This was required because the participant had no detectable JEV antibodies and was required to deploy to a JE endemic area. (2) A total of 125 participants did not attend the annual follow-up visit over 5 years, principally due to military deployments, leaving the service or posting outside the visitation range of the single center study site.
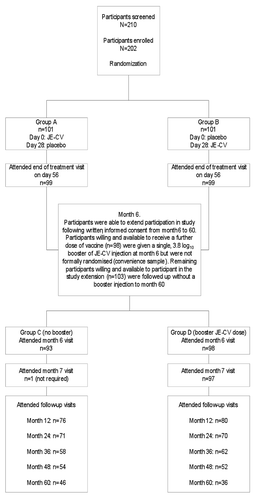
Figure 2 Kaplan-Meier survival analysis showing seroprotection (PRNT50 > 10) over time for 1-dose (Group C) and 2-dose (Group D) JE-CV groups from month 6 to 60. Kaplan-Meier survival analysis was used to determine the proportion of participants maintaining seroconversion to JE-CV from month 6 to 60 with separate analyses undertaken for participants given a single dose of vaccine and those given two doses of vaccine (log-rank test). Participant numbers “at risk”, “failed” and “censored” reflect those who attended each visit and a particular study population (i.e. safety or ITT populations) (). Month 7 was not performed for the single dose participant with a serology value at month 7. PRNT50 values reported at < 10 were converted to a PRNT50 = 5. If a PRNT50 value was missing for a visit and the participant returned for the next visit and was seroprotected at that time; the participant was assumed to have remained seroprotected at the time of the missing visit. If a PRNT50 value was missing for a visit and the participant returned for the next visit and was not seroprotected (i.e. PRNT50 < 10) at that time, the participant was assumed to have not been seroprotected at the time of the missing visit. For participants who missed two consecutive visits, only the data up to the missing visits was used for the Kaplan-Meier estimate.
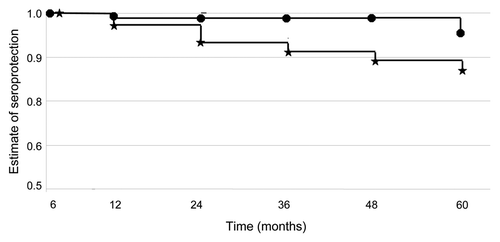
Figure 3 GMT and GMT ratio for 1-dose (Group C) and 2-dose (Group D) from month 6 to 60. The geometric mean ratio was calculated by ANOVA and was considered significant if the 95% CI did not contain the value one. Significant differences (p < 0.05) in the anti-JE-CV GMT from month 12 through month 48 are indicated by an asterisks.
Table 1 Treatment related adverse events occurring in ≥1% of healthy participants following treatment with a single dose of JE-CV or placebo at Month 0, and following a booster dose of JE-CV at month 6 (safety population)
Table 2 Seroprotection and seroconversion to vaccine (JE-CV) from day 28 to month 60 in healthy participants (ITT population) treated with a single dose of JE-CV and placebo followed or not by a booster dose of JE-CV at month 6
Table 3 Kaplan-Meier estimate for persistence of neutralizing antibodies (PRNT50 ≥10) to vaccine (JE-CV) from month 6 to 60 in healthy volunteers (ITT population) treated with a single dose of JE-CV and placebo followed or not by a booster dose of JE-CV at month 6
Acknowledgements
The authors gratefully acknowledge the assistance of Dr. Thomas Monath, Dr. Scott Kitchener and the former staff of Acambis Inc., as study Sponsors. Corporal Andrew Baron, Warrant Officer Derek Davis, Major Mark Kirshbaum, Corporal Natalie Lehmann staff of the Australian Army Malaria Institute and the Australian Defence Force for data entry, sample collection, study coordination, pharmacy support and flavivirus serology. Staff at the Center for Vaccine Development, Institute of Molecular Biosciences, Mahidol University at Salaya, Thailand for JEV serology. Simon Coggins and staff at Pharmaceutical Product Development (PPD) Inc., Wilmington, USA; for full service, contract research support as well as Staff at Sanofi Pasteur for critically reviewing the manuscript.
References
- Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry 2000; 68:405 - 415
- Sanofi Pasteur Inc. Package Insert Japanese Encephalitis Virus Vaccine Inactivated JE-VAX® 2010 Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM123761.pdf
- World Health Organization. Global Advisory Committee on Vaccine Safety. Live attenuated SA 14-14-2 Japanese encephalitis (JE) vaccine 2010 Available at: http://www.who.int/vaccine_safety/topics/japanese_encephalitis/live_attenuated/en/index.html
- Intercell AG. Package Insert Japanese Encephalitis Vaccine, Inactivated, Adsorbed IXIARO 2010 Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142570.pdf
- Global advisory committee on vaccine safety 9–10 June 2005. Wkly Epidemiol Rec 2005; 80:242 - 248
- Monath TP, Soike K, Levenbook I, Zhang ZX, Arroyo J, Delagrave S, et al. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax™-JE) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and non-structural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine 1999; 17:1869 - 1882
- Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, et al. Clinical proof of principle for chimerivax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002; 20:1004 - 1018
- Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (chimerivax-je): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis 2003; 188:1213 - 1230
- Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva 2–3 September 2004. Vaccine 2005; 23:5205 - 5211
- Japanese Encephalitis Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm.Rep 2010; 59:1 - 27
- Duggan ST, Plosker GL. Japanese encephalitis vaccine (inactivated, adsorbed) [IXIARO®]. Drugs 2009; 69:115 - 122
- Beasley DWC, Li L, Suderman MT, Guirakhoo F, Trent DW, Monath TP, et al. Protection against Japanese encephalitis virus strains representing four genotypes by passive transfer of sera against ChimeriVax™-JE experimental vaccine. Vaccine 2004; 22:3722 - 3726
- Sohn YM, Tandan JB, Yoksan S, Ji M, Ohrr M. A 5-year follow-up of antibody response in children vaccinated with single dose of live attenuated SA14-14-2 Japanese encephalitis vaccine: Immunogenicity and anamnestic responses. Vaccine 2008; 26:1638 - 1643
- Tandan JB, Ohrr H, Sohn YM, Yoksan S, Ji M, Nam CM, et al. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: A case-control study in Nepalese children 5 years after immunization. Vaccine 2007; 25:5041 - 5045
- Ferguson M, Kurane I, Wimalaratne O, Shin J, Wood D. WHO informal consultation group. WHO informal consultation on the scientific basis of specifications for production and control of inactivated Japanese encephalitis vaccines for human use, Geneva, Switzerland 1-2 June 2006. Vaccine 2007; 25:5233 - 5243
- Wills M, Sil B, Cao J, Yu Y, Barrett A. Antigenic characterization of the live attenuated Japanese encephalitis vaccine virus SA14-14-2: a comparison with isolates of the virus covering a wide geographic area. Vaccine 1992; 10:861 - 872
- Kurane T, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains. Vaccine 2000; 18:33 - 35
- Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 1967; 99:285 - 290