Abstract
Rapid uptake of new vaccines can improve health and wealth and contribute to meeting Millennium Development Goals. In the past, however, the introduction and use of new vaccines has been characterized by delayed uptake in the countries where the need is greatest. Based on experience with accelerating the adoption of Hib, pneumococcal and rotavirus vaccines, we propose here a framework for new vaccine adoption that may be useful for future efforts. The framework organizes the major steps in the process into a continuum from evidence to policy, implementation and finally access. It highlights the important roles of different actors at various times in the process and may allow new vaccine initiatives to save time and improve their efficiency by anticipating key steps and actions.
Rapid uptake of new vaccines and health technologies can help to improve health and wealth and contribute to meeting Millennium Development Goals.Citation1,Citation2 In the past, however, the introduction and use of new vaccines has often been characterized by rapid uptake in the countries where the disease burden is least and delayed uptake in the countries where the disease burden is greatest.Citation3 Differences in the economic power of these countries are an obvious contributor to the delays, however experience with ‘economics-only solutions’ like the provision of free vaccines have not overcome the problem and as a result, have illustrated that the obstacles are more diverse than economics alone.
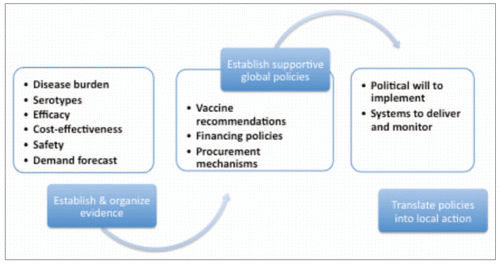
Based on experience with accelerating the adoption of Hib, pneumococcal and rotavirus vaccines and an existing set of WHO guidelines for new vaccine introduction, (http://whqlibdoc.who.int/hq/2005/WHO_IVB_05.18.pdf) we propose here a policy framework for considering the issues and challenges to accelerating new vaccine adoption. The framework organizes the major steps in the process into a continuum from evidence to policy, through, implementation and finally access (). It acknowledges the fact that this process is multi-disciplinary and involves multiple stakeholders including epidemiologists, vaccine scientists, economists, clinicians, behavioral scientists, advocates, policy analysts, communications specialists, politicians, health workers, communities, vaccine manufacturers, international agencies, donors and more. As a policy framework it focuses primarily on the policy-level and recognizes that significant programmatic and operational issues and challenges that are key to ultimate vaccine access are not covered in this document.
Evidence to Policy
The vaccine introduction process is grounded in establishing a sound evidence base on the epidemiology and burden of the disease (including the distribution of serotypes or strains if relevant to vaccine policies) and the safety, efficacy and relative cost-effectiveness of the vaccine as a solution. In short, this process needs to reach a technical consensus that, based on the evidence, the vaccine is proven safe and effective for preventing the target disease.
In the next stage of this process, policy-makers will want to understand the costs involved and the likely health impact over time. For these discussions, forecasting the volume and timing of vaccine demand based on the evidence is critical. It is not too early at this point to begin a dialogue with industry to discuss the packaging and presentation requirements that would be needed for a successful introduction in the systems that exist in target countries. Critical to success in this phase, and throughout the process, is the ability to move on parallel streams of activity at once. Moving sequentially will reduce risk of failure at any stage but may also prove costly in terms of the speed of progress.
The technical evidence base then needs to be transformed into specific, actionable policies, preferably with timelines and measurable indicators or outcomes. Generally this requires linking with formal policy-making bodies like the World Health Organization's Strategic Advisory Group of Experts (SAGE) on immunization or domestic technical advisory groups such as the Government of India's National Technical Advisory Group on Immunization or a national academy of pediatrics. In countries where these committees are absent or in need of strengthening, it is important to support their development into robust policy forming bodies. The policy-making process will then require the synthesis of relevant evidence and effective communication of that evidence to policy-makers in specifically developed materials. In this step, the research community that generated the evidence-base generally must connect with a policy community and begin to translate their data into messages and summaries that are consistent with and concordant with national health goals. In this step, expertise in communications and policy development is essential.
In our experience with GAVI countries, the policies that are most critical to a successful process are: (i) a strong, evidence-based recommendation for vaccine use, (ii) development of credible, predictable financing policies that overcome the economic obstacles to vaccine use and (iii) a procurement framework that allows a rapid, uncomplicated process from expressed country demand to provision of vaccines in countries.
Successful policy formation is not enough, however, and efforts must continue after policy recommendations are made. This is especially true when the policies are formed at a global and/or regional level, and then require implementation at local levels where officials may not have been involved in the global policy process or be unaware of the evidence used to support the policy. Many safe, effective interventions with supportive global policies go unimplemented every year in developing countries, which highlights the need for efforts to assure that national policy decisions are followed by efforts to assure implementation of the policy at all levels (e.g., state, district, municipal).
Policy to Implementation and Access
Ultimately, then, the successful vaccine introduction process requires the generation of political will locally to make the implementation of these policies a priority and the capacity of local systems to deliver the vaccine. Access is not achieved if the local health systems are unable to reliably deliver the vaccine to those who need them the most. At this point, the role of local politicians and civil society is likely to increase as the civil society voices raise demands and the politicians aim to respond to those, and other demands. This is particularly true because a broader base of support, beyond immunization systems alone, is often needed to ensure success and build awareness about the larger benefits to overall population health.
Also, at this point in the process the focus on implementation generally requires careful attention to the integration with local health systems and may involve adaptation of previously generic information or tools to the local situation, i.e., away from a ‘one size fits all’ and into a ‘customized’ approach and the involvement of several government ministries in addition to the Health Ministry. It will almost certainly require additional training of local staff, strengthening of core immunization system functions like cold chain, logistics, waste disposal and surveillance for adverse events following immunization and for the vaccine preventable disease outcomes of interest. In the event of multiple suppliers of similar but not identical vaccines (e.g., 10-valent vs. 13-valent pneumococcal conjugate or monovalent versus 5-valent rotavirus vaccines), it will be important to build strong local planning capacity, and in some countries, procurement policies and mechanisms. Also importantly, in countries with local vaccine manufacturing capacity, these issues may be complicated by economic issues and require efforts to involve the local suppliers in the solutions.
Supporting Observations
The basis for this framework is a series of observations and experiences on what has and has not generated progress along this continuum. The first observation was that initial delays in Hib vaccine adoption were later reversed by implementation of a broader approach to supporting vaccine adoption. This natural “experiment” provides useful insights. In the absence of a strongly supportive WHO recommendation for vaccine useCitation4 and with ongoing controversies about whether there was a substantial burden of Hib disease in some areas of the worldCitation5 GAVI's initial offer in 2000 of free Hib vaccine for 5 years to GAVI countries did not generate demand for the vaccine beyond a small group of early-adopter countries between 2000–2004. Among the accomplishments of the HibInitiative, it supported the revision of the WHO Hib vaccine policy from a weak, permissive statement into a firm recommendation calling for universal vaccine introduction in all countries, and helped communicate the vast evidence on Hib disease burden and vaccine efficacy, safety and cost-effectiveness and the cost of delay in ways that addressed the needs of local policy-makers.Citation6 The result was a rapid increase in applications from GAVI countries for Hib conjugate vaccines. This experience illustrates how, when added to the supportive financing and procurement policies, efforts to develop advocacy based on evidence-driven recommendations for use can produce rapid improvements in vaccine adoption.
Learning from Hib vaccines, the efforts on pneumococcal and rotavirus vaccines have been characterized by even more rapid demand for these vaccines in GAVI countries than was observed with Hib vaccines, but at the same time illustrate the challenges of procurement mechanisms. In the case of pneumococcal and rotavirus, the vaccines received strong evidence-based recommendations for routine use in all countries from WHO Strategic Advisory Group of Experts prior to GAVI accepting applications for the vaccines.Citation7,Citation8 This reflects a significant acceleration of this key policy as compared to Hib vaccine, for which the strong WHO recommendation came six years after GAVI began accepting applications for the vaccine. Additionally, due to focused efforts of vaccine-specific initiatives (pneumoADIP and Rotavirus Vaccine Program) that were constituted earlier in the framework, countries were also better informed about the benefits of pneumococcal and rotavirus vaccines prior to development of recommendations, including their potential to address pneumonia and diarrhea, the two leading causes of child mortality worldwide.
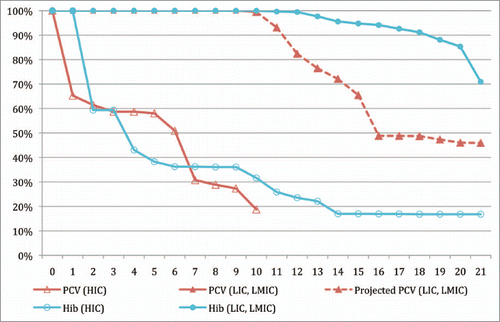
Consequently, the rates of GAVI applications for pneumococcal and rotavirus vaccines have accelerated significantly as compared to historical precedent. A comparison of the observed rollout of Hib conjugate vaccines as compared to the projected rollout of pneumococcal vaccines illustrates this point (). Using the date of actual and projected vaccine introduction, for example, it can be shown that while the introduction of Hib and pneumococcal conjugate vaccines progressed at basically the same rate in high income countries, the projected uptake of pneumococcal conjugate vaccine in low and middle income countries shows a significant improvement over the historical uptake of Hib conjugates.
Conclusions
This proposed framework is based on observations of the process and drivers of new vaccine adoption in GAVI-eligible countries from a group of individuals who have been involved in many, but not all, parts of it. As such, it may or may not be applicable to non-GAVI countries like the USA or Brazil or to non-vaccine interventions like drugs or diagnostics. This framework assumes that vaccines are being developed or are already in existence and does not aim to propose solutions for accelerating vaccine development processes. Furthermore, none of the vaccines we worked on (Hib, pneumococcal and rotavirus) have successfully completed the continuum everywhere. As such, it remains unclear whether a vaccine initiative that begins with this framework from the outset will be completely effective or substantially faster or more efficient than past efforts or other approaches.
Finally, as a proposed framework, we hope it will be used as a guide and that colleagues in the field will contribute suggestions, refining and improving it based on their own experience and research. Over time, we expect it will become more accurate and reliable, and provide a set of transparent steps that can be successfully navigated as a result of preparation, anticipation, and coordination of the needed actors and actions.
Figures and Tables
Figure 2 Figure total. Comparison of coverage with Hib and pneumococcal conjugate vaccines in high-income vs. low and middle income countries over time. Y-axis. Proportion of global birth cohort that lack access to vaccines. X-axis. Years from first country introduction. Red line, open triangles. Pneumococcal conjugate vaccine access in high income countries. Red line, solid triangles. Pneumococcal conjugate vaccine access in low and middle income countries (actual coverage in solid line, projected coverage in dashed line). Blue line, open circle. Hib conjugate vaccine access in high income countries. Blue line, closed circle. Hib conjugate vaccine access in middle income countries. Sources: Country introduction data: International Vaccine Access Center. Vaccine Information Management System (VIMS). Johns Hopkins Bloomberg School of Public Health. Avail at: http://www.jhsph.edu/ivac/vims.html. Last accessed Jul 12, 2010. 2008 DTP 3 Coverage Rates: World Health Organization. WHO Vaccine Preventable Diseases Monitoring System (WHO/UNICEF Best Estimates). Jul 10, 2009. Last accessed Jul 12, 2010 at: http://www.who.int/immunization_monitoring/data/data_subject/en/index.html. 2008 Country Birth Cohorts: UNICEF. The State of the World's Children 2010. Accessed Jul 12, 2010 at: http://www.unicef.org/sowc/; Income groupings: World Bank, 2008.
References
- Bloom DE, Canning D, Weston M. The value of vaccination. Wrld Econ 2005; 6:15
- Barnighausen T, Bloom DE, Canning D, O'Brien J. Accounting for the full benefits of childhood vaccination in south africa. S Afr Med J 2008; 98:842 - 846
- Levine OS, Cherian T, Shah R, Batson A. PneumoADIP: An example of translational research to accelerate pneumococcal vaccination in developing countries. J Health Popul Nutr 2004; 22:268 - 274
- World Health Organization. Conclusions and recommendations from the strategic advisory group of experts to the department of immunizations, vaccines, and biologicals. Wkly Epidemiol Rec 2006; 81:2 - 11
- WHO. WHO position statement on hib conjugate vaccines. Wkly Epidemiol Rec 2004; 79:173
- WHO. WHO position paper on haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec 2006; 81:445 - 452
- WHO. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec 2007; 82:93 - 104
- WHO. Rotavirus vaccines: An update. Wkly Epidemiol Rec 2009; 84:533 - 540