Abstract
The study was planned to assess and compared immunogenicity and safety of an indigenous DTPw-Hib combination vaccine (Shan 4) with EasyFour, the available DTwP-Hib vaccine in India. Overall 210 healthy infants, six to eight weeks of age were randomized to receive three doses of either Shan 4 or EasyFour at 6, 10 and 14 weeks of age. Antibodies were analyzed prior to and four to six weeks post third vaccine dose. Solicited and unsolicited local and systemic events in the follow up period after each dose were recorded. Post vaccination 100% of the infants in Shan 4 and EasyFour groups had seroprotective concentrations of Anti PRP-T IgG antibodies, IgG anti-diphtheria toxoid antibodies and IgG anti-tetanus toxoid antibodies. Following third dose of vaccination 86.99% subjects in the Shan 4 group and 73.85% subjects in the EasyFour group seroconverted for anti-pertussis antibody titres. Two Serious Adverse Events (SAEs) were reported during the course of the study, all unrelated to the respective vaccine administered. Most commonly reported adverse events in both the groups were pain at injection site, mild fever (< 103° F) and minor swelling at injection site. The study proved that Shan 4 was safe and immunogenic compared to the available licensed vaccine.
Introduction
Diphtheria, Tetanus and Pertussis are common infections still prevalent in the developing countries even with the reported global DTP3 vaccine coverage being 81%.Citation1 In 2008 globally reported cases of diphtheria declined to 7,084 from 8,229 in 2005.Citation2 On the contrary in India the reported diphtheria cases increased to 6,081 in 2008 from 3,354 in 2007.Citation3 The number of reported cases of pertussis in India decreased from an alarming 70,729 in the year 2007 to 44,180 in 2008.Citation4 From a global perspective the incidence of reported cases of pertussis decreased to 136,331 in 2008 from 152,535 in the year 2007.Citation5 Reported tetanus cases world over decreased to 16,609 in 2008 from 17,012 in 2007,Citation6 as compared to a decrease in reported cases to 3,714 (2008) from 7,005 (2007) in India.Citation7
The incidence of Hib disease has been reported to have declined dramatically in developed countries since the introduction of the Hib conjugate vaccines.Citation8–Citation10 In developing countries, however, the experience with conjugate Hib vaccines is limited and complicated by the fact that the vaccines are administered in two vaccination schedules. Studies from the Brazil,Citation11 GambiaCitation12,Citation13 and ChileCitation14,Citation15 where the vaccine was administered to children from the age of 2 months show the vaccines to be immunogenic and protective against Hib disease. The data is even more limited for the vaccines being administered at 6, 10 and 14 weeks, the current World Health Organization (WHO) Expanded Programme on Immunization (EPl) schedule of vaccination. Recent studies from the PhilippinesCitation16 and South AfricaCitation17 have indicated that such vaccines are safe and immunogenic when administered according to this schedule.
Addition of antigens to existing DTP vaccine is an effective way to rapidly achieve better coverage against other childhood pathogens like hepatitis B and Haemophilus influenzae type b (Hib). Combination vaccines also have advantages of lesser number of injections, decreased cost and better compliance. At the same time it is essential to ensure that interactions between antigens do not affect efficacy, safety and immunogenicity of individual components. The current tetravalent vaccine (Shan 4) is India's first indigenously developed DTwP-Hib tetravalent vaccine containing Hib-tetanus toxoid conjugate in a fully liquid formulation. This study was undertaken to evaluate and compare the safety and immunogenicity of an indigenously developed DTPw-Hib liquid tetravalent vaccine manufactured by Shantha Biotechnics Ltd., with a licensed vaccine in Indian infants.
Objectives
Primary Objective of the study was to assess the immune response of each of the four components of the combination vaccines. These responses were measured as percentage of infants who developed antibody titres to D, T, P and Hib above the defined cut-off levels, four to six weeks after the third dose of the tetravalent vaccines. These immune responses were represented in the form of Geometric Mean Titres (GMT) of the antibodies four to six weeks after the last dose of the vaccines. Secondary Objective of the study was to evaluate the safety of the vaccines by assessment of the reactogenicity and tolerance after each dose of the vaccines.
Results
Characteristics of study population.
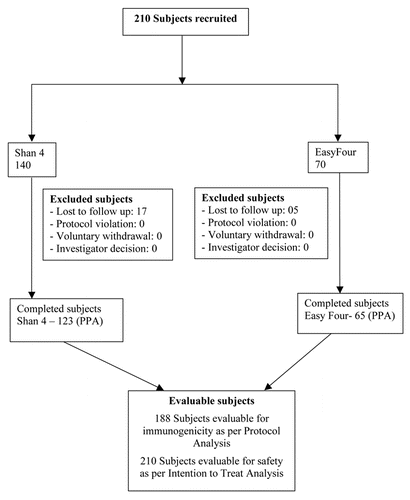
The 140 subjects randomized to the Shan 4 group and 70 to the EasyFour group were comparable with respect to the baseline demographic parameters such as Age, Body Weight, Head Circumference and Body length as represented in . The flow of the 210 subjects recruited in the study is represented in .
Immunogenicity evaluation.
The seroprotection and post immunization GMT for all components in the Shan 4 group were comparable with licensed vaccine group and no significant differences were observed ().
It was observed that 100% subjects in the Shan 4 and EasyFour™ groups had the pre-vaccination titers ≥0.1 IU/ml (Protective levels) for tetanus toxoid, which may be related to the vaccination of the mothers with two doses of tetanus toxoid during gestation leading to passive transfer of antibodies in the neonates. Such a trend has also been observed in published data from studies of similar vaccines.Citation18 All these subjects had sero-protective levels after the third dose of the vaccines.
Prior to first dose of vaccine 9.75% subjects in the Shan 4 group had anti-pertussis antibody titers more than protective levels (≥9 panbio units) as compared to just 1.53% in the EasyFour group. Following third dose of vaccination 86.99% subjects in the Shan 4 group and 73.85% subjects in the EasyFour group seroconverted, a statistically significant difference [Mean difference of proportions 0.2055; 95% CI 0.02672, 0.3821; p = 0.0281]. The post vaccination anti Pertussis antibody titres for Shan 4 and EasyFour groups were comparable and did not show any significant difference (p = 0.1120; Mean Difference 1.895; 95% CI −0.4479, 4.238).
To evaluate immunity to Hib disease, a post vaccination titer ≥0.15 µg/mL is considered to be indicative of short-term protection and ≥1.0 µg/mL suggests long term protection. PRP-T antibody titres four to six weeks after the third dose of the vaccines were comparable between the two groups and did not show any significant difference (p = 0.1135; Mean Difference 4.847; 95% CI −10.860, 1.166).
The difference between the upper limits of 95% confidence intervals for seroconversion between the various components for Shan 4 and EasyFour were within the pre-defined non inferiority limits of 10% thus establishing non inferiority of the two vaccines.
Reactogenicity evaluation.
Overall 608 doses were administered 403 in Shan 4 and 205 in EasyFour™ groups respectively to 210 subjects and 583 diary cards were returned. The common local and systemic reactions and adverse events generally reported were pain at injection site, minor swelling at injection site and fever. Fever was mild in nature, subsequently subsided within 6–12 hours following vaccination and did not recur. Only five subjects in the Shan 4 group and two from EasyFour group had fever ≥103°F. Most commonly report systemic adverse events were Irritability, crying and drowsiness. There were two Serious Adverse Events reported from Shan 4 group (diagnosed as bronchopneumonia and bronchiolitis). Both the subjects were appropriately treated, recovered completely and completed the vaccination schedule. In the opinion of the respective study teams, both the events were not considered to be related to the study vaccines and appropriate reports were submitted to the local Ethics Committees, ethics committees at other study centers and the National Regulatory authorities as per GCP guidelines. All Adverse Events Following Immunization (AEFI) are shown in .
Discussion
The ‘enotion’ of co-administering multiple antigens in a single injection is now a widely accepted means of maximizing the efficiency and cost-effectiveness of infant immunization programs. Combined vaccines also offer an opportunity to improve disease control by maximizing coverage. More inclusive combination vaccine might improve vaccination coverage and decrease cost but could compromise safety.
In present study we have evaluated the new indigenous DTwP-Hib tetravalent combination vaccine manufactured by Shantha Biotechnics Ltd., when administered according to a 6-10-14 week EPI schedule. It demonstrated efficacy and safety of the new DTwP-Hib vaccine compared to other licensed tetravalent combination vaccine EasyFour™ in terms of the immune response to all antigens and safety profile.
The results of this study demonstrate a good immunogenicity and reactogenicity profile for the new combined DTwP-Hib vaccine (Shan 4). Statistical comparison based on the result showed that in terms of the antibody response to all the four antigens Shan 4 vaccine was non inferior to licensed comparator tetravalent combined vaccine (EasyFour). Additionally Shan 4 was well tolerated by the infants and the rates of local and systemic AEFIs were comparable between both the vaccines. Although it has to be noted, that the trial was not powered enough to detect rare adverse events that may be associated with the use of the vaccine.
The safety and immunogenicity results of this study were comparable to the results published using similar vaccines ().Citation11–Citation17,Citation19,Citation20 In fact the antibody titers of PRP-T indicative of long term protection (>1.0 µg/mL one month after third dose) were much higher with Shan 4 as compared to those observed with similar vaccines (Combined and Monovalent) when administered in an identical schedule.
The results of this study demonstrate that the new combined tetravalent DTwP-Hib vaccine (Shan 4, Shantha Biotechnics Limited, India) has the potential to provide protection against four major pathogens with a minimum number of injections and potentially improved immunization coverage for Hib vaccines. Inspite of the availability of pentavalent vaccines (DTwPHepB-Hib) commercially, this vaccine would prove to be very useful in scenarios where the first dose of hepatitis B vaccine is administered at birth followed by second and third doses at 6 weeks and 10 weeks of age, as a part of pentavalent combination vaccines. The tetravalent (DTwPHib) vaccine can be utilized to complete the EPI schedule by vaccinating as third dose at 14 weeks of age. Additionally the utility of the DTwPHib vaccines at 15–18 months of age need not be highlighted, when there is a recommendation for booster doses of DTP and Hib.
Material and Methods
Study design and procedure.
Seven centers across India randomly recruited 210 subjects for this single blind, randomized, comparative, non-inferiority study in a 2:1 ratio to receive three doses of either Shan 4 or EasyFour (DTwP-Hib vaccines). The randomization code was generated using the SAS version 8.2 software package. Protocols and the other relevant study documents were approved by the Drugs Controller General of India (DCGI) and local institutional ethics committees prior to the start of the study, which was conducted according to the guidelines, laid in Declaration of Helsinki and Good Clinical Practice as per ICH and Indian GCP. Subjects were recruited following a written informed consent provided by parents or guardians. The largest clinically acceptable difference between two vaccine groups for demonstration of non inferiority was assumed not to exceed 10% in terms of anti-PRP seroprotection rate. Hence with a type I error of 5% and type II error of 10% (i.e., power = 90%) 105 evaluable subjects per group were necessary, but in anticipation of drop outs, 140 subjects were enrolled for Shan 4 group. Since the randomization was carried out in a 2:1 ratio, 70 subjects received the comparator DTPw-Hib vaccine (EasyFour). The randomization ratio of 2:1 was chosen to obtain more safety data for the study vaccine, Shan 4.
All subjects received three doses of 0.5 ml of one of the vaccine (as per randomization group) by intramuscular route in the antero-lateral region of thigh at age six to eight weeks, 10 to 12 weeks and 14 to 16 weeks after birth. Immunization was done using a disposable syringe and a 23G needle of one inch length. Study subjects also received concomitant Oral Polio Vaccine (OPV) according to Universal Immunization programme.
Study participants.
Healthy infants in the age group six to eight weeks, born to mothers after a normal gestation period between 36 and 42 weeks, were enrolled for the study. Infants having evidence of disease or fever, history of allergic disease or persistent hematological, hepatic, renal, cardiac or respiratory disease and signs of a CNS disorder at the time of vaccination, major congenital or hereditary immunodeficiency and history of allergic disease were excluded from the study. Also the administration of immunoglobulin or any blood products since birth and use of any investigational or un-registered drug or vaccine other than the study vaccine (with the exception of OPV, BCG and Hepatitis B vaccine) prior to the first dose of the study vaccines or during the study period were not recruited. Infants who had been administered a birth dose of the hepatitis B vaccine, as per the study center protocol, were not excluded from the study but a record of this was made in the Case Record Forms and these infants received the second and third dose of the hepatitis B vaccine as per the respective study center schedule. In infants who did not receive the birth dose of the hepatitis B vaccine, consistent with the immunization protocol of the respective study site, three doses of the hepatitis B vaccine were administered on the same date as the study vaccines.
Serological analysis for immunogenicity.
Blood samples (1.5 ml) for the estimation of antibody titers were collected before and four to six weeks after the third dose of the vaccines. The antibodies were estimated for all components by Enzyme Linked Immunosorbent Assay (ELISA). The trial sera were labeled with a unique identifier number at the study sites so that all the serological assays could be performed in a blinded manner. IgG Anti-diphtheria toxin and IgG anti-tetanus toxin antibodies were determined using an ELISA, by Nova Tec kit (Nova Tec Immundiagnostica GmbH, Technologie & Waldpark, Germany), with seroprotection defined as a titer level ≥0.1 IU/mL for both Anti-diphtheria toxin antibodies and anti-tetanus toxin antibodies. A whole-cell ELISA (IgG PANBIO ELISA Panbio Limited, Australia) was used to detect IgG antibodies to B. pertussis. A correlate of protection has yet to be established for pertussis, therefore seroconversion was defined as a post vaccination titer more than or equal to the pre-vaccination titer in initially seropositive subjects and in case of initial seronegative subjects, the response was considered according to assay cut off. (<9 panbio units-NEGATIVE; 9–11 panbio units—EQUIVOCAL; >11 panbio units—POSITIVE). The Hib ELISA (BINDAZYME, Human anti Haemophilus influenzae enzyme immunoassay kit, Binding Site Limited, Birmingham UK) specifically detects IgG antibodies against type b capsular polysaccharide of H. influenzae (anti-PRP) and was calibrated against FDA standard. Hib seroprotection rates were determined for both titer levels ≥0.15 µg/mL and .1.0 µg/mL. All the assays were performed at Metropolis Health Services (India) Private Limited, Mumbai, India.
Reactogenicity and safety assessments.
Subjects were observed at the study sites for minimum of 30 minutes upto maximum of three hours after vaccination to detect and treat any immediate reaction. Parents or guardians recorded pre-specified local and systemic reactions, and solicited and unsolicited adverse events on diary cards for three days and 30 days respectively following vaccination. Solicited local reactions included redness, swelling, tenderness and induration. Solicited systemic reactions included fever, persistent crying, vomiting, diarrhea and loss of appetite. Axillary temperature was measured daily using a standard digital thermometer provided by the sponsor. Serious adverse events were documented from enrollment to last follow-up visit. All adverse events were reported and recorded as per Brighton case definitions issued by Brighton Collaboration. Following each dose of the vaccine, the infants with body temperature ≥40.4°C, persistent screaming or crying for three hours within 48 hours of vaccination, seizures, encephalopathy or hypersensitivity reaction were excluded from receiving subsequent doses of the vaccines.
Study vaccines.
The DTwP-Hib tetravalent combination vaccine (Shan 4, Shantha Biotechnics Ltd., Hyderabad, India) comprised of Diphtheria toxoid (≥30 IU), Tetanus toxoid (≥60 IU), Whole cell B. pertussis (≥4 IU), 0.01 mg of purified capsular polysaccharide of Hib covalently bound to 20–40 mcg of Tetanus toxoid [PRP-T], Thiomersal (≤0.05 mg) as preservative, Aluminum phosphate gel equivalent to Al+++ (0.425 mg) as an adjuvant and Sodium Chloride (4.5 mg) in 0.5 ml single dose. The comparator vaccine used in this study, EasyFour (Panacea Biotech Ltd., New Delhi, India) contained 30 IU Diphtheria toxoid, 7.5 Lf (40 to 60 IU) Tetanus toxoid, 4 IU inactivated whole cell B. pertussis, 10 mcg Hib oligosaccharides conjugated to CRM197 protein, 0.025 mg Thiomersal as a preservative and 0.25 mg Aluminum salt as an adjuvant in 0.5 ml single dose.
Statistical analysis.
All Statistical tests were computed using SAS 8.2 software package. For continuous variables, descriptive statistics (minimum, maximum, range, mean and standard deviation) and for discrete variables, counts with percentages were presented. Pre-vaccination and post-vaccination antibody titers were compared using t-test and proportion of subjects demonstrating seroprotection/seroconversion were compared using Fischer's exact test (value of p < 0.05 was considered statistically significant). Pre and post vaccination Geometric Mean Titres (GMT) were also computed using the same statistical software. To prove the non-inferiority of study vaccine with respect to comparator vaccine differences of <10% between the upper limits of the 95% CI of the seroconversion rates for each antigen was set as the criteria. Immunogenicity analysis was based on the ‘Per Protocol’ population (eligible immunized subjects who completed the study and provided pre and post vaccination blood samples), where as safety analysis was based on ‘Intention To Treat’ population (All subjects who were given at least one dose of the vaccine).
Conclusion
We conclude from this study that the indigenously developed DTPw-Hib tetanus toxoid conjugate liquid combination vaccine (Shan 4) is similar in its safety and immunogenicity profile to the licensed tetravalent vaccine. As compared to the vaccines made in developed countries, this vaccine can be utilized to provide a better coverage at a lesser cost in the developing countries where incidence of these diseases is still very high.
Financial disclosure
This work was funded by Shantha Biotechnics Limited.
Figures and Tables
Table 1 Demographic profile of subjects recruited
Table 2 Seroconversion and GMT: anti-diphtheria, anti-tetanus, anti-pertussis and anti-PRP antibodies
Table 3 Local and systemic adverse events following immunization
Table 4 Comparative Hib immunogenicity data four weeks after third dose of either monovalent Hib conjugate vaccine or combined Hib conjugate vaccine
Acknowledgements
We are grateful to all the subjects who volunteered to participate in the study.
Contributions: M.S.D. and R.R. conceived and designed the study. Drs. S.B., R.J., V.K., H.R.P., S.N.R., G.R.S. and N.S. performed study procedures and were involved in the acquisition of data. M.M. and M.S.D. contributed to the analysis and interpretation of data. All the authors were involved in the initial drafting of the manuscript. M.S.D. and R.R. were involved in the finalization of the manuscript. R.R. will stand as the guarantor for the paper.
Competing interests: R.R., M.S.D. and M.M. were all employees of the manufacturer of the experimental vaccine (Shantha Biotechnics Limited) during the planning, analysis and interpretation of the study. Drs. S.B., R.J., V.K., H.R.P., S.N.R., G.R.S. and N.S. had no financial interests in the vaccine or the manufacturer but received research funding to undertake the study.
References
- World Health Organization. Global Immunization Data October 2008 http://www.who.int/immunization/newsroom/GID_english.pdf (2009, date last accessed)
- World Health Organization. Immunization Surveillance, assessment and monitoring: Diphtheria http://www.who.int/immunization_monitoring/diseases/diphteria/en/index.html (2009, date last accessed)
- World Health Organization. Diptheria reported cases: India http://www.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidencedip.htm (2009, date last accessed)
- World Health Organization. Pertussis reported cases: India http://www.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidenceper.htm (2009, date last accessed)
- World Health Organization. Immunization Surveillance, assessment and monitoring: Pertussis http://www.who.int/immunization_monitoring/diseases/pertussis/en/index.html (2009, date last accessed)
- World Health Organization. Immunization Surveillance, assessment and monitoring: Tetanus http://www.who.int/immunization_monitoring/diseases/tetanus/en/index.html (2009, date last accessed)
- World Health Organization. Tetanus (total) reported cases: India http://www.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidencette.htm (2009, date last accessed)
- Peltola H, Kilpi T, Anttila M. Rapid disappearance of Haemophilus influenzae type b meningitis after routine childhood immunization with conjugate vaccines. Lancet 1992; 340:592 - 594
- Adams WG, Deaver KA, Cochi Sl, Plikaytis BD, Zell ER, Broom CV, et al. Decline in Haemophilus influenzae type b (Hib) diseases in the Hib vaccine era. JAMA 1993; 269:221 - 226
- Centers for Disease Control. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987–1995. MMWR 1996; 45:905 - 906
- Martins Rde M, Camacho LA, Marcovistz R, Noronha TG, Maia Mde L, dos Santos EM, et al. Immunogenicity, reactogenicity and consistency of production of a Brazilian combined vaccine against diphtheria, tetanus, pertussis and Haemophilus influenzae type b. Mem Inst Oswaldo Cruz 2008; 103:711 - 718
- Mulholland EK, Hoestermann A, Ward JI, Maine N, Ethevenaux C, Greenwood BM. The use of Haemophilus influenzae type b tetanus toxoid conjugate vaccine mixed with diphtheria-tetanus-pertussis vaccine in Gambian infants. Vaccine 1996; 14:905 - 909
- Mulholland K, Hilton S, Agedbola K, Usen S, Uparaugo A, Omosigho C, et al. Randomised trial of Haemophilus influenzae type b-tetanus protein conjugate for prevention of pneumonia and meningitis in Gambian infants. Lancet 1997; 349:1191 - 1197
- Hoppenbrouwers K, Lagos R, Swennen B, Ethevenaux C, Knops J, Levine MM, et al. Safety and immunogenicity of a Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) and diphtheria-tetanus-pertussis (DTP) combination vaccine administered in a dual chamber syringe to infants in Belgium and Chile. Vaccine 1998; 16:921 - 1011
- Lagos R, Horwitz I, Toro J, Martin OS, Abrego P, Bustamante C, et al. Large scale, post licensure, selective vaccination of Chilean infants with PRP-T conjugate vaccine: practicality and effectiveness in preventing invasive Haemophilus influenzae type b infections. Pediatr lnfect Dis J 1996; 15:216 - 222
- Capeding MRZ. lmmunogenicity of Haemophilus influenzae conjugate vaccines in developing countries. JAMA 1994; 5:40 - 43
- Hussey G, Malan H, Hughes J, Eley B, Piollet M, Charrondiere M, et al. Safety and Immunogenicity of TETRACTHIB (a vaccine combining DTP vaccine and Haemophilus influenzae type b conjugate vaccine) administered to infants at 6, 10 and 14 weeks of age. S Afr Med J 2002; 92:53 - 57
- Rao R, Dhingra MS, Bavdekar S, Behera N, Daga SR, Dutta AK, et al. A Comparison of the Immunogenicity and Safety of Indigenously Developed Liquid (DTPwHB-Hib) Pentavalent Combination Vaccine (Shan 5) with EasyFive (Liquid) and Tritanrix + Hiberix (lyophilized) in Indian Infants Administered According to the EPI Schedule. Human Vaccines 2009; 5:425 - 429
- Watemberg N, Dagan R, Arbelli Y, Belmaker I, Morag A, Hessel L, et al. Safety and immunogenicity of Haemophilus type b tetanus protein conjugate vaccine, mixed in the same syringe with diphtheria-tetanuspertussis vaccine in young infants. Pediatr Infect Dis J 1991; 10:758 - 763
- Avendano A, Ferreccio C, Lagos R, Horwitz I, Cayazzo M, Fritzell B, et al. Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine does not depress serologic responses to diphtheria, tetanus or pertussis antigens when co-administered in the same syringe with diphtheria-tetanus-pertussis vaccine at two, four and six months of age. Pediatr Infect Dis J 1993; 12:638 - 643