Abstract
Background. Immunization is a successful and cost-effective method for preventing disease, yet many adolescents do not receive recommended vaccines. We assessed correlates of uptake of three vaccines (tetanus booster, meningococcal, and human papillomavirus [HPV] vaccines) recommended for adolescent females. Methods. We examined cross-sectional data from 647 parents of 11-20 year-old females from North Carolina who completed the Carolina HPV Immunization Measurement and Evaluation (CHIME) Project follow-up survey in late 2008. Analyses used ordinal and binary logistic regression. Results. Only 17% of parents indicated their daughters had received all three vaccines. Eighty-seven percent of parents indicated their daughters had received tetanus booster vaccine, 36% reported vaccination against meningococcal disease, and 36% reported HPV vaccine initiation. Daughters aged 13-15 years (OR=1.70, 95% CI: 1.09–2.64) or 16-20 years (OR=2.28, 95% CI: 1.51–3.44) had received a greater number of these vaccines compared to daughters aged 11-12 years. Daughters who had preventive care visits in the last year (OR=4.81, 95% CI: 3.14–7.34) or whose parents had at least some college education (OR=1.90, 95% CI: 1.29–2.80) had also received a greater number of these vaccines. Conclusions. Few daughters, particularly 11-12 years olds, had received all three vaccines recommended for adolescent females. Ensuring annual preventive care visits and increasing concomitant administration of adolescent vaccines may help increase vaccine coverage.
Introduction
Immunization is one of the most beneficial and cost-effective methods for preventing disease,Citation1 with an estimated 20 million prevented deaths from infections worldwide over the last two decades.Citation2 Diseases that can be prevented by vaccines recommended for adolescents include tetanus, diphtheria, pertussis, and certain types of meningococcal disease and human papillomavirus (HPV) infection. Tetanus and diphtheria are uncommon in the US largely because of successful immunization programs,Citation3,Citation4 but the others remain significant public health concerns. In the US, pertussis rates have increased over the last few decades with more than 10,000 cases reported in 2007,Citation3,Citation5 an estimated 1,400–2,800 cases of invasive meningococcal disease occur annually with a case fatality ratio of 10%–14%,Citation6,Citation7 and HPV is a common sexually transmitted infectionCitation8 that accounts for thousands of cancers annually.Citation9 Since 2005, new vaccines for these diseases have been licensed for use and recommended for adolescents by the US Advisory Committee on Immunization Practices (ACIP), including tetanus, diphtheria and acellular pertussis (Tdap) vaccine (licensed for use in 2005), meningococcal conjugate vaccine (MCV4; licensed for use in 2005) and quadrivalent HPV vaccine (licensed for use in 2006).Citation6,Citation10,Citation11
For 2008, the year of our study, ACIP recommended the following immunization schedule for adolescents aged 11–18 years.Citation12 A booster dose of Tdap vaccine should be administered to adolescents aged 11–12 years who completed the recommended childhood vaccination series for tetanus, diphtheria and pertussis and have not received a tetanus and diphtheria toxoids (Td) vaccine booster dose. Adolescents aged 13–18 years who did not receive Tdap while they were 11–12 years old or received only Td should also receive a dose of Tdap in certain cases.Citation10 While Tdap is the preferred vaccine, Td can be administered in situations where Tdap is not available.Citation10 One dose of MCV4 should be administered at 11–12 years of age with catch-up vaccination for adolescents aged 13–18 years who have not been previously vaccinated. Meningococcal polysaccharide vaccine (MPSV4) is an acceptable alternative to MCV4, though administration of MCV4 is preferred. The three-dose quadrivalent HPV vaccine regimen should be given routinely to all females aged 11–12 years, with catch-up vaccination for older females (aged 13–26 years). The bivalent HPV vaccine was not available in the US in 2008.
While these recommended vaccines provide substantial health benefits, many adolescents may never receive them. The 2008 National Immunization Survey-Teen (NIS-Teen) found that 28% of 13–17 year-old adolescents had not received any doses of tetanus booster vaccine (Tdap, Td or unknown tetanus booster vaccine type) since age 10, 58% had not received meningococcal vaccine (MCV4 or unknown meningococcal vaccine type), and 63% of female adolescents had not received any doses of HPV vaccine.Citation13 Although Healthy People 2010 did not set goals for HPV or meningococcal vaccination, current tetanus booster vaccination rates are well below the goal of 90% coverage.Citation14
Recent research has identified correlates of HPV vaccination among adolescent females, including older age, having health insurance coverage, recent utilization of healthcare, doctor's recommendation to get vaccinated, and their parents' beliefs about the vaccine.Citation15–Citation19 Less is known about correlates of tetanus booster and meningococcal vaccination among adolescents, though NIS-Teen data suggest uptake of these vaccines may differ by age and race.Citation13 Furthermore, to our knowledge, the existing literature has not examined uptake of all three recommended adolescent vaccines, the preferred public health outcome. We aimed to characterize vaccination coverage of tetanus booster, meningococcal and HPV vaccines among adolescent females from southeastern North Carolina. We examined characteristics of adolescent females, their parents and their households as potential correlates of vaccination.
Results
Of 1,220 eligible parents contacted, 889 (73%) completed baseline interviews.Citation20 Of 873 baseline respondents eligible for follow-up, 650 (74%) completed follow-up interviews.Citation21 Three parents prematurely ended their follow-up interviews. Thus, we report data obtained during follow-up interviews with parents on vaccination histories of 647 adolescent females aged 11–20 years (who were aged 10–18 years at the time of baseline interviews).
Most parents were 40 years of age or older (79%), female (94%), non-Hispanic white (74%) or non-Hispanic African American (20%), had at least some college education (81%), and reported a household income of $60,000 or more (56%) (). Half lived in rural areas. The daughters' mean age was 16 years old (range 11–20), and most parents indicated their daughters had private health insurance (82%) and had a preventive care visit in the last year (83%).
Few parents (17%, 111/647) reported their daughters had received all three recommended adolescent vaccines. However, most indicated their daughters had received one (42%, 273/647) or two (33%, 212/647) of these vaccines (). Most parents reported their daughters had received tetanus booster vaccine (Td or Tdap; 87%; 565/647), but fewer reported their daughters had received meningococcal vaccine (MCV4 or MPSV4; 36%; 234/647) or initiated the HPV vaccine series (36%; 231/647). Eight percent (51/647) of parents reported their daughters had not received any of the three recommended adolescent vaccines.
Correlates of receiving recommended adolescent vaccines.
In bivariate analyses, daughters who were older or had preventive care visits in the last year had received a greater number of the three recommended adolescent vaccines (). Daughters whose parents had at least some college education or reported a household income of $60,000 or more (compared to parents who reported a household income of less than $60,000) had also received more of these vaccines.
Multivariate analyses yielded similar results. Daughters aged 13–15 years (OR = 1.70, 95% CI: 1.09–2.64) or 16–20 years (OR = 2.28, 95% CI: 1.51–3.44) had received a greater number of these recommended adolescent vaccines compared to daughters aged 11–12 years. Daughters who had preventive care visits in the last year (OR = 4.81, 95% CI: 3.14–7.34) or whose parents had at least some college education (OR = 1.90, 95% CI: 1.29–2.80) had also received a greater number of these vaccines.
Correlates of tetanus booster, meningococcal and HPV vaccination.
In multivariate analyses, tetanus booster vaccination was more common among daughters aged 16–20 years compared to those aged 11–12 years (OR = 2.00, 95% CI: 1.09–3.67) (). Parents were also more likely to report tetanus booster vaccination if their daughters had preventive care visits in the last year (OR = 2.94, 95% CI: 1.74–4.95) or had also received meningococcal vaccine (OR = 2.66, 95% CI: 1.44–4.90).
For meningococcal vaccination, daughters aged 16–20 years were more likely to have been vaccinated compared to those aged 13–15 years in multivariate analyses (OR = 1.68, 95% CI: 1.13–2.50) (). Meningococcal vaccination was also more common among daughters who had received tetanus booster vaccine (OR = 2.85, 95% CI: 1.53–5.33) or at least one dose of HPV vaccine (OR = 2.29, 95% CI: 1.60–3.27).
In multivariate analyses, daughters aged 13–15 years (OR = 2.31, 95% CI: 1.32–4.04) or 16–20 years (OR = 2.04, 95% CI: 1.21–3.44) were more likely to have received one or more doses of HPV vaccine compared to those aged 11–12 years (). Parents were also more likely to report HPV vaccination if their daughters had preventive care visits in the last year (OR = 7.24, 95% CI: 3.55–14.78), their daughters had also received meningococcal vaccine (OR = 2.27, 95% CI: 1.60–3.23) or the parents had at least some college education (OR = 1.69, 95% CI: 1.04–2.73).
Sensitivity analyses.
Few parents responded “don't know” to vaccination items for tetanus booster (n = 15) and HPV (n = 9) vaccines, while 99 parents indicated they did not know if their daughters had received meningococcal vaccine. We conducted sensitivity analyses to explore the effect of classifying daughters whose parents responded “don't know” to vaccination items as “not vaccinated.” When we excluded these daughters from analyses, the results did not meaningfully change for any of the outcomes examined (data not shown), giving us confidence in our findings.
Discussion
Despite national recommendations for HPV, tetanus booster and meningococcal vaccines,Citation12 vaccination levels among adolescent females in these five North Carolina counties were suboptimal, with just 17% of parents indicating their daughters had received all three vaccines. To our knowledge, this represents the first estimate of having received all three of these recommended vaccines among adolescent females. The percentage of parents who reported their daughters had received tetanus booster vaccine was slightly below the Healthy People 2010 goal of 90% vaccine coverage (set for adolescents aged 13–15 years),Citation14 and meningococcal and HPV vaccination levels were much lower.
HPV vaccine uptake in our study was highly similar to the 2008 NIS-Teen estimate for North Carolina (36% vs. 34%), as was meningococcal vaccine uptake (36% vs. 31%).Citation13 However, tetanus booster vaccine uptake was noticeably higher in our study (87% vs. 64%).Citation13 While the difference in tetanus booster vaccine uptake may be partly due to reliance on parental reports of daughters' vaccination histories (NIS-Teen estimates are based on provider records), we believe it is more likely due to the recent Tdap school requirements in North Carolina. Beginning in August 2008, the state required all 6th grade students attending public schools (and 12 year olds in non-public schools) to receive a booster dose of Tdap, provided it had been five or more years since their last dose of Td.Citation22 North Carolina also required individuals enrolling in a college or university for the first time on or after July 1, 2008 to receive a booster dose of Tdap, provided Td or Tdap had not been administered within the past 10 years.Citation22 As data collection for this study occurred exclusively after these requirements went into effect (whereas NIS-Teen was conducted both before and after), our results may simply reflect the effectiveness of the new Tdap school requirements. School requirements do not currently exist in North Carolina for HPV or meningococcal vaccination.Citation22
Interestingly, the correlates of vaccination were similar across the four outcomes examined in our analyses. Daughters whose parents indicated they had preventive care visits in the last year were often more likely to have received recommended adolescent vaccines. However, while most (83%) daughters had a preventive care visit in the last year, relatively few of these daughters had received all three vaccines, suggesting missed opportunities to vaccinate.Citation23 Developing and implementing a standard of care and structured visits for adolescents, including an adolescent immunization platform,Citation24 may decrease such missed opportunities. Physician vaccination reminder systems can also help increase vaccination levels,Citation25,Citation26 yet remain underused by healthcare providers.Citation27 Furthermore, almost 20% of parents indicated their daughters did not have a preventive care visit within the last year, despite recommendations that all adolescents should have such visits annually.Citation28,Citation29 For these adolescents, alternative vaccination settings, such as school-based health centers, may be an option to consider for increasing vaccination.Citation30
Adolescents who received one of the recommended vaccines examined in this study were often more likely to have received other recommended adolescent vaccines examined (e.g., receipt of tetanus booster vaccine was correlated with meningococcal vaccination). Concomitant administration of adolescent vaccines could capitalize on the tendency of adolescents who get one vaccine to also get others. ACIP currently recommends administering tetanus booster and meningococcal vaccines during the same healthcare visit if both are indicated and available.Citation10 ACIP also currently states that HPV vaccine can be administered at the same visit as other adolescent vaccines, because concomitant administration is likely to increase the number of adolescents receiving vaccines on schedule.Citation11 Although parents and healthcare providers have expressed concerns about concomitant administration of childhood vaccines,Citation31,Citation32 most parents have allowed their children to receive multiple recommended vaccines during the same visit.Citation33 Additional research on the acceptability of concomitant administration of adolescent vaccines to both adolescents and parents is needed.
Vaccination tended to be lower among younger adolescents in our study, even though all three vaccines have the same target age group of 11–12 year-old adolescents. Other studies have also reported lower levels of HPV and meningococcal vaccination among younger adolescents.Citation15,Citation34 While some unvaccinated 11–12 year olds may have not yet had the opportunity to receive all three vaccines but will eventually receive them, it is concerning that many may not. About one-third of all pertussis cases occur among 11–18 year olds,Citation10 and adolescents ages 11–19 years have rates of meningococcal disease higher than the general population.Citation6 College students living in dormitories also face high rates of meningococcal disease.Citation6 About 9% of females ages 14–19 years have serologic evidence of infection with at least one HPV type contained in the quadrivalent vaccine.Citation35 Increasing vaccination among younger adolescents may help reduce the prevalence of these diseases among adolescents.
Our study has several important strengths including interviewing a large sample of parents, examination of three recommended adolescent vaccines, and a good response rate. While our assessment of vaccination relied on parental reports that may be subject to recall and social desirability error, previous research has shown parents can accurately recall their young children's influenza vaccination status.Citation36 Furthermore, HPV and meningococcal vaccine uptake was comparable to estimates from the 2008 NIS-Teen, as discussed previously.Citation13 We did not collect information regarding which meningococcal (MPSV4 or MCV4) or tetanus booster (Tdap or Td) vaccines adolescents received, the timing of vaccine delivery, or the presence of existing conditions that may contraindicate vaccination. We also did not collect information on some constructs that may be important to vaccination behaviors or specific reasons why daughters had not received all vaccines. Since we interviewed parents from only one geographic region who had a landline telephone and spoke English, the generalizability of the findings to populations that have different characteristics is not yet known.
Vaccination coverage among adolescent females was suboptimal, with few parents indicating their daughters had received all three recommended adolescent vaccines: tetanus booster, meningococcal and HPV vaccines. Ensuring annual preventive care visits and reducing missed opportunities for vaccination at existing visits, perhaps by increasing concomitant administration of adolescent vaccines, may help increase vaccine uptake.
Materials and Methods
Study design.
This study is part of the Carolina HPV Immunization Measurement and Evaluation (CHIME) Project that investigated HPV vaccine decision making in an area where women are at relatively high risk of invasive cervical cancer. In one component of the CHIME Project, caregivers of adolescent females participated in a two-phase longitudinal study described in detail in references 20 and 21. This report includes crosssectional data from the follow-up interviews, since data on adolescent vaccines (other than HPV vaccine) were not collected at baseline.
In brief, eligible counties in North Carolina, US had (1) rates of invasive cervical cancer substantially higher than the national average (i.e., incidence >10 cases/100,000 women annually from 1993–2003 and mortality >4 cases/100,000 women annually from 1994–2004), (2) 20% or more African American residents and (3) at least 1,500 girls in the targeted age range of 10–18 years (to allow for a minimum number of caregivers). We selected four rural counties (Duplin, Harnett, Sampson and Wayne) and a fifth urban county (Cumberland) that were geographically clustered in the southeastern part of the state.Citation20
In these five counties, trained interviewers from the University of North Carolina Survey Research Unit contacted a probability sample of households using random-digit-dialing (5%) or a non-overlapping targeted-list frame of directory-listed residential telephone numbers with available recent household demographic information (95%). They oversampled households likely to contain an adolescent female in the targeted age range of 10 to 18 years old, households likely to be African American and rural telephone exchanges.Citation37
Trained personnel used computer-assisted telephone interviewing equipment to interview parents, grandparents, or any other individual who self-identified as being responsible for the adolescent's care. Interviewers attempted to speak with female caregivers, if possible, but interviewed male caregivers if a female caregiver was unavailable. Interviewers prioritized female caregivers as they may be more knowledgeable about their daughters' vaccination histories. We refer to caregivers as parents throughout the paper. If a household contained more than one 10–18 year-old female, interview software randomly selected one as the index child for the interviews. Interviewers conducted baseline interviews between July and October 2007 and follow-up interviews between October and November 2008. Parents gave verbal consent for the study and received $10 US for their participation in each survey. The Institutional Review Board at the University of North Carolina approved the study.
Measures.
The follow-up survey collected data on uptake of three vaccines recommended for adolescent females:Citation12 meningococcal vaccine (MCV4 or MPSV4), tetanus booster vaccine (Tdap or Td) and HPV vaccine. For meningococcal vaccine, interviewers asked parents, “Has [name] received a meningitis shot, sometimes called Menactra or Menomune?” The survey assessed tetanus booster vaccination using the item, “Has [name] received a tetanus booster, also called Td or Tdap shot? Tetanus boosters are given every 10 years. The first booster is usually given around 11 or 12 years of age.” For HPV vaccine, interviewers asked parents, “Has [name] had any shots of the HPV vaccine?” Interviewers also provided parents with alternate names for HPV vaccine (e.g., cervical cancer vaccine) at the beginning of the survey. While we acknowledge that three doses of HPV vaccine may be required for full vaccine effectiveness, we focus on HPV vaccine initiation (one or more doses received) in this report.
For each vaccine, we classified daughters as “vaccinated” (parents responded “yes” to the appropriate vaccination item) or “not vaccinated” (parents responded “no” or “don't know” to the appropriate vaccination item). We also counted how many of these three recommended adolescent vaccines each daughter had received (possible range = 0–3). Interviewers collected information on various demographic and health-related factors, which we examined as potential correlates of vaccination. We based urbanicity on the census block where the parent was living.Citation37
Data analysis.
The primary outcome was a four-level ordinal variable indicating how many of the three vaccines (tetanus booster, meningococcal and at least one dose of HPV vaccine) daughters had received. We used ordinal logistic regression models to identify bivariate correlates. We then entered statistically significant bivariate correlates (p < 0.05) into a multivariate ordinal logistic regression model. Analyses met the assumption of proportional odds using methods described by Hosmer and Lemeshow.Citation38
In subsequent analyses, we examined tetanus booster vaccination, meningococcal vaccination and HPV vaccination as additional separate outcomes using binary logistic regression. For each outcome, we constructed models using the previously described procedure, though we report only multivariate findings to conserve space. All analyses were unweighted and conducted using SPSS 17.0 (Chicago, IL) and Intercooled Stata Version 11.0 (College Station, TX). Statistical tests were two-tailed using a critical alpha of 0.05.
Abbreviations
| MCV4 | = | meningococcal conjugate vaccine |
| MPSV4 | = | meningococcal polysaccharide vaccine |
| Tdap | = | tetanus, diphtheria and acellular pertussis vaccine |
| Td | = | tetanus and diphtheria toxoids vaccine |
| HPV | = | human papillomavirus |
| ACIP | = | Advisory Committee on Immunization Practices |
| CHIME Project | = | Carolina HPV Immunization Measurement and Evaluation Project |
| OR | = | odds ratio |
| NIS-Teen | = | National Immunization Survey-Teen |
| CI | = | confidence interval |
| ref. | = | referent group |
Figures and Tables
Figure 1 Adolescent vaccines received as reported by parents (n = 647). Figure does not show 51 parents (8%) who reported their daughters had not received any of these vaccines. Tetanus booster vaccine includes tetanus, diphtheria and acellular pertussis (Tdap) vaccine or tetanus and diphtheria toxoids (Td) vaccine. Meningococcal vaccine includes meningococcal conjugate vaccine (MCV4) or meningococcal polysaccharide vaccine (MPS V4).
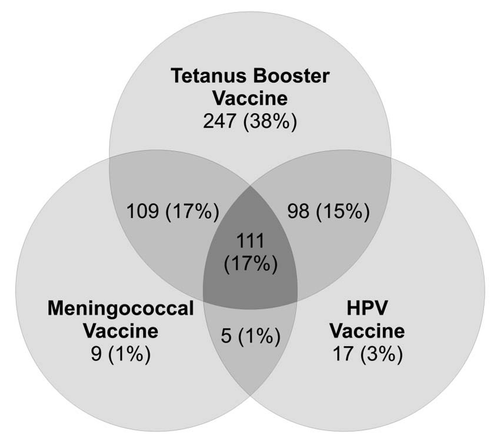
Table 1 Characteristics of parents and their daughters in the follow-up study (n = 647)
Table 2 Correlates of receiving recommended adolescent vaccines (tetanus booster,Table Footnotea meningococcal,Table Footnoteb and HPV vaccines) (n = 647)
Table 3 Correlates of receiving each recommended adolescent vaccine (n = 647)
Acknowledgements
This study was funded by the Centers for Disease Control and Prevention (S3715-25/25), the American Cancer Society (MSRG-06-259-01-CPPB), and the Cancer Control Education Program at Lineberger Comprehensive Cancer Center (Grant No. R25 CA57726). Although we do not believe we have any conflicts of interest, we wish to share the following information in the interest of full disclosure. Authors have received research grants from Merck & Co., Inc. (N.B. and P.R.) and GlaxoSmithKline (N.B.), but neither has received honoraria or consulting fees from these companies. These funds were not used to support this research study.
References
- Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med 2006; 31:52 - 61
- UNICEF and WHO. Immunization summary 2006 2006; Available at: http://www.unicef.org/publications/files/Immunization_Summary_2006.pdf
- Centers for Disease Control and Prevention. Summary of notifiable diseases-United States 2007. MMWR 2007; 56
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases 2008; Atlanta GA Centers for Disease Control and Prevention
- Hitchcock WP. Rationale for use of Tdap booster vaccines for adolescent immunization: overview of efficacy, safety and clinical use. Clin Pediatr (Phila) 2006; 45:785 - 794
- Bilukha OO, Rosenstein N. National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54:1 - 21
- Bilukha O, Messonnier N, Fischer M. Use of meningococcal vaccines in the United States. Pediatr Infect Dis J 2007; 26:371 - 376
- Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates 2000. Perspect Sex Reprod Health 2004; 36:6 - 10
- Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 2008; 113:3036 - 3046
- Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, et al. Preventing tetanus, diphtheria and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1 - 34
- Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1 - 24
- Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0–18 years—United States 2008. MMWR 2008; 57:1 - 4
- Centers for Disease Control and Prevention (CDC). National, state and local area vaccination coverage among adolescents aged 13–17 years—United States 2008. MMWR Morb Mortal Wkly Rep 2009; 58:997 - 1001
- Department of Health and Human Services. Healthy People 2010; 1:14 Immunization and Infectious Diseases. Available at: http://www.healthypeople.gov/document/pdf/Volume1/14Immunization.pdf.
- Gottlieb SL, Brewer NT, Sternberg MR, et al. Human papillomavirus vaccine initiation in an area with elevated rates of cervical cancer. J Adolesc Health 2009; 45:430 - 437
- Chao C, Slezak JM, Coleman KJ, Jacobsen SJ. Papanicolaou screening behavior in mothers and human papillomavirus vaccine uptake in adolescent girls. Am J Public Health 2009; 99:1137 - 1142
- Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents' health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med 2009; 69:475 - 480
- Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. J Adolesc Health 2009; 45:453 - 462
- Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination and self-reported barriers to vaccination. J Womens Health (Larchmt) 2009; 18:1679 - 1686
- Hughes J, Cates JR, Liddon N, Smith JS, Gottlieb SL, Brewer NT. Disparities in how parents are learning about the human papillomavirus vaccine. Cancer Epidemiol Biomarkers Prev 2009; 18:363 - 372
- Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of HPV vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2010; In press
- North Carolina Immunization Branch. Immunize North Carolina Available at: http://www.immunizenc.com/Default.htm. Updated 2009.
- Lee GM, Lorick SA, Pfoh E, Kleinman K, Fishbein D. Adolescent immunizations: missed opportunities for prevention. Pediatrics 2008; 122:711 - 717
- Middleman AB. Adolescent immunizations: policies to provide a shot in the arm for adolescents. J Adolesc Health 2007; 41:109 - 118
- Briss PA, Rodewald LE, Hinman AR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents and adults. The Task Force on Community Preventive Services. Am J Prev Med 2000; 18:97 - 140
- Jacobson VJ, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev 2005; 3941
- Tierney CD, Yusuf H, McMahon SR, et al. Adoption of reminder and recall messages for immunizations by pediatricians and public health clinics. Pediatrics 2003; 112:1076 - 1082
- American Medical Association. Guidelines for Adolescent Preventive Services (GAPS). Recommendations Monograph 1997;
- Rosen DS, Elster A, Hedberg V, Paperny D. Clinical preventive services for adolescents: position paper of the Society for Adolescent Medicine. J Adolesc Health 1997; 21:203 - 214
- Daley MF, Curtis CR, Pyrzanowski J, et al. Adolescent immunization delivery in school-based health centers: a national survey. J Adolesc Health 2009; 45:445 - 452
- Madlon-Kay DJ, Harper PG. Too many shots? Parent, nurse and physician attitudes toward multiple simultaneous childhood vaccinations. Arch Fam Med 1994; 3:610 - 613
- Woodin KA, Rodewald LE, Humiston SG, Carges MS, Schaffer SJ, Szilagyi PG. Physician and parent opinions. Are children becoming pincushions from immunizations?. Arch Pediatr Adolesc Med 1995; 149:845 - 849
- Melman ST, Nguyen TT, Ehrlich E, Schorr M, Anbar RD. Parental compliance with multiple immunization injections. Arch Pediatr Adolesc Med 1999; 153:1289 - 1291
- Enger KS, Stokley S. Meningococcal conjugate vaccine uptake, measured by Michigan's immunization registry. J Adolesc Health 2007; 40:398 - 404
- Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16 and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis 2009; 200:1059 - 1067
- Shinall MC Jr, Plosa EJ, Poehling KA. Validity of parental report of influenza vaccination in children 6 to 59 months of age. Pediatrics 2007; 120:783 - 787
- US Census Bureau. Census glossary Available at: http://factfinder.census.gov/home/en/epss/glossary_a.html.
- Hosmer DW, Lemeshow S. Applied Logistic Regression 2000; Second Ed. New York John Wiley & Sons Inc