Abstract
Schistosomiasis is a major neglected tropical disease of public health importance to a billion people. An estimated 200 million people are currently infected; an additional 779 million individuals are at risk to acquire the infection in 74 countries. Despite many years of implementation of mass anti-parasitic drug therapy programs and other control measures, this disease has not been contained and continues to spread to new geographic areas. The discovery of a protective vaccine still remains the most potentially effective means for the control of this disease, especially if the vaccine provides long-term immunity against the infection. A vaccine would contribute to the reduction of schistosomiasis morbidity through induced immune responses leading to decrease in parasite load and reduced egg production. This vaccine could be administered to children between the ages of 3 and 12 years to prevent severe infection in a particularly high risk population. This review summarizes the current status of schistosomiasis vaccine development.
Introduction
The human parasitic disease schistosomiasis is caused by intravascular diacious digenetic trematodes of the genus Schistosoma. Schistosoma spp are responsible for several distinct infections. The first step in human infection involves penetration of the skin by infectious larvae (cercariae) which come out from a freshwater intermediate snail host. Inside the human host, cercariae develop into juvenile worms or schistosomula, undergo a complex migration and finally develop into sexually mature egg-laying worms in the hepatic portal system. The eggs are passed out from the human body in feces or urine, depending on the species of the parasite. The eggs hatch on contact with water and release free-swimming larvae (miracidia) which infect the intermediate snail host. This chronic infection can persist for decades causing disease of intestinal or urinogenital system. Intestinal schistosomiasis is caused by Schistosoma mansoni and occurs in Africa, the Eastern Mediterranean, the Caribbean and South America. In addition, S. intercalatum causes a form of intestinal schistosomiasis that has been reported in central African countries. Yet another form of intestinal schistosomiasis, known as Oriental or Asiatic, is caused by the S. japonicum group of parasites (including S. mekongi in the Mekong river basin). S. japonicum is endemic to South-East Asia and the Western Pacific region. Finally, S. hematobium is responsible for urinary schistosomiasis and is endemic to Africa and the Eastern Mediterranean. Cumulatively, schistosomiasis is a significant cause of morbidity for an estimated 200 million people with an additional 779 million individuals at risk for infection.Citation1,Citation2 Approximately 10% of infected individuals (21 million people) suffer from severe hepatic periportal fibrosis, which can lead to portal hypertension, hepatosplenomegaly and esophageal varices and is the leading cause of death from schistosomiasis.Citation3 Even mild to moderate fibrosis is correlated with poor nutritional status, anemia, fatigue, impaired cognitive ability, and increased susceptibility to infection with pathogens, such as HIV-1.Citation4-Citation6 Certain estimates of disability adjusted life years (up to 70 million disability adjusted life years per annum) have shown schistosomiasis to be a more debilitating infection than even malaria.Citation4-Citation6
Current Control Strategies
Schistosomiasis and diseases caused by soil-transmitted helminths have been targeted for eradication by the World Health Organization, and the World Health Assembly, resolution 54.19 (WHA 54.19). Despite global efforts to reduce disease through chemotherapy via praziquantel (a drug developed in 1970s), infection rates continue to be high in endemic regions and overall prevalence either remains unchanged or has increased since the introduction of global efforts to eradicate helminth infections.Citation1,Citation4 The procurement at low cost and continuous supply of praziquantel (PZQ) for repeated doses by all those at risk of schistosomiasis remains a lingering concern.Citation7,Citation8 Even though schistosomiasis and geohelminths inflict a devastating toll on the inhabitants of a large number of underdeveloped countries, national control programs have not been instituted in many endemic countries. It has been estimated that 1–2 billion PZQ tablets will be required to treat the 400 million people per year in sub-Saharan Africa alone for at least 5 y at a cost of $US 100 million.Citation4 Due to the high cost, lack of infrastructure and other logistical problems in many areas of the developing world, treatment with PZQ has primarily been restricted to children attending school. However, enrollment in school is usually a fraction of all school-aged children, resulting in large numbers of untreated children.Citation6,Citation7 Furthermore, even if the infrastructure for repeated PZQ doses were developed and maintained, history tells us that intensive PZQ treatment fails to eliminate disease. In Kenya, a well-characterized cohort has been treated since 1995, in some cases up to 25 times and still disease transmission persists in a relatively small area of Lake Victoria.Citation9 PZQ-based morbidity control for schistosomiasis has been useful but there are distinct disadvantages associated with this strategy. These include little impact on the reduction of disease transmission and the inherent danger of development of large scale drug resistance.Citation10-Citation12 Potential emergence of drug resistance makes long-term planning solely based on PZQ uncertain. In addition, PZQ cannot provide protection from re-infection.Citation13,Citation14 Other efforts to reduce the impact of schistosomiasis in endemic regions have focused on control of intermediate snail hosts and improved sanitation, with minimal success.Citation6 Calls for using limited funds to develop multi-faceted programs that integrate chemotherapy with vaccines need to be answered.Citation15 There is now general agreement among experts in the field that durable and sustained reduction in the disease spectrum and transmission can only be obtained through long-term protection via vaccination linked with chemotherapy.Citation10,Citation15,Citation16 An effective anti-schistosome vaccine would contribute greatly to a decrease in morbidity associated with schistosomiasis via protective immune responses leading to reduced worm burdens and decreased egg production.Citation10,Citation12,Citation15-Citation18
Is a vaccine possible for a complex helminth like schistosomes?
Diseases caused by different infectious disease pathogens for which no effective vaccines exist, such as HIV, malaria, tuberculosis and parasitic helminths remain uncontrolled.Citation19 Attempts at developing vaccines for these diseases have proved challenging for a number of reasons.Citation19 Obstacles that must be overcome include the need for the elicitation of an immune response greater than that induced by natural infection as well as an understanding of mechanisms of protective immunity in humans. Vaccine development against parasitic helminths in particular has been hampered by a poor understanding of the complex interactions between the human host and parasites, including the mechanisms behind protective immunity.
An effective vaccine against a complex metazoan schistosome parasite can be developed. This belief is based on the following: (1) the immunization of mice with one dose of irradiated cercariae results in 50% - 70% reduction in adult worm burden which can be increased to over 80% with two or three immunizationsCitation20; (2) in non-permissive animal models (e.g., rats and rhesus macaques), worm elimination proceeds via a coordinated immune response by the hostCitation21,Citation22; and (3) human populations following exposure in endemic areas invariably develop some degree of protection naturally.Citation23,Citation24 Moreover, this belief is strongly reinforced by the successful introduction of several effective anti-parasite vaccines (both against protozoa and helminths) in veterinary practice.Citation25
Vaccinology for human helminth infections is defined by a paradigm that differs from that for bacterial or viral infections. Namely, sterile immunity is likely not achievable, but immunotherapy should confer sufficient levels of protection to reduce worm burdens to an intensity associated with healthy growth and development of children.Citation26 A vaccine that would limit cercariae penetration and/or adult worm maturation would reduce egg accumulation, and thus, control pathology, the major source of morbidity. Additionally, the prevailing opinion is that a vaccine that confers an initial 50% protection in humans should be effective in reducing overall morbidity and mortality,Citation10,Citation15,Citation27 and in all likelihood would be an appropriate first generation schistosomiasis vaccine.Citation28
Possible mechanisms of protection in animal models and humans
The mechanism of protection (elimination of adult worms) in the mouse model has been well studied using multitudes of irradiated cercarial vaccine regimens and the topic has been extensively reviewed.Citation29,Citation30 Briefly, this immune mediated protection appears to be a highly orchestrated series of events starting in the skin, involving draining lymph nodes and lungs where most larvae are killed, 3 weeks post-challenge.Citation30,Citation31 Vaccination with irradiated cercariae results in the development of various effector responses that can range from Th1-type cell-mediated response to parasite-specific antibodies–all of these responses play some role in the immune killing of worms.Citation29,Citation30,Citation32
In humans, evidence from multiple studies documents an age-acquired resistance to re-infection in older children and adults.Citation5,Citation33-Citation35 Individuals living in endemic communities display a characteristic left-skewed convex distribution by age of prevalence and intensity of infection for schistosomes.Citation36,Citation37 Factors predictive of resistance in multiple immuno-epidemiologic studies include a high concentration of serum parasite-specific IgE, increased circulating CD23+ B cells, eosinophilia, and secretion of IL-5 in response to crude worm extracts.Citation9,Citation38-Citation41 However, whether any of these immune responses is directly involved in worm killing has not been elucidated and there are too few reports that delve into the mechanistic aspects of protective immunity.
Vaccine Candidates and Search of Functionally Important Vaccine Candidates
More than 100 schistosome antigens have been identified, of which over a quarter have shown some level of protection in the murine model of schistosomiasis. The list of antigens and associated prophylactic efficacy has been extensively reviewed in the last few years.Citation10,Citation12,Citation16,Citation27,Citation42-Citation45 From all of these antigens, only one S. hematobium antigen, Sh-28-GST, has advanced into clinical trials, though this occurred over a decade ago and detailed findings are not available.Citation46 Another important schistosome antigen, Sm-14, a fatty acid binding protein has also been developed for clinical trials.Citation47,Citation48
It is our belief that functionally important antigens will serve as appropriate targets for a schistosome vaccine. This is because schistosomes interact closely with their host, performing functions such as immune evasion, nutrient uptake and attachment. We believe that host-exposed schistosome proteins that undertake such essential functions will be effective targets for a schistosomiasis vaccine.Citation16 In this regard, our group has targeted a host interactive schistosome protein, calpain (Sm-p80) which plays an important role in the surface membrane renewal of schistosomes, a phenomenon which is widely considered to be a mechanism employed by hemo-helminths to evade host immunity.Citation49
We believe that a systematic and methodical approach is critical toward developing a vaccine for schistosomiasis. Over the past 20 y, we have attempted to follow this strategy toward developing Sm-p80 into a viable vaccine candidate. At present, to our knowledge, Sm-p80 is the sole schistosome vaccine candidate that has been tested for its prophylactic, antifecundity and therapeutic efficacy in different vaccine formulations and approaches (e.g., naked DNA alone; recombinant protein with adjuvants; and prime with DNA, followed by boosting with protein plus adjuvants) in two experimental animal models (mouse and baboon) of infection and disease.Citation16,Citation50-Citation62 Furthermore, the validity of Sm-p80 as a viable vaccine candidate has been reinforced by the work of five “research groups” who have independently demonstrated reproducible and consistent protective efficacy in mice following challenge infection using calpain or its peptides as an antigen (Nagoya City University Medical School, Nagoya, JapanCitation63,Citation64; The John P. Robarts Research Institute and University of Western Ontario, London, CanadaCitation65,Citation66; Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MDCitation67; Cairo University, Cairo, EgyptCitation68,Citation69; Texas Tech University Health Sciences Center, Lubbock, TXCitation50-Citation62).
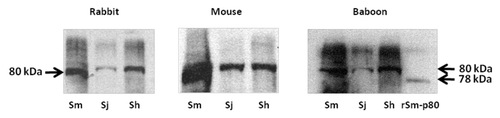
Sm-p80-based vaccine formulations have three protective effects: worm reduction, antifecundity effect, and protection against acute schistosomiasis.Citation50-Citation62 Some of the recent data generated by our group also demonstrate therapeutic effects of Sm-p80-based vaccine via killing of established adult worms in the nonhuman primate host. Protection and antifecundity levels recorded with Sm-p80 vaccine formulation(s) in both mice and baboons are approaching levels previously recorded only with the irradiated cercarial vaccine approach. Specifically, worm reductions of up to 70% in mice and up to 60% in baboons have been demonstrated, using recombinant protein formulations containing toll-like receptor (TLR) agonists (TLR4, TLR7/8 and TLR9). Complete elimination of egg-induced organ pathology has been achieved using Sm-p80-based vaccine with 100% reductions in liver/intestine egg counts in both mice and baboons. Human correlate studies have revealed Sm-p80-reactivity with IgG in human sera from schistosome infected individuals. Additionally, lack of prevailing Sm-p80-specific IgE in a high risk/infected population was observed, thus minimizing the risk of hypersensitivity reaction following vaccination with Sm-p80 in humans. Proof-of-concept studies in rodent and nonhuman primate animal models have been completed. Sm-p80 protein has successfully been made in 50 L fermenters, purified to homogeneity with high yields and with Food and Drug Administration acceptable endotoxin levels by PREMAS Biotech Pvt. Ltd. (Gurgaon, India), with reproducible efficacy in animals as observed using the protein generated in the laboratory. Based on these data, it is our assessment that Sm-p80, in a recombinant protein formulation, is now ready to be moved into the next phase, i.e., scale up and cGMP production in preparation for Phase I/II human clinical trials. Overall, we believe that calpain has a great potential as an important vaccine antigen for the reduction of the morbidity associated with both S. mansoni and S. japonicumCitation63,Citation64,Citation70 infections. The immunobiological goals of a Sm-p80-based vaccine would be blocking an essential parasite function via IgG. Furthermore, our preliminary studies indicate that calpain may also be useful for S. hematobium infection ().
Figure 1. Western blot analyses of three species of schistosomes. Parasite extracts of Schistosoma mansoni (Sm), S. japonicum (Sj) and S. hematobium (Sh) were separated via polyacrylaimde gel electrophoresis and resultant immunoblots were probed with (first panel) rabbit polyclonal antibody raised by using peptide of S. mansoni calpain (position 737–758); (second panel) mouse sera obtained from animals vaccinated with Sm-p80; (third panel) sera from baboons immunized with Sm-p80. S. hematobium calpain (Sh-p80) has 95% and S. japonicum calpain (Sj-p80) 85% sequence homology with S. mansoni calpain (Sm-p80). This preliminary study indicates that immunogenic epitopes of Sm-p80 which may play a role in vaccinemediated protection may be conserved in the three species of schistosomes. Thus Smp80 based vaccine formulation may have potential for cross-species protection.
Another important vaccine candidate in this regard includes the tetraspanins (TSP-1/2), which are present on the apical syncytial surface of S. mansoni and have been identified and used in a defined vaccine formulation that upon administration provided protection ranging from 29–61% and reduction in egg burden from 50–61% in mice.Citation71 It is speculated that, like their human counterparts, TSP-1/2 are involved in cell-cell interactions and maintenance of membrane integrity,Citation71 and may thus be functionally important antigens as vaccine candidates.Citation43,Citation72 Recently, though it has been reported that TSP-2, is not a reliable target for S. japonicum.Citation73
Conclusions and Future Directions
Unquestionably, the development of an effective, protective schistosomiasis vaccine would be of immense public health importance. This vaccine can be administered to children in order to prevent severe infection in the following years of high risk (3–12 y of age). This age group of children and young adolescents correspond to those ages in which contact with infected water is maximal. Booster doses of schistosomiasis vaccine may not be necessary since subsequent exposure to infective larvae could provide continuous re-stimulation to immunity. Such a vaccine would greatly reduce the need for logistically difficult and expensive drug-based programs and will save millions of lives. There is a very good likelihood of having a starter vaccine by the next decade. It is also important to realize that the most appropriate clinical endpoint for vaccine efficacy is reduction in morbidity associated with schistosomiasis. To this effect, emphasis should also be placed on exploring the therapeutic potential of antigens in addition to the conventional prophylactic and antifecundity efficacy estimations. Obviously, further research is required on the development of novel adjuvant vehicles as well as cocktail vaccine formulations to enhance protection levels with the eventual aim of 100% worm reduction. Furthermore, it would be sagacious if partly characterized antigens are not rushed pre-maturely into clinical trials without first testing their prophylactic and therapeutic potential in both rodent and nonhuman primate systems. There are several reasons for this cautious approach. For example, Lebens et al.Citation18 have pointed out, and we concur, that some of the proposed vaccination strategies based on studies performed only in the mouse model could have undesirable effects in some individuals if taken to human clinical trials. This is partly because of a developing paradigm which suggests that mechanisms of protection in the permissive mouse model of schistosomiasis cannot completely be generalized to human protection. This belief is based on the failure to know exactly whether a successful schistosome vaccine should induce Th1 or Th2 or both responses that will result in acceptable protection/resistance. Overall, while schistosomiasis represents a major public health burden in areas of the world least-equipped to shoulder it, the future is bright for the development of a vaccine to combat this disease, but more funding and resources should be dedicated to these efforts.
Acknowledgments
This work is supported by a grant from the NIAID/NIH (R01AI071223) and Thrasher Research Fund (Award No. 02824–5) to Afzal A. Siddiqui; and NIAID/NIH (R21AI074843) grant to Lisa Ganley-Leal. We also thank Dr Fred Lewis for providing S. japonicum and S. hematobium worms that were used for the study. We appreciate the efforts of Dr. Gul Ahmad and Afzal Ahrorov for making the figure. We thank Dr. David Straus for critically reviewing the manuscript and for his thoughtful comments.
Reference
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet 2006; 368:1106 - 18; http://dx.doi.org/10.1016/S0140-6736(06)69440-3; PMID: 16997665
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med 2007; 357:1018 - 27; http://dx.doi.org/10.1056/NEJMra064142; PMID: 17804846
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 2006; 6:411 - 25; http://dx.doi.org/10.1016/S1473-3099(06)70521-7; PMID: 16790382
- Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis 2010; 10:733 - 6; http://dx.doi.org/10.1016/S1473-3099(10)70099-2; PMID: 20705513
- Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, et al. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet 2002; 360:592 - 6; http://dx.doi.org/10.1016/S0140-6736(02)09781-7; PMID: 12241930
- King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop 2010; 113:95 - 104; http://dx.doi.org/10.1016/j.actatropica.2009.11.012; PMID: 19962954
- Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de SN et al. Helminth Infections: Soil-transmitted Helminth Infections and Schistosomiasis. 2006.
- Hotez PJ, Engels D, Fenwick A, Savioli L. Africa is desperate for praziquantel. Lancet 2010; 376:496 - 8; http://dx.doi.org/10.1016/S0140-6736(10)60879-3; PMID: 20709217
- Black CL, Muok EM, Mwinzi PN, Carter JM, Karanja DM, Secor WE, et al. Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. J Infect Dis 2010; 202:399 - 405; http://dx.doi.org/10.1086/653828; PMID: 20560767
- Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis 2008; 2:e244; http://dx.doi.org/10.1371/journal.pntd.0000244; PMID: 18575619
- Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, Botros S, et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology 2009; 136:1825 - 35; http://dx.doi.org/10.1017/S0031182009000493; PMID: 19281637
- McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev 2008; 21:225 - 42; http://dx.doi.org/10.1128/CMR.00046-07; PMID: 18202444
- Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, et al. The genome of the blood fluke Schistosoma mansoni. Nature 2009; 460:352 - 8; http://dx.doi.org/10.1038/nature08160; PMID: 19606141
- James CE, Hudson AL, Davey MW. An update on P-glycoprotein and drug resistance in Schistosoma mansoni. Trends Parasitol 2009; 25:538 - 9; http://dx.doi.org/10.1016/j.pt.2009.09.007; PMID: 19850522
- Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach?. Trends Parasitol 2005; 21:112 - 7; http://dx.doi.org/10.1016/j.pt.2005.01.001; PMID: 15734657
- Siddiqui AA, Ahmad G, Damian RT, Kennedy RC. Experimental vaccines in animal models for schistosomiasis. Parasitol Res 2008; 102:825 - 33; http://dx.doi.org/10.1007/s00436-008-0887-6; PMID: 18259777
- Hagan P, Sharaf O. Schistosomiasis vaccines. Expert Opin Biol Ther 2003; 3:1271 - 8; http://dx.doi.org/10.1517/14712598.3.8.1271; PMID: 14640953
- Lebens M, Sun JB, Czerkinsky C, Holmgren J. Current status and future prospects for a vaccine against schistosomiasis. Expert Rev Vaccines 2004; 3:315 - 28; http://dx.doi.org/10.1586/14760584.3.3.315; PMID: 15176948
- Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 2011; 473:463 - 9; http://dx.doi.org/10.1038/nature10124; PMID: 21614073
- Smythies LE, Betts C, Coulson PS, Dowling MA, Wilson RA. Kinetics and mechanism of effector focus formation in the lungs of mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasite Immunol 1996; 18:359 - 69; http://dx.doi.org/10.1046/j.1365-3024.1996.d01-115.x; PMID: 9229389
- Cutts L, Wilson RA. Elimination of a primary schistosome infection from rats coincides with elevated IgE titres and mast cell degranulation. Parasite Immunol 1997; 19:91 - 102; http://dx.doi.org/10.1046/j.1365-3024.1997.d01-184.x; PMID: 9076811
- Wilson RA, Langermans JA, Van Dam GJ, Vervenne RA, Hall SL, Borges WC, et al. Elimination of Schistosoma mansoni Adult Worms by Rhesus Macaques: Basis for a Therapeutic Vaccine?. PLoS Negl Trop Dis 2008; 2:e290; http://dx.doi.org/10.1371/journal.pntd.0000290; PMID: 18820739
- Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 1991; 349:243 - 5; http://dx.doi.org/10.1038/349243a0; PMID: 1898985
- Hagan P. Reinfection, exposure and immunity in human schistosomiasis. Parasitol Today 1992; 8:12 - 6; http://dx.doi.org/10.1016/0169-4758(92)90303-J; PMID: 15463518
- Meeusen EN, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. [table.] Clin Microbiol Rev 2007; 20:489 - 510; http://dx.doi.org/10.1128/CMR.00005-07; PMID: 17630337
- Olson CL, Acosta LP, Hochberg NS, Olveda RM, Jiz M, McGarvey ST, et al. Anemia of inflammation is related to cognitive impairment among children in Leyte, the Philippines. PLoS Negl Trop Dis 2009; 3:e533; http://dx.doi.org/10.1371/journal.pntd.0000533; PMID: 19847303
- Bergquist R. Prospects of vaccination against schistosomiasis. Scand J Infect Dis Suppl 1990; 76:60 - 71; PMID: 2102021
- Todd CW, Colley DG. Practical and ethical issues in the development of a vaccine against schistosomiasis mansoni. Am J Trop Med Hyg 2002; 66:348 - 58; PMID: 12164288
- Bickle QD. Radiation-attenuated schistosome vaccination–a brief historical perspective. Parasitology 2009; 136:1621 - 32; http://dx.doi.org/10.1017/S0031182009005848; PMID: 19327194
- Hewitson JP, Hamblin PA, Mountford AP. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol 2005; 27:271 - 80; http://dx.doi.org/10.1111/j.1365-3024.2005.00764.x; PMID: 16138848
- Kumar P, Ramaswamy K. Vaccination with irradiated cercariae of Schistosoma mansoni preferentially induced the accumulation of interferon-gamma producing T cells in the skin and skin draining lymph nodes of mice. . Parasitol Int. 1999; 48:109 - 19; http://dx.doi.org/10.1016/S1383-5769(99)00008-2; PMID: 11269272
- Jankovic D, Wynn TA, Kullberg MC, Hieny S, Caspar P, James S, et al. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. J Immunol 1999; 162:345 - 51; PMID: 9886405
- Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, et al. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol 1992; 22:1483 - 94; http://dx.doi.org/10.1002/eji.1830220622; PMID: 1601036
- Dunne DW, Butterworth AE, Fulford AJ, Ouma JH, Sturrock RF. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem Inst Oswaldo Cruz 1992; 87:Suppl 4 99 - 103; http://dx.doi.org/10.1590/S0074-02761992000800014; PMID: 1343933
- Pinot de Moria A, Fulford AJ, Kabatereine NB, Ouma JH, Booth M, Dunne DW. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: the influence of age, sex, ethnicity and IgE. PLoS Negl Trop Dis 2010; 4:e820; http://dx.doi.org/10.1371/journal.pntd.0000820; PMID: 20856909
- Kurtis JD, Friedman JF, Leenstra T, Langdon GC, Wu HW, Manalo DL, et al. Pubertal development predicts resistance to infection and reinfection with Schistosoma japonicum. Clin Infect Dis 2006; 42:1692 - 8; http://dx.doi.org/10.1086/504326; PMID: 16705573
- Olds GR. New insights into the observed age-specific resistance to reinfection with Schistosoma japonicum. Clin Infect Dis 2006; 42:1699 - 701; http://dx.doi.org/10.1086/504331; PMID: 16705574
- Butterworth AE, Dunne DW, Fulford AJ, Thorne KJ, Gachuhi K, Ouma JH, et al. Human immunity to Schistosoma mansoni: observations on mechanisms, and implications for control. Immunol Invest 1992; 21:391 - 407; http://dx.doi.org/10.3109/08820139209069381; PMID: 1428017
- Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, Andove J, Hightower AW, Karanja DM, et al. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun 2006; 74:2169 - 76; http://dx.doi.org/10.1128/IAI.74.4.2169-2176.2006; PMID: 16552047
- Leenstra T, Acosta LP, Wu HW, Langdon GC, Solomon JS, Manalo DL, et al. T-helper-2 cytokine responses to Sj97 predict resistance to reinfection with Schistosoma japonicum. Infect Immun 2006; 74:370 - 81; http://dx.doi.org/10.1128/IAI.74.1.370-381.2006; PMID: 16368992
- Mwinzi PN, Ganley-Leal L, Black CL, Secor WE, Karanja DM, Colley DG. Circulating CD23+ B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments. J Infect Dis 2009; 199:272 - 9; http://dx.doi.org/10.1086/595792; PMID: 19072134
- Bergquist R, Utzinger J, Chollet J, Shu-Hua X, Weiss NA, Tanner M. Triggering of high-level resistance against Schistosoma mansoni reinfection by artemether in the mouse model. Am J Trop Med Hyg 2004; 71:774 - 7; PMID: 15642970
- Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, Loukas A, et al. Vaccines to combat the neglected tropical diseases. Immunol Rev 2011; 239:237 - 70; http://dx.doi.org/10.1111/j.1600-065X.2010.00976.x; PMID: 21198676
- Capron A, Dessaint JP, Capron M, Pierce RJ. Vaccine strategies against schistosomiasis. Mem Inst Oswaldo Cruz 1992; 87:Suppl 4 19 - 27; http://dx.doi.org/10.1590/S0074-02761992000800003; PMID: 1343894
- Hagan P. Schistosomiasis–a rich vein of research. Parasitology 2009; 136:1611 - 9; http://dx.doi.org/10.1017/S003118200999093X; PMID: 19691867
- Capron A, Riveau G, Capron M, Trottein F. Schistosomes: the road from host-parasite interactions to vaccines in clinical trials. Trends Parasitol 2005; 21:143 - 9; http://dx.doi.org/10.1016/j.pt.2005.01.003; PMID: 15734662
- Tendler M, Vilar MM, Brito CA, Freire NM, Katz N, Simpson A. Vaccination against schistosomiasis and fascioliasis with the new recombinant antigen Sm14: potential basis of a multi-valent anti-helminth vaccine?. Mem Inst Oswaldo Cruz 1995; 90:255 - 6; http://dx.doi.org/10.1590/S0074-02761995000200022; PMID: 8531667
- Tendler M, Simpson AJ. The biotechnology-value chain: development of Sm14 as a schistosomiasis vaccine. Acta Trop 2008; 108:263 - 6; http://dx.doi.org/10.1016/j.actatropica.2008.09.002; PMID: 18834847
- Siddiqui AA, Zhou Y, Podesta RB, Karcz SR, Tognon CE, Strejan GH, et al. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta 1993; 1181:37 - 44; PMID: 8457603
- Ahmad G, Zhang W, Torben W, Haskins C, Diggs S, Noor Z, et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res 2009; 105:1767 - 77; http://dx.doi.org/10.1007/s00436-009-1646-z; PMID: 19809833
- Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80-based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunol 2009; 31:156 - 61; http://dx.doi.org/10.1111/j.1365-3024.2008.01091.x; PMID: 19222788
- Ahmad G, Zhang W, Torben W, Damian RT, Wolf RF, White GL, et al. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine 2009; 27:2830 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.02.096; PMID: 19366570
- Ahmad G, Zhang W, Torben W, Noor Z, Siddiqui AA. Protective effects of Sm-p80 in the presence of resiquimod as an adjuvant against challenge infection with Schistosoma mansoni in mice. Int J Infect Dis 2010; 14:e781 - 7; http://dx.doi.org/10.1016/j.ijid.2010.02.2266; PMID: 20630783
- Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF et al. Preclinical Prophylactic Efficacy Testing of Sm-p80-Based Vaccine in a Nonhuman Primate Model of Schistosoma mansoni Infection and Immunoglobulin G and E Responses to Sm-p80 in Human Serum Samples From an Area Where Schistosomiasis Is Endemic. J.Infect.Dis. 2011.
- Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, et al. Enhancement of Sm-p80 (large subunit of calpain) induced protective immunity against Schistosoma mansoni through co-delivery of interleukin-2 and interleukin-12 in a DNA vaccine formulation. Vaccine 2003; 21:2882 - 9; http://dx.doi.org/10.1016/S0264-410X(03)00159-2; PMID: 12798631
- Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, et al. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect Immun 2003; 71:3844 - 51; http://dx.doi.org/10.1128/IAI.71.7.3844-3851.2003; PMID: 12819068
- Siddiqui AA, Pinkston JR, Quinlin ML, Kavikondala V, Rewers-Felkins KA, Phillips T, et al. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite 2005; 12:3 - 8; PMID: 15828575
- Siddiqui AA, Pinkston JR, Quinlin ML, Saeed Q, White GL, Shearer MH, et al. Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine 2005; 23:1451 - 6; http://dx.doi.org/10.1016/j.vaccine.2004.09.018; PMID: 15670880
- Torben W, Ahmad G, Zhang W, Siddiqui AA. Role of antibodies in Sm-p80-mediated protection against Schistosoma mansoni challenge infection in murine and nonhuman primate models. Vaccine 2011; 29:2262 - 71; http://dx.doi.org/10.1016/j.vaccine.2011.01.040; PMID: 21277404
- Zhang W, Ahmad G, Torben W, Noor Z, Le L, Damian RT, et al. Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine. J Infect Dis 2010; 201:1105 - 12; http://dx.doi.org/10.1086/651147; PMID: 20187746
- Zhang W, Ahmad G, Torben W, Siddiqui AA. Sm-p80-based DNA vaccine made in a human use approved vector VR1020 protects against challenge infection with Schistosoma mansoni in mouse. Parasite Immunol 2010; 32:252 - 8; http://dx.doi.org/10.1111/j.1365-3024.2009.01181.x; PMID: 20398225
- Zhang W, Ahmad G, Torben W, Siddiqui AA. Schistosoma mansoni antigen Sm-p80: Prophylactic efficacy of a vaccine formulated in human approved plasmid vector and adjuvant (VR 1020 and alum). Acta Trop 2011; 118:142 - 51; http://dx.doi.org/10.1016/j.actatropica.2011.01.010; PMID: 21334302
- Ohta N, Kumagai T, Maruyama H, Yoshida A, He Y, Zhang R. Research on calpain of Schistosoma japonicum as a vaccine candidate. Parasitol. Int. 2004; 53:175 - 81; http://dx.doi.org/10.1016/j.parint.2004.01.007; PMID: 15081949
- Zhang R, Yoshida A, Kumagai T, Kawaguchi H, Maruyama H, Suzuki T, et al. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect Immun 2001; 69:386 - 91; http://dx.doi.org/10.1128/IAI.69.1.386-391.2001; PMID: 11119528
- Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine 1997; 15:1631 - 40; http://dx.doi.org/10.1016/S0264-410X(97)00081-9; PMID: 9364694
- Hota-Mitchell S, Clarke MW, Podesta RB, Dekaban GA. Recombinant vaccinia viruses and gene gun vectors expressing the large subunit of Schistosoma mansoni calpain used in a murine immunization-challenge model. Vaccine 1999; 17:1338 - 54; http://dx.doi.org/10.1016/S0264-410X(98)00391-0; PMID: 10195769
- Jankovic D, Aslund L, Oswald IP, Caspar P, Champion C, Pearce E, et al. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol 1996; 157:806 - 14; PMID: 8752932
- El Ridi R, Tallima H. Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine 2009; 27:666 - 73; http://dx.doi.org/10.1016/j.vaccine.2008.11.039; PMID: 19056448
- El Ridi R, Tallima H, Mahana N, Dalton JP. Innate immunogenicity and in vitro protective potential of Schistosoma mansoni lung schistosomula excretory-secretory candidate vaccine antigens. Microbes Infect 2010; 12:700 - 9; http://dx.doi.org/10.1016/j.micinf.2010.04.012; PMID: 20452455
- Osada Y, Kumagai T, Hato M, Suzuki T, El-Malky M, Asahi H, et al. Establishment of Schistosoma japonicum calpain-specific mouse T cell hybridomas and identification of a T cell epitope that stimulates IFNgamma production. Vaccine 2005; 23:2813 - 9; http://dx.doi.org/10.1016/j.vaccine.2004.10.042; PMID: 15780729
- Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med 2006; 12:835 - 40; http://dx.doi.org/10.1038/nm1430; PMID: 16783371
- Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol 2010; 8:814 - 26; http://dx.doi.org/10.1038/nrmicro2438; PMID: 20948553
- Zhang W, Li J, Duke M, Jones MK, Kuang L, Zhang J, et al. Inconsistent Protective Efficacy and Marked Polymorphism Limits the Value of Schistosoma japonicum Tetraspanin-2 as a Vaccine Target. PLoS Negl Trop Dis 2011; 5:e1166; http://dx.doi.org/10.1371/journal.pntd.0001166; PMID: 21655308