Abstract
In the United States, therapeutic vaccines may provide considerable benefit to cancer patients. Yet, there has been no assessment of whether vaccines currently in the research and development pipeline reflect the burden of disease and current survival patterns for different malignancies. The authors used data from the National Cancer Institute, Surveillance Epidemiology and End Results (SEER) database, and clinicaltrials.gov registry to characterize the vaccine development pipeline with respect to 5 measures of disease burden and treatment effectiveness for cancer: annual incidence, annual mortality, five-year survival rate, recent change in five-year survival (1999-2006 vs 1990-1992), and five-year mortality estimate (=annual incidence*[1 - 5-yr survival rate]). In 2011, the authors identified 231 active clinical trials for therapeutic cancer vaccines. Of these trials, 81 vaccines are currently in Phase I, 140 in Phase II, and 10 vaccines in Phase III. Vaccine trials for melanoma are most common (n=40), followed by breast cancer (34), lung cancer (30), and prostate cancer (22). Correlation analyses revealed that only annual cancer incidence is significantly associated with current therapeutic cancer vaccine trial activity (r=.60; p=.003). Annual mortality, 5-year survival rate and 5-year mortality estimates were not associated with vaccine trial activity. The authors conclude that therapeutic cancer vaccine clinical trials correspond with disease incidence in the U.S., but not with measures of mortality and survival that reflect the effectiveness of currently available treatment modalities. Future development of therapeutic vaccines for cancer may benefit patients more if there is stronger complementarity with other therapeutic options.
Introduction
Conventional therapies for treatment of malignant disease—including chemotherapy, surgery and radiation—have greatly improved the survival rates of many cancers over recent decades. However, these therapies often fail to reach tumor remission and are frequently associated with incapacitating and sometimes life-threatening side effects.Citation1-Citation3
Therapeutic cancer vaccines, which prompt a targeted immune response against tumor-specific antigens and tumor-associated antigens, offer substantial promise as future treatments for cancer patients—chiefly due to their potential to initiate and sustain immune responses that may either restrain or entirely eliminate a tumor and thereby extend patient survival.Citation2 In addition, the highly specific nature of the triggered immune response may avoid many of the adverse events and side effects associated with existing cancer therapies.Citation4 In April 2010 the US. Food and Drug Administration (FDA) approved the first therapeutic cancer vaccine, sipuleucel-T (Provenge; Dendreon), for the treatment of patients with asymptomatic or minimally symptomatic hormone-resistant prostate cancer.
Licensure of sipuleucel-T, based on survival benefit in clinical trials,Citation5 was regarded a major milestone for therapeutic cancer vaccines in the United States.Citation6 Yet, there has been no assessment of therapeutic cancer vaccines currently in clinical trials, which would help clinicians and their patients understand therapeutic and research opportunities in the near future. Nor has there been an assessment of the extent to which research and development in this expanding niche reflects the incident burden of disease or the current survival patterns for different malignancies in the United States. The population benefit of cancer vaccines may be greatest, as a therapeutic class, if research and development is concentrated and promoted most strongly for common cancers where survival rates are currently lower than average for oncology treatments overall.Citation7
In this study we examine the pipeline of therapeutic cancer vaccines currently undergoing clinical trials, using publicly available data.
Results
Overall Clinical Research Activity for Therapeutic Cancer Vaccines – 2011
In 2011, we identified 231 active clinical trials for therapeutic cancer vaccines (). Of the active clinical trials, 81 are in Phase I, 140 have reached Phase II, and 10 vaccines are currently in Phase III. By cancer type, active therapeutic vaccine trials for melanoma are most numerous (n = 40), followed by breast cancer, lung cancer, and prostate cancer.
Table 1. Therapeutic cancer vaccines currently in human clinical trials, by disease target
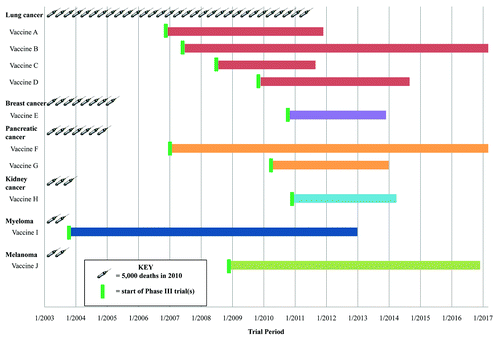
Of the 10 active Phase III trials, 4 target lung cancer, 2 target pancreatic cancer, and there is one trial each for breast cancer, melanoma, multiple myeloma, and kidney cancer (). Two of the studies (Lung A and Lung C) are projected to be completed by the end of 2011, and 3 more by the end of 2013. National trial identifier codes are provided in for interested physicians and patients who may wish to learn more about the active therapeutic cancer vaccine trials currently in phase III.
Figure 1. Development targets and timelines for therapeutic cancer vaccines in phase III clinical trials. Data abstracted by the authors from National Cancer Institute clinical trials databaseCitation8 and clinicaltrials.gov.Citation10 National clinical trials (NCT) information is provided to readers for reference regarding each vaccine.
Association of Therapeutic Vaccine Trial Activity with Disease Burden and Survival Trends
According to surveillance statistics (), cancers range in annual incidence in the US from about 8,000 (testis, Hodgkin’s lymphoma) to more than 220,000 (lung and bronchus). Annual mortality for cancers in the US ranges from about 300 (testis) to more than 157,000 (lung and bronchus).
Table 2. Cancer types and corresponding measures of burden and survival in the United States
According to the latest national five-year survival data, survival at 5 y from diagnosis is 67.8 percent for all cancer sites overall, which is a 7.1-percentage-point absolute improvement from a decade ago (11.7 percent relative improvement). Five-year survival rates for specific cancers () are highest for prostate cancer (99.6 percent) and lowest for pancreas (5.7 percent). Absolute recent improvement in five-year survival is greatest for non-Hodgkin’s lymphoma (17.3 percentage points) and least for cancer of the larynx (-3.4 percentage points).
In combination, annual incidence and five-year survival data yield estimates of five-year mortality—that is, the therapeutic window of need and consequent opportunity for cancer vaccines. Five-year mortality is estimated to range from approximately 300 for cancers of the testis to more than 185,000 for cancers of the lung and bronchus ().
Pairwise correlations of therapeutic cancer vaccine trial activity with measures of disease burden and cancer survival are presented in . Annual cancer incidence is significantly associated with current therapeutic cancer vaccine trial activity (r = 0.60; p = 0.003). Regression analyses demonstrated that, on average, there is an additional clinical trial for each 10,000-case increase in the annual number of incident cases. Annual incidence was found to explain 32% of the variance in the number of therapeutic cancer vaccine trials.
Table 3. Correlation analysis of clinical trials activity for therapeutic cancer vaccines with measures of cancer disease burden and survival
The number of therapeutic cancer vaccine trials was less strongly correlated with annual mortality (r = 0.39); this finding did not reach statistical significance (p = 0.07).
The three measures of cancer survival that we examined were not associated with clinical trials activity ().
Discussion
The licensure of sipuleucel-T in 2010 as a therapeutic vaccine for prostate cancer may signal a very active new era of treatment innovation for malignancies. In this analysis of current clinical trial data, we find that therapeutic vaccines are currently in development for 13 of 22 cancer types specified by the NCI. In addition, of 10 vaccines currently in phase III clinical trials, 8 are expected to complete their trial periods by the end of 2016. Even allowing for common trial delays and the expected duration (one to two years) of the FDA licensure period itself, it appears possible that care of cancers of the prostate, breast, lung, pancreas, and kidney and multiple myeloma and melanoma may be expanded with therapeutic vaccine options as early as 2020.
Such a projection begs the question: are therapeutic vaccines likely to meaningfully alter the burden of malignancies in the United States and provide clinically worthwhile alternatives to currently available therapies? Importantly, we found that current therapeutic vaccine development for cancers is strongly associated with annual incidence—and not with annual mortality, current or recent changes in five-year survival rates, or five-year mortality estimates. While notable outliers, such as melanoma and brain cancer, have a large number of active clinical trials relative to their incidence, across all cancers investigated incidence was the only statistically significant factor we found that corresponded with cancer development. In the broadening niche of therapeutic cancer vaccines, annual incidence appears to provide the strongest appeal for vaccine innovators at this moment in time.
Prostate cancer vaccines are perhaps the best illustration of this finding. The five-year survival rate for prostate cancer stands at > 99 percent, the most favorable in the US—indicating that available therapeutic approaches are helping the vast majority of men diagnosed, and offering an incremental advantage for another treatment approach in the majority of cases. Nonetheless, sipuleucel-T has already been licensed and there are 22 more prostate cancer vaccines already in clinical trials (many targeting treatment-resistant forms of this malignancy). Apparently, the attraction for vaccine developers is the large number of men who develop prostate cancer each year; even a small fraction of men who fail currently available therapies for prostate cancer is a large number of potential candidates for vaccine therapy.
Lung cancer—the most common cancer in the US—presents a distinctly different opportunity for therapeutic vaccines. First, in stark contrast with prostate cancer, five-year survival for cancers of the lung and bronchus is 16.4 percent. Second, this NCI category includes multiple different cancer subtypes—e.g., small cell, squamous cell, adenocarcinoma—that vary considerably in their genetic origins, clinical prognoses and effectiveness of currently available therapies. In fact, this large category is perhaps better viewed as a set of smaller subcategories, each of which may offer a distinct target for vaccine development. At this time, the 4 vaccine candidates currently in phase III trials for lung cancer are all developed for non-small cell treatment.
Pancreatic cancer presents a third example of current therapeutic vaccine development. Cancer of the pancreas occurs only one-fifth as frequently as cancers of the lung, breast, and prostate, and currently has the lowest five-year survival rate of all cancer types in the US. Our analysis would suggest that lower incidence would translate to less therapeutic vaccine development—and that is true (n = 11) in comparison with prostate (23), lung (30) and breast (37) cancers. On the other hand, there are already two therapeutic vaccines for pancreatic cancer in phase III trials, one with a projected end date as soon as 2014. In cases such as pancreatic cancer, where currently available therapies offer only modest or little benefit, therapeutic vaccines may offer marked incremental value to patients in the future.
It appears that progress in cancer vaccines can serve the greatest benefit to patients if drug developers choose to target malignancies that are not as successfully treated by existing therapies.Citation7 Even a mild to moderately successful vaccine that targets malignancies with dire prognoses, such as pancreatic and lung cancer, may have more potential to increase patient outcomes than a wildly successful vaccine that targets less aggressive cancers such as prostate tumors. Cancer incidence should still be a key factor when considering potential drug targets, but by also considering current treatment outcomes, one can identify malignancies that afflict the largest numbers of patients as well as have the poorest prognoses (e.g., lung cancers).
Importantly, our analysis here emphasizes the application of new therapeutic vaccine technologies from the perspective of patients who may potentially benefit. This perspective, which we may call ‘end-of-the-pipeline,’ is not the only part of the story. Factors related to initiating and facilitating new therapeutic vaccine development may also play a role. In the case of cancer vaccines, the identification of tissue-specific targets (e.g., lactalbumin for breast cancer; prostate-specific antigen for prostate cancer) may make vaccine development not only more feasible but safer as well. Where tissue-specific targets are more difficult to identify, vaccine development efforts may need to be amplified in order to realize the population benefit described above.
Our findings must be interpreted in the context of certain limitations. First, it would be clinically relevant to compare the therapeutic effects and side effects of newly available therapeutic vaccines with established therapies for cancer. However, this form of comparative effectiveness analysis must await the outcomes of phase III trials for the products in the pipeline. Importantly, in the case of sipuleucel-T, a comparative effectiveness analysis (i.e., vs. other therapeutic options) was not performed (the trial was placebo-controlled).Citation5 It would be beneficial in the years ahead if therapeutic cancer vaccine trials implemented head-to-head comparisons vs. existing therapies, rather than placebo, in order to directly compare benefits and risks for these new treatments. While cancer vaccines differ in their mechanism of action compared with existing cancer treatments such as chemotherapy, commonly measured outcomes, like survival, would be useful parameters to compare across treatments.
A second limitation of this analysis is that clinical trials provide a valuable but incomplete perspective on the extent of research and development activity, which extends back into preclinical (animal models) and research-and-discovery phases. Based on recently published data about the relative balance of products in preclinical vs. clinical trials within the rapidly growing pipeline of prophylactic vaccines,Citation11 the number of therapeutic cancer vaccine candidates in preclinical stages may well exceed the numbers currently in human trials. Nonetheless, the progress of vaccine candidates through the development pipeline to clinical trials indicates the products with the greatest likelihood of eventual licensure. Therefore, we believe that the analysis presented here, while only a part of the complete development picture, is the most relevant part for patients and physicians alike.
In summary, current projections indicate that several therapeutic vaccines for cancer may be licensed for use in the US by the end of this decade. Our finding that therapeutic vaccine development is generally more active for more common cancers fits conventional wisdom in pharmaceutical development. Nonetheless, for therapeutic cancer vaccines to fundamentally change how cancer care is provided, and how many patients survive who would have died with other available treatments, vaccine developers will need to attend to measures such as annual mortality and five-year survival that are not currently driving factors within this new niche.
Methods
Study Sample – Therapeutic Cancer Vaccines
We determined the numbers of active therapeutic cancer vaccine trials using the clinical trial search database of the National Cancer Institute (NCI).Citation8 We implemented search criteria “treatment,” “vaccine therapy,” and “active” for the trial type, treatment/intervention, and trial status search fields, respectively. By NCI definition, to be “active” a trial must currently be enrolling patients. We collected trial data in the period January–May 2011.
We identified therapeutic cancer vaccine types as those specified by NCI as being in active clinical trials for therapeutic vaccines.Citation9 These types include: bladder cancer, brain tumors, breast cancer, cervical cancer, kidney cancer, leukemia, lung cancer, non-Hodgkin and Hodgkin lymphoma, melanoma, multiple myeloma, pancreatic cancer, and prostate cancer. We added these cancer types in the cancer type/condition search field for their respective searches, in order to assign vaccine candidates to specific cancer targets.
Cancer Vaccine Trials and Corresponding Disease Burdens
From the NCI database search elaborated above, we identified the active vaccine trials and procured the National Clinical Trial identifier (NCT ID) for each. Using the NCT ID, we then used the NIH ClinicalTrials.gov database to obtain more data about each trial including start date and projected end date.Citation10
Using the most recent data available, we determined 2010 estimates of new cases and deaths by cancer type in the USCitation1 Using the most recent publicly available data from SEER (seer.cancer.gov/), we determined the five-year survival rates by cancer type in the US for the two most recent measurement periods specified by SEER: 1999–2006 and 1990–1992. From these data, we calculated the absolute and relative recent changes in five-year survival for different cancer types.
As an additional measure, we also estimated mortality burden at five years, which we calculated as: [annual incidence] * [1 – five-year survival rate].
Data Analyses
The central research question in this study was whether therapeutic cancer vaccine development activity (as measured by number of products in human trials) is related to disease burden and survival patterns. We measured burden and survival in 5 ways: (1) annual incidence (hypothesis: incidence is positively associated with vaccine development), (2) annual mortality (hypothesis: mortality is positively associated with vaccine development), (3) latest 5-y survival rate (hypothesis: survival is negatively associated with vaccine development), (4) recent change in 5-y survival rate (hypothesis: change in survival is negatively associated with vaccine development), (5) 5-y mortality estimate (hypothesis: mortality is positively associated with vaccine development).
We used pairwise correlation statistics and linear regression to examine the association of vaccine development activity with each of the 5 different measures above. All analyses were performed using Stata 9 (Stata Corp., College Station, TX). As an analysis of de-identified data from publicly available sources, this study was exempt from human subjects considerations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
REFERENCES
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277 - 300; http://dx.doi.org/10.3322/caac.20073; PMID: 20610543
- Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. J Biomed Biotechnol 2010; 2010:596432; http://dx.doi.org/10.1155/2010/596432; PMID: 20706612
- Aldrich JF, Lowe DB, Shearer MH, Winn RE, Jumper CA, Kennedy RC. Vaccines and immunotherapeutics for the treatment of malignant disease. . Clin Dev Immunol 2010; 2010:697158; http://dx.doi.org/10.1155/2010/697158; PMID: 20936120
- Andersen MH, Junker N, Ellebaek E, Svane IM, Thor Straten P. Therapeutic cancer vaccines in combination with conventional therapies. J Biomed Biotechnol 2010; 2010:237623; http://dx.doi.org/10.1155/2010/237623; PMID: 20617155
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al, IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411 - 22; http://dx.doi.org/10.1056/NEJMoa1001294; PMID: 20818862
- Brower V. Approval of Provenge seen as first step for cancer treatment vaccines. J Natl Cancer Inst 2010; 102:1108 - 10; http://dx.doi.org/10.1093/jnci/djq295; PMID: 20668267
- Davis MM, Dayoub EJ. A strategic approach to therapeutic cancer vaccines in the 21st century. JAMA 2011; 305:2343 - 4; http://dx.doi.org/10.1001/jama.2011.814; PMID: 21642687
- National Cancer Institute clinical trials database. http://www.cancer.gov/clinicaltrials/search. Accessed February 27, 2011.
- National Cancer Institute. Cancer vaccine fact sheet. http://www.cancer.gov/cancertopics/factsheet/Therapy/cancer-vaccines. Accessed February 27, 2011.
- U.S. National Institutes of Health. ClinicalTrials.gov. http://www.clinicaltrials.gov. Accessed February 27, 2011.
- Davis MM, Butchart AT, Coleman MS, Singer DC, Wheeler JRC, Pok A, et al. The expanding vaccine development pipeline, 1995-2008. Vaccine 2010; 28:1353 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.11.007; PMID: 19932670