Abstract
Objective
To evaluate the general safety of zoster vaccine (ZV) in adults ≥60 years old.
Patients/Methods
Subjects were enrolled in a 1:1 ratio to receive 1 dose of ZV or placebo. Subjects were followed for serious adverse experiences (SAEs) for 42 days (primary follow-up period) and 182 days (secondary follow-up period) postvaccination. Relative-risks (ZV/placebo) for SAEs during both safety periods were calculated. Study period: 17-Sep‑2007 to 09-Jan-2009.
Results
Overall, 5,983 subjects received ZV and 5,997 received placebo. Within the primary 42-day follow-up period, 84 ZV subjects and 67 placebo subjects reported SAEs. The estimated risk of SAEs within 42 days was 1.41% for ZV versus 1.12% for placebo, with a relative-risk of 1.26 (95% CI 0.91,1.73); indicating no statistically significant difference between groups, meeting the pre-specified success criterion. During the 182-day follow-up period, 340 ZV subjects and 300 placebo subjects reported SAEs. The estimated risk of SAEs within 182 days was 5.68% for ZV versus 5.01% for placebo, with a relative-risk of 1.13 (95% CI 0.98,1.32), indicating no statistically significant difference between groups. Two subjects in the ZV group reported SAEs deemed by the investigator to be vaccine-related (uveitis and sciatica; onset Day 5 and 4, respectively). One subject in the placebo group reported a SAE deemed by the investigator to be vaccine-related (lumbar radiculopathy; onset Day 51). There were 24 fatal SAEs in the ZV group and 17 in the placebo group (relative risk = 1.41; CI: 0.77, 2.60); 6 and 5, respectively, with SAE onset during the primary 42-day follow-up period. No deaths were deemed vaccine-related.
Conclusions
ZV and placebo groups had similar safety profiles in terms of SAEs during the primary (Day 1 to 42) and secondary (Day 1 to 182) follow-up periods.
Introduction
Herpes zoster (HZ) is caused by reactivation of varicella-zoster virus (VZV), which remains latent in sensory ganglia following primary VZV infection. Clinically, HZ is characterized by a painful, unilateral, dermatomal, vesicular rash and may be complicated by post-herpetic neuralgia (PHN) in 10–20% of cases.Citation1,Citation2 Zoster vaccine (ZV; ZOSTAVAX®; zoster vaccine live, Merck), a live attenuated VZV vaccine, is the only currently-licensed intervention for prevention of HZ in people ≥ 50 y of age in the United States (US) and other countires.Citation3-Citation6 As demonstrated in the Shingles Prevention Study (SPS), ZV has been shown to reduce the incidence of HZ, PHN and the burden of illness due to HZ.Citation7,Citation8
In previous clinical studies, including the SPS, the safety of ZV was evaluated in subjects ≥ 50 y of age (including unpublished data, Merck).Citation7-Citation17 In the SPS, all 38,546 subjects were followed for serious adverse experiences (SAEs) from Day 1 to 42 postvaccination.Citation7,Citation8 The Adverse Event Monitoring Substudy (AEMS) of the SPS enrolled a subset of 6,616 subjects who were followed for 42 d postvaccination by means of a Vaccination Report Card (VRC). Beyond Day 42 postvaccination, all subjects in the SPS were followed for vaccine-related SAEs and deaths. The number (%) of subjects in the AEMS who reported one or more SAE was higher in the ZV group (64; 1.9%) as compared with the placebo group (41; 1.3%), with a relative risk of 1.53 (95% CI: 1.04,2.25). However, no difference was seen in the number (%) of subjects from the overall study (including the Routine Safety Monitoring Cohort) reporting one or more SAE between the ZV group (255; 1.4%) and placebo group (254; 1.4%), with a relative risk of 1.01 (95% CI: 0.85,1.20).
This study evaluated the general safety of ZV in adults ≥ 60 y old by assessing the rates of SAEs in 6,000 subjects who received ZV compared with the rates of SAEs in 5,999 subjects who received placebo. This was the first study on the safety of ZV with a full six months of active postvaccination SAE monitoring.
Results
Participant Accounting and Demographics
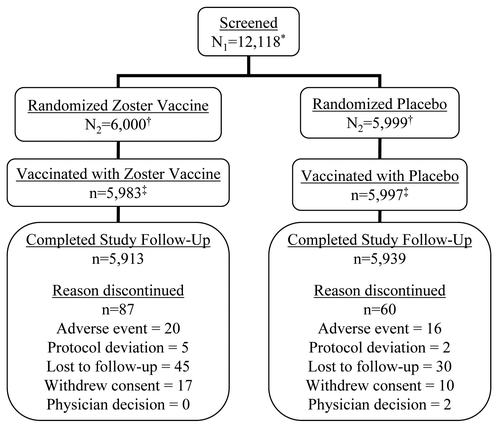
Of the 12,118 screened subjects, 11,999 were eventually randomized; 6,000 to the ZV group and 5,999 to the placebo group. The 119 screened, but not randomized subjects were not included in the analyses. Of the 11,999 randomized subjects, 19 were not vaccinated and not included in the analyses. Additionally, 10 subjects randomized to the ZV group received placebo, and 5 subjects randomized to the placebo group received ZV due to errors at the study sites. Thus, 5,983 subjects actually received ZV and 5,997 subjects actually received placebo (). For all analyses, cross-treated (i.e., randomized to ZV and received placebo, or randomized to placebo and received ZV) subjects were considered according to the vaccine received, and not the vaccine assigned.
A total of 98.9% (11,852/11,980) of the vaccinated subjects completed both primary (42 d) and secondary (182 d) safety follow-up. Eighty-seven (87) subjects discontinued from the study in the ZV group and 60 (60) subjects discontinued from the study in the placebo group. Twenty (20) subjects in the ZV and 16 subjects in the placebo group discontinued the study due to a fatal SAE within the secondary safety follow-up period; three deaths in the ZV group and 4 in the placebo group occurred during the primary safety follow-up period. None of these events were deemed by the investigators to be vaccine-related.
Subjects in the ZV and placebo groups were generally comparable with respect to baseline characteristics (). The mean age at enrollment was 70.5 y for the ZV group and 70.4 y for the placebo group. The overall study group was chiefly comprised of Caucasians (96.2%) residing in the US (88.7%) and living in independent residences (96.9%). A higher percentage of females than males were enrolled. Both vaccination groups were generally balanced with respect to age, race/ethnicity, gender, and type of residence.
Table 1. Demographics
Approximately 94% of subjects in each vaccination group reported prior and/or concomitant therapies. Similar to those therapies used prior to study entry, the most frequently reported concomitant therapies were agents acting on the renin angiotensin system, lipid modifying agents, and analgesics; each with an incidence rate ≥ 40% in both vaccination groups. Approximately 98.6% of subjects in each vaccination group had one or more underlying medical condition: the most commonly reported medical conditions by system organ class were vascular disorders, musculoskeletal and connective tissue disorders, and metabolism and nutrition disorders.
Safety
Within the primary 42-d follow-up period, 84 (1.4%) ZV subjects and 67 (1.1%) placebo subjects reported SAEs (). Systemic SAEs that were reported most frequently were those in the cardiac disorders system organ class (ZV: 0.3%; placebo: 0.3%) and the neoplasms (benign, malignant, and unspecified) system organ class (ZV: 0.3%; placebo: 0.2%). Specific SAEs were reported in low frequencies (≤ 0.1%) among subjects in both vaccination groups; the types of SAEs were not significantly different across vaccination groups. Estimated risk of SAEs within 42 d () was 1.41% for ZV vs. 1.12% for placebo, with a relative risk of 1.26 (95% CI 0.91, 1.73) indicating no statistically significant difference in SAE risk between vaccination groups, meeting the pre-specified success criterion.
Table 2. Serious Adverse Experience (SAE) Summary - According to Follow-Up Period
Table 3. Analysis of Proportion of Subjects Reporting ≥ 1 Serious Adverse Experiences
During the 182-d follow-up period, 340 (5.7%) ZV subjects and 300 (5.0%) placebo subjects reported SAEs (). Systemic SAEs were most frequently reported in the cardiac disorders system organ class (ZV: 1.2%; placebo: 1.2%) and the neoplasms (benign, malignant, and unspecified) system organ class (ZV: 1.3%; placebo: 1.0%). The most commonly reported SAE was atrial fibrillation at 0.4% in the ZV group and 0.2% in the placebo group. Estimated risk of SAEs within 182 d () was 5.68% for ZV vs. 5.01% for placebo, with a relative risk of 1.13 (95% CI 0.98, 1.32); indicating no statistically significant difference in SAE risk between vaccination groups.
The relative risk (ZV/placebo) for SAEs during the primary 42 d follow-up period was calculated according to age group and region. The relative risk by age group was 1.66 (95% CI: 0.96, 2.85) for subjects 60 to 69 y of age, 1.03 (95% CI: 0.63, 1.69) for subjects 70 to 79 y of age, and 1.18 (95% CI: 0.63, 2.21) for subjects ≥ 80 y of age. The relative risk by region was 1.23 (95% CI: 0.88, 1.72) for subjects residing in the US, and 1.51 (95% CI: 0.57, 4.04) for non-US subjects. The 95% CI of relative risk for each category contained the relative risk of 1, indicating no significant difference of SAE risk exists between the two vaccination groups for each subgroup. Furthermore, the data suggested no statistical trends of treatment difference in SAE risk among age groups or regions. The p-values for the interaction terms were large, 0.450 for treatment-by-age and 0.728 for treatment-by-region, and the 95% CIs of relative risk for age groups or regions overlapped substantially.
Two subjects in the ZV group reported SAEs deemed vaccine-related by the investigator (uveitis and sciatica; onset Day 5 and Day 4, respectively). After receiving additional clinical information, the investigator determined that the case of uveitis may have been related to a new diagnosis of Crohn’s disease and not to study vaccine, but the investigator had been unblinded to treatment group at the time that this change in assessment was made. One subject in the placebo group reported a SAE deemed vaccine-related by the investigator (lumbar radiculopathy; onset Day 51). There were 24 fatal SAEs in the ZV group and 17 in the placebo group during the study (relative risk = 1.41; CI: 0.77, 2.60); 6 and 5, respectively with SAE onset during the primary 42-d follow-up period (). None of these deaths were deemed by the investigator to be vaccine-related.
Table 4. Listing of SAEs Resulting in Death (Day 1 to Day 42)
Discussion
The results from the current study are consistent with findings from previous clinical trials, in which ZV has been evaluated for safety in participants 60 y of age and above in approximately 21,000 adults.Citation7-Citation17 In these studies, the overall incidence of systemic clinical adverse experiences (AEs) following a dose of ZV is similar to that following a dose of placebo. The incidence of vaccine-related systemic clinical AEs is slightly higher after a dose of ZV than after a dose of placebo, but no particular systemic AE is consistently reported at a higher rate among ZV recipients across studies.
In the largest of these trials, the SPS, subjects received a single dose of either ZV (n = 19,270) or placebo (n = 19,276), and were actively followed for safety outcomes through Day 42 postvaccination and passively followed for safety after Day 42.Citation7,Citation8 The AEMS of the SPS (n = 3,345 received ZV and n = 3,271 received placebo) was designed to ensure complete collection of AEs, even those that are mild or might be missed due to subject recall bias. To facilitate collection of these data, subjects in the AEMS were asked to record all injection-site and clinical complaints occurring from Days 0 to 42 postvaccination on a VRC. Safety follow-up and reporting procedures for SAEs were the same for SPS subjects in the AEMS and those not in the AEMS. All subjects on study were instructed to notify site personnel immediately if they experienced a SAE. To further ensure complete reporting of AEs all study subjects were contacted on or around Day 43 postvaccination.
In the AEMS, the rate of SAEs was increased in the group of subjects who received ZV (1.9%) as compared with the group of subjects who received placebo (1.3%). However, in the overall SPS study population, which included the subjects in the AEMS, SAEs occurred at a similar rate (1.4%) in subjects vaccinated with ZV or placebo. The difference in SAE rates observed in the AEMS was not attributable to either a specific AE term or to AEs occurring within a body system (as defined by the Coding Symbols from a Thesaurus of Adverse Reaction Terms, or COSTART, classification system). The SPS investigators concluded that the statistically significant difference in SAE rates between the ZV and placebo groups observed in the AEMS was most likely due to chance and did not represent vaccine-related events.
The findings from the current study are generally consistent with SAE frequencies observed among the overall study cohort of the SPS. For both the current study and the SPS, the risk of SAEs in the ZV group relative to the placebo group was not statistically different. For the current study, both ZV and placebo had similar safety profiles in terms of SAEs in both the Day 1 to Day 42 and the Day 1 to Day 182 follow up periods.
In addition to the primary safety evaluation, the proportion of subjects reporting SAEs occurring through Day 182 postvaccination in the current study were summarized by age group (60 to 69, 70 to 79, and ≥ 80 y of age), by subjects’ type of residence (e.g., independent residential, assisted living, or nursing home) (data not shown), by region (e.g., US and non-US), and by vaccination lot (data not shown). No significant differences of proportions of subjects reporting SAEs between the two vaccination groups were noted when summarized by these variables. Furthermore, pre-specified analyses suggested no statistical trends of treatment difference in SAE risk among age groups nor regions; the p-values for the interaction terms were large, 0.450 for treatment-by-age and 0.728 for treatment-by-region, and the 95% CIs of relative risk for age groups or regions overlapped substantially.
In this study, two subjects in the ZV group reported SAEs deemed vaccine-related by the investigator (uveitis and sciatica; onset Day 5 and Day 4, respectively). After the investigator was unblinded to treatment group, additional information on the case of uveitis became available suggesting that this event was related to a new diagnosis of Crohn’s disease and not to study vaccine. One subject in the placebo group reported a SAE deemed vaccine-related by the investigator (lumbar radiculopathy; onset Day 51). In the SPS, only 2 possibly vaccine-related SAEs within 42 d postvaccination were reported in subjects vaccinated with ZV, both of which occurred in the Routine Safety Monitoring Cohort. These events were polymyalgia rheumatica, which became symptomatic on Day 3 postvaccination, and an exacerbation of pre-existing asthma on Day 2 postvaccination. In addition, 3 possibly vaccine-related SAEs (anaphylaxis; Goodpasture’s syndrome; polymyalgia rheumatica) were reported among placebo recipients in the SPS.
In summary, this study demonstrated the safety of ZV compared with placebo over a 6 mo follow-up period in patients ≥ 60 y of age, and confirmed the safety profile, with regards to SAEs, previously documented in other ZV trials including the SPS.
Patients and Methods
Study Design and Population
This randomized, double-blind, placebo-controlled, age-stratified study enrolled 11,999 subjects ≥ 60 y old in 1:1 ratio to receive 1 dose of ZV or placebo (NCT00550745). The study was conducted at 46 sites in Canada, Germany, Spain, the United Kingdom, and the US from 17 September 2007 to 9 January 2009. The protocol was approved by the Ethical Review Committee of each site and conducted in conformance with applicable country/local requirements. Informed consent was obtained prior to vaccination. An independent Data Monitoring Committee was established for continuous safety oversight during the study.
Afebrile (< 101.0°F/ 38.3°C) male or female subjects ≥ 60 y of age were eligible for the study. Subjects were to have: no history of hypersensitivity reaction to gelatin, neomycin, or any other component of the vaccine; no prior receipt of any varicella or zoster vaccine; no live vaccinations from 4 weeks prior to vaccination or expected through the 42-d postvaccination period; no inactivated vaccinations within 7 d prior to vaccination or expected through the 42-d postvaccination period with the exception of influenza vaccine; no intercurrent illness that might interfere with the interpretation of the study or prevent the subject from completion of the study; no immune dysfunction caused by a medical condition, no use of immunosuppressive therapy; no concomitant use of systemic antiviral therapy with activity against herpes viruses; and no participation in an investigational drug or vaccine study within 30 d prior to vaccination or expected through the 42-d postvaccination period.
This was an estimation study with no formal statistical hypotheses. The sample size of 6,000 randomized subjects per vaccination group was calculated to have an 80% chance to detect a 1.5-fold increase in relative risk (ZV/placebo) if the incident rate was 1.3% in the placebo group. This study had an 85% chance to detect a 1.5-fold increase in relative risk if the incidence rate in the placebo group was 1.5% and a 94% chance if the incidence rate in the placebo group was 2.0%. If the true underlying relative risk was 1.53, as was observed in the SPS AEMS,Citation5,Citation6 this study would have an 84% chance to detect the increased risk.
Measures
In this study, subjects were followed for clinical SAEs for 42 d postvaccination (primary safety follow-up period) and for 6 mo postvaccination (Day 1 to Day 182 postvaccination; secondary safety follow-up period). Subjects were also instructed to call the study site immediately, at any time during the study, if they experienced a clinical AE that resulted in a hospitalization, prolonged a hospitalization, was a cancer or an overdose, or was another severe, unexpected, or life-threatening event that could potentially have been considered serious. Subjects were called by the study staff at 6 weeks, 4 mo, and 6 mo postvaccination, using a prespecified telephone script, to determine if the subject had a previously unreported clinical AE that met one or more of the criteria for SAEs. Injection-site AEs, systemic AEs, rashes, and temperatures were only collected if they were serious in nature.
Vaccine Descriptions
The lyophilized ZV and placebo were supplied to the study centers in 0.7-mL single-dose vials and stored between 2 to 8°C. The ZV and placebo were reconstituted with sterile diluent immediately prior to administration, and were indistinguishable from each other in appearance. Placebo was the vaccine stabilizer of ZV with no live virus. All subjects received a single subcutaneous injection of either ZV or placebo.
Endpoints
The primary safety endpoint was the proportion of subjects reporting one or more SAEs within 42 d postvaccination and the secondary safety endpoint was the proportion of subjects reporting one or more SAEs within 182 d postvaccination. Non-SAEs were not collected.
Statistical Analysis
The safety analysis included all subjects who were vaccinated and had safety follow-up. The primary safety evaluation is represented by the proportion of subjects reporting one or more SAEs during the primary safety follow-up period (1 to 42 d postvaccination) in each vaccination group. This study estimated the safety profiles of ZV and placebo with respect to the risk of SAEs occurring within 42 d postvaccination. The relative risk (ZV/placebo) of subjects reporting one or more SAEs and its associated 95% CI for all subjects with safety follow-up were calculated using a stratified method with a sample size weight proportional to the number of subjects in each age group (60 to 69, 70 to 79, and ≥ 80 y of age) and region (US and non-US).Citation18 If there were no subjects reporting SAEs in any one of the 6 age by region strata the primary analysis would be adjusted by age group only. For the primary safety endpoint, the protocol defined study success criteria were that either the lower bound of the 95% CI of the relative risk of a SAE (ZV/placebo) for all-subject population is ≤ 1 or the point estimate of the relative risk (ZV/placebo) is ≤ 1.25. For the secondary safety evaluation, the proportion of subjects reporting one or more SAEs through Month 6 postvaccination (Day 1 to Day 182 postvaccination) were summarized by vaccination group and age group.
A test of treatment-by-age-stratification interaction was also performed as an exploratory analysis to further evaluate the age trend using a generalized linear model with binomial distribution and log-link function with the following covariates: age group (60 to 69, 70 to 79, and ≥ 80 y of age), vaccination group (ZV or placebo), region (US and non-US), and the interaction of vaccination group by age group. To further evaluate the effect of region (US and non-US) difference, a test of treatment-by-region was performed using a generalized linear model with binomial distribution and log-link function with the following covariates: age group, vaccination group, region (US and non-US), and the interaction of vaccination group by region.
| Abbreviations: | ||
| AE | = | adverse experience |
| AEMS | = | Adverse Event Monitoring Substudy |
| CI | = | confidence interval |
| HZ | = | herpes zoster |
| PHN | = | post-herpetic neuralgia |
| SAE | = | serious adverse experience |
| SPS | = | Shingles Prevention Study |
| US | = | United States |
| VRC | = | vaccine report card |
| VZV | = | varicella zoster virus |
| ZV | = | zoster vaccine |
Acknowledgments
The authors would like to thank:All the subjects who participated in this study, ICON Clinical Research (North Wales, PA, and Eastleigh, UK) for study conduct, and Data Monitoring Committee Members for study oversight
Financial Disclosure
Other than employees of Merck (as indicated on the title page), all authors have been investigators for the sponsor. KSR has also received speaker fees and consultancy payments from the sponsor. Employees may hold stock and/or stock options in the company.
Author Contributions
Murray, Reisinger, and Kerzner: enrollment of subjects and/or data collection, analysis and interpretation of data, and preparation of manuscript. Stek, Sausser, Xu, and Parrino: analysis and interpretation of data, and preparation of manuscript. Chan, Wang, and Annunziato: study concept and design, analysis and interpretation of data, and preparation of manuscript. The report was primarily drafted by Murray, Stek, and Parrino. All co-authors approved the final version of the manuscript.
Sponsor’s Role
This study was funded by Merck Sharp and Dohme Corp. (sponsor). In conjunction with the external investigators, this study was designed, executed, and analyzed by the sponsor. Although the sponsor formally reviewed a penultimate draft, the opinions expressed are those of the authorship and may not necessarily reflect those of the sponsor. All co-authors approved the final version of the manuscript.
ZOSTAVAX® Protocol 020 Study Group
C. Albert (Niedersachsen, Germany), C. Andrews (San Antonio, TX), R. Biedenbender (Norfolk, VA), K. Charani (Tucson, AZ), H. Charles (ElyCardiff, UK), S. Christensen (Salt Lake City, UT), D. DeSantis (Tempe, AZ), D. Dinh (Garden Grove, CA), A. Dowell (Point Claire, Quebec), J. Ervin (Kansas City, MO), M. Eyck (Berlin, Germany), L. Ferguson (Truro, Nova Scotia), T. Fiel (Tempe, AZ), D. Fried (Warwick RI), J. Goehas (Chicago, IL), L. Gilderman (Pembroke Pines, FL), D. Haworth (Lancashire, UK), L. Helman (South Bend, IN), D. Henry (Salt Lake City, UT), B. Kerzner (Baltimore, MD), J. Kirstein (West Jordan, UT), J. Lawless (Camillus, NY), P. Lee (Houston, TX), L. Martínez (Barcelona, Spain), D. Morin (Bristol, TN), A. Murray (Greensboro, NC), L. Murray (Pinellas Park, FL), K. Reisinger (Pittsburgh, PA), S. Rodstein (Edina, MN), J. Rosen (Miami, FL), J. Rubino (Raleigh, NC), N. Sandoval (Tucson, AZ), D. Schadendorf (Baden-Württemberg, Germany), K. Schmader (Durham, NC), B. Seidman (Delray Beach, FL), S. Shapr (Nashville, TN), R. Sharma (East Sussex, UK), G. Shockey (Mesa AZ), C. Strout (Mt. Pleasant, SC), M. Terns (Barcelona, Spain), M. Turner (Boise, ID), S. Tyring (Houston, TX), M. Van Cleeff (Cary, NC), V. von Behren (Hessen, Germany), C. Woodruff (Birmington, AL), P. Zickler (Surrey, BC)
References
- Creed R, Satyaprakash A, Tyring SK. Varicella Zoster Virus. In: Tyring SK, Yen Moore A and Lupi O (eds). Mucocutaneous Manifestations of Viral Diseases, 2ed, London: Informa Healthcare; 2010:98-122.
- Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37; http://dx.doi.org/10.1186/1741-7015-8-37; PMID: 20565946
- Zostavax (zoster vaccine, live) [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2009. Available at: http://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf. Accessed 31-May-2011.
- Centers for Disease Control and Prevention. Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1 - 5; PMID: 18185492
- National Health & Medical Research Council. The Australian immunisation handbook. Williams A, ed. Canberra: Australian Government Publishing Service, 2008.
- EMEA Committee for Medicinal Products for Human Use. Product information for ZOSTAVAX™. http://www.emea.europa.eu/humandocs/PDFs/EPAR/zostavax/H-674-PI-en.pdf. Accessed 27-Mar-2009.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271 - 84; http://dx.doi.org/10.1056/NEJMoa051016; PMID: 15930418
- Simberkoff MS, Arbeit RD, Johnson GR, Oxman MN, Boardman KD, Williams HM, et al. Safety of Zoster Vaccine in the Shingles Prevention Study: A Randomized Trial. Ann Intern Med 2010; 152:545 - 54; PMID: 20439572
- Levin MJ, Oxman MN, Bobrove AM, Hayden FG, Hayward AR, Irwin M, et al. Dose ranging safety and immunogenicity study of live attenuated Varicella-Zoster virus (VZV) vaccine (Oka/Merck) administered to adults 60 years of age or older: GER-3. South Med J 2005; 98:S55; http://dx.doi.org/10.1097/00007611-200510001-00151
- Schmader KE, Bobrove AM, Levin MJ, Oxman M, Tucker R, Reisinger K, et al. Immunogenicity & Safety of Varicella-Zoster Virus (VZV) Vaccine Administered to Older Adults With or Without Diabetes Mellitus (DM) or Chronic Obstructive Pulmonary Disease (COPD). In: Program and abstracts of the Annual Scientific Meeting of the American Geriatrics Society; May 5, 2006; Chicago, IL. Abstract #227638.
- Macaladad N, Marcano T, Guzman M, Moya J, Jurado F, Thompson M, et al. Safety and immunogenicity of a high-potency zoster vaccine in varicella-zoster virus seronegative and low-seropositive healthy adults. Vaccine 2007; 25:2139 - 44; http://dx.doi.org/10.1016/j.vaccine.2006.11.011; PMID: 17250932
- Lange J, Tyring SK, Peters PH, Nunez M, Poland G, Levin MJ, et al. Immunogenicity, Kinetics of VZV-Specific CD4+ T-cell γ-IFN Production and Safety of a Live Attenuated Oka/Merck Zoster Vaccine in Healthy Adults ≥ 60 Years of Age. In: Program and abstracts of the 44th Annual Meeting if the Infectious Diseases Society of America; October 12-15, 2006; Toronto, Canada. Abstract #857.
- Tyring SK, Diaz-Mitoma F, Padget LG, Nunez M, Poland G, Cassidy WM, et al. Safety and tolerability of a high-potency zoster vaccine in adults >/=50 years of age. Vaccine 2007; 25:1877 - 83; http://dx.doi.org/10.1016/j.vaccine.2006.10.027; PMID: 17227688
- Gilderman LI, Lawless JF, Nolen TM, Sterling T, Rutledge RZ, Fernsler DA, et al. A Double-Blind, Randomized, Controlled, Multicenter Safety and Immunogenicity Study of a Refrigerator-Stable Formulation of ZOSTAVAX®. [Comment in: Clin Vaccine Immunol. 2009;16:1381; author reply 1381-2] Clin Vaccine Immunol 2008; 15:314 - 9; http://dx.doi.org/10.1128/CVI.00310-07; PMID: 18077611
- Kerzner B, Murray AV, Cheng E, Ifle R, Harvey PR, Tomlinson M, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007; 55:1499 - 507; http://dx.doi.org/10.1111/j.1532-5415.2007.01397.x; PMID: 17908055
- MacIntyre CR, Egerton T, McCaughey M, Parrino J, Campbell BV, Su SC, et al. Concomitant Administration of Zoster and Pneumococcal Vaccines in Adults ≥60 Years Old. Hum Vaccin 2010; 6:894 - 902; http://dx.doi.org/10.4161/hv.6.11.12852; PMID: 20980796
- Mills R, Tyring SK, Levin MJ, Parrino J, Li X, Coll KE, et al. Safety, Tolerability, and Immunogenicity of Zoster Vaccine in Subjects With a History of Herpes Zoster (HZ). Vaccine 2010; 28:4204 - 9; http://dx.doi.org/10.1016/j.vaccine.2010.04.003; PMID: 20416263
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4:213 - 26; http://dx.doi.org/10.1002/sim.4780040211; PMID: 4023479