Abstract
The human hookworms Necator americanus and Ancylostoma duodenale remain among the most common infections of humans in areas of rural poverty in the developing regions of the world, with an estimated 1 billion people infected with one or more of these parasites. Herein, we review the nearly 100 years of research, development, animal testing, and fieldwork that have led to our current progress in recombinant hookworm vaccines. We begin with the identification of hookworm at the start of the 20th century in Southern US, then discuss the progress in developed countries to eliminate human hookworm infection, and then the industrial development and field use in the 1970s a canine hookworm vaccine(Ancylostoma caninum), and finally our progress to date in the development and clinical testing of an array of recombinant antigens to prevent human hookworm disease from N. americanus infection. Special attention is given to the challenges faced in the development of a vaccine against a blood-feeding nematode, including the epidemiology of infection (high prevalence of infection), pathogenesis (chronic infection that increases with the age of the host), and a robust immune response that fails to confer the protection in the host and a concomitant absence of correlates of protection by a successful vaccine could be developed and tested. Finally, we provide the optimal and acceptable profiles of a human hookworm vaccine, including the proposed indication, target population, and route of administration, as developed by the Human Hookworm Vaccine Initiative, the only group currently working on vaccines targeting this parasite.
Understanding the Impact of Hookworm Infection
Human hookworms are some of the world’s most common parasitic helminths and are prevalent in South and Central America, sub-Saharan Africa, and East Asia. Until half a century ago, hookworms were also prevalent in the United States, southern Europe, and Australia.Citation1,Citation2 As a result of considerable economic growth and development, especially in the years following World War II, which included programs of mass chemotherapy with anthelminthic medications, improved sanitation and health education, the soil-transmitted helminths (STHs), which also include Ascaris lumbricoides and Trichuris trichiura, as well as the focus of the current review the hookworms Necator americanus and Ancylostoma duodenale were eliminated from most of these regions. However, the STHs, especially the hookworms, remain the most common infections of humans in areas of rural poverty in the developing regions of the world, with an estimated 1 billion people infected with one or more of these parasites. The epidemiology of hookworm infection (either Necator americanus or Ancylostoma duodenale) as it relates to the rationale for a hookworm vaccine has been extensively reviewed by us elsewhere.Citation3-Citation5 In brief, current control efforts focus on the administration of anti-nematode drugs (“anthelminthics”) from the benzimidazole (BMZ) class of drugs, either albendazole or mebendazole.Citation3-Citation5 While BMZ class anthelminthics are highly effective at removing established (“patent”) infections, they do not confer lasting protection against new infection—hence, individuals resident in hookworm endemic areas are rapidly re-infected even after successful treatment. For this reason, the extensive use of chemotherapy has failed to interrupt and, in some cases, even limit transmission of these blood-feeding nematode parasites—with individuals becoming re-infected to their previous levels of infection within 12 mo of treatment.Citation3-Citation5 Current control efforts center on mass drug administration (MDA), which refers to the administration of BMZ class anthelminthics to entire populations resident in endemic areas irrespective of their individual infection status, has also raised concerns about the rates of drug failure (mebendazole) when used in a single dose and the possible emergence of drug resistance, as seen in veterinary uses of the BMZ class of drugs.Citation6 These concerns have stimulated efforts to develop a hookworm vaccine that would provide long‐term protection against disease due to N. americanus, the most prevalent hookworm worldwide.Citation3-Citation5
History of Hookworm Infection
Evidence of hookworm infections stems back to Pharaonic times in the Old World and to pre-Columbian times in the New World.Citation7 While A. duodenale was identified over 1.5 centuries ago, N. americanus was more recently discovered only a century ago in the southern United States.Citation7 Shortly after the turn of the 20th century, Stiles and his colleagues conducted several scientific experiments and issued public health reports concerning hookworm infection in the United States, and its impact on the population, especially school-aged children.Citation8-Citation10 Prompted by these reports, the Rockefeller hookworm eradication campaign was soon underway in the southern United States and quickly expanded globally.Citation11 Despite some successes, the overall impact of the campaign on reducing the prevalence and intensity of hookworm infection remains controversial.Citation12,Citation13
In 1947, Norman StollCitation14 made the first global estimate of the number of individuals infected with human hookworm when he claimed that nearly one third of the world’s population (over 600 million cases at that time) was infected with hookworm, which would make hookworm one of the most common human infections.Citation14,Citation15 Over the next half of a century, efforts have been undertaken to reduce the prevalence of hookworm in regions as diverse as North America, Asia, sub-Saharan Africa and Latin America through a combination of improvements in infrastructure and widespread anthelminthic drug treatment.Citation16 Today, endemic hookworm has been essentially eliminated from the United States.Citation17,Citation18 Economic development in countries such as Taiwan, Japan, and South Korea during the 1960–1970s also resulted in significant control of hookworm transmission, with national prevalence levels currently below 1%.Citation14 Additionally, over the past 20 y, the People Republic of China has successfully decreased the national hookworm infection prevalence levels, especially in Eastern China.Citation19 Latin America and the Caribbean have experienced noticeable decreases in the prevalence of hookworm infections over time with the implementation of various national treatment programs coupled with economic growth.Citation20 Today, hookworm remains highly prevalent in regions (especially rural) of the low- and middle-income countries of Africa, Asia and the Americas where economic development has not yet taken place, and is not expected to anytime soon.Citation15 Despite the successful control of hookworm in several parts of the world, an estimated 576–740 million cases still exist worldwide, a figure very close to Stoll’s original estimate more than 60 y ago.Citation21
Natural History of Hookworm
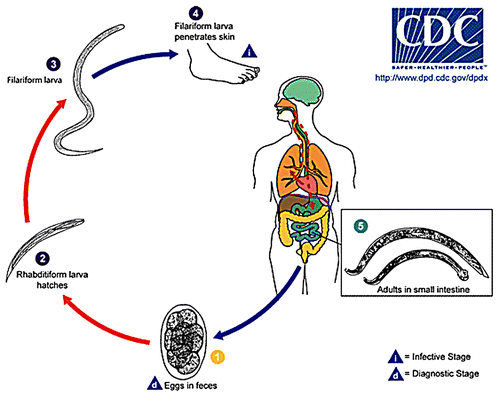
The life cycles of N. americanus and A. duodenale as they relate to vaccine development have been reviewed extensively by us elsewhereCitation3-Citation5 and are summarized in . Briefly, humans become infected with hookworms when third-stage infective larvae (L3) penetrate the skin and then migrate into subcutaneous venules and lymphatics before traveling via the afferent circulation to the pulmonary capillary bed. From there, L3 enter the respiratory tree through the alveolae and ascend the bronchioles and the bronchi, pass over the epiglottis, and enter the gastrointestinal tract. In the small intestine, L3 molt twice to become adult male and female hookworms where they can live for five years or more. The adult worms, approximately one centimeter in length, attach to the mucosa and submucosa, rupture capillaries and arterioles, and then feed on host blood and mucosal tissues. The hookworms mate and female worms then produce thousands of eggs that exit the body in the feces. The eggs hatch in soil with adequate moisture and high temperature and then molt twice into L3 that seek higher ground to come into contact with human skin.
Figure 1. The life cycle of the human hookworms as presented by www.cdc.gov/parasites/hookworm/biology.html Hookworm eggs are passed in the stool and larvae hatch in 1 to 2 days (1). The released larvae grow in the feces or soil and after 5 to 10 days become infective third-stage larvae as those used by Miller and other to form an irradiated larval vaccine (irL3) (2-3). On contact with the human host, L3 penetrate the skin and are carried through the blood vessels to the heart and then to the lungs (4) where they penetrate pulmonary alveoli, ascend the bronchial tree to the pharynx and are swallowed. The larvae reach the he lumen of the small intestine and mature into blood-feeding adults (5), by attaching to the intestinal wall. Several of the vaccine candidate antigens mentioned in this review target this stage, including Na-GST-1 and Na-AP R-1.
Intestinal blood loss is the main clinical manifestation of human hookworm infection.Citation3-Citation5 Heavily and even moderately infected patients with underlying iron or protein nutritional deficiencies can develop hookworm disease—the clinical entity that specifically refers to the resulting iron deficiency anemia [IDA], which is a microcytic and hypochromic, anemia, that occurs when hookworms feeding on the blood host with poor nutritional background.Citation3-Citation5 Hookworm-induced blood loss is estimated to be as high as 9·0 mL per day in heavy infections, with hookworm burdens of 40–160 worms sufficient to cause anemia.Citation22 In school-aged children and adults in resource-poor countries, where host iron stores are often lower than those in developed countries, there is a well-established relationship between the intensity of hookworm infection, intestinal blood loss, and anemia.Citation3-Citation5
The Human Immune Response to Hookworm Infection
As with other helminths that infect humans, the robust immune response is induced by hookworm infection fails to confer protection against new or established infection. This phenomenon has been extensively reviewed elsewhere for hookwormCitation23 and for helminths in general, as well as the consequences it has on the discovery and development of vaccines against these parasites. The major impediment to the development of vaccines for these infections is that there is no evidence of protection in humans.
An important reason for this “major impediment” is the natural history of hookworm infections: individuals can be infected for years, starting as young as 12 mo of age, with hookworms sometimes infecting up to 70% of individuals at risk in endemic areas (i.e., 70% an entire village may be infected). As such, it is difficult to find individuals in endemic areas who have not been continuously infected for months, years, or even decades. More specifically, it is nearly impossible to find hookworm-näive individuals (i.e., individuals who have never been infected), follow them longitudinally and observe them for incident or “new” infection in order to elucidate the manner in which the human immune response evolves over time to this pathogen. Hence, most of our knowledge of the human immune responses to hookworm infection comes from individuals who have failed to mount a protective immune response against the pathogen, leaving us without a model for a “successful” immune response that could be used to predict vaccine-induced protection or at least determine a “correlate of protection” (CoP).Citation24,Citation25 The lack of a CoP profoundly effects the pre-clinical and clinical development of hookworm vaccines in variety of ways; for example, the lack of a CoP makes difficult to test the variation in “potency” of different manufactured lots of a hookworm vaccine as we do not know the biologically effective level of antibody needed to induce protection. or if one even exists Additionally, the lack of CoP or a model of protection necessitates the inclusion of much higher sample sizes in phase 2 and 3 trials of candidate hookworm vaccines, since the endpoints must be clinically-based rather than an easier-to-measure CoP.
While host immune response to hookworm infection is robust and comprehensive in scope, activating both strong humoral and cellular immune responses, it fails to elicit protection. Naturally-infected humans mount strong antibody responses against hookworms, involving all isotypes and most IgG subclasses, with IgG4 and IgE exhibiting the greatest increases. These antibody responses can be detected to all stages of the pathogen in the host (L3, L4, and L5).Citation26,Citation27 It is well accepted that a T-helper type 2 (Th2) cellular immune responses is elicited during hookworm infection (as with other helminth infections such as schistosomiasis, onchocerciasis and filariasisCitation28-Citation32). As part of this Th2 response, individuals develop elevated levels of total IgE and parasite-specific IgE, increased levels of interleukin [IL]-4, IL-5, IL-10, and IL-13, and an increase in eosinophils and mast cells. There has been a long-standing hypothesis that parasite-specific IgE can have a partially protective effect against helminth infections such as Schistosoma spp.Citation33,Citation34 Our own studies have shown that elevated levels of IgE to the recombinant Ac-ASP-2 (Ancylostoma caninum Ancylostoma Secreted Protein-2), are associated with a decreased risk of heavy hookworm infection.Citation35
The Th2 response during helminth infection is induced against a background of potent, parasite-induced immuno-regulation, referred to as a “modified” Th2 response.Citation28-Citation32 This response consists of alternatively activated macrophages, Foxp3+ CD4 regulatory T [Treg] cells, and CD4+ Tr1-IL-10 producing T cells.Citation28-Citation32 The effect of this response is to create an immune environment so extensively downregulated that it may protect the host not only from the strong inflammatory effects of helminth infections, but also against the effects of other IgE-mediated disorders such as atopy, asthma, and anaphylaxis.Citation32 Reduced allergic responses have been shown in studies of infection of mice with various helminth infections (see ErbCitation36 for review). Moreover, epidemiological evidence suggests that hookworm infection is associated with reduced skin reactivity to common allergens and a lowered risk of extrinsic asthma.Citation37 As seen with other helminth infections, hookworm is associated with a systemic down-modulation of immune responsive, with measurable attenuation of responses to bystander antigens to immunization with routinely administered vaccines.
As mentioned above, there is no CoP (as defined either by QinCitation38 or by PlotkinCitation24,Citation25) that has been identified for human hookworm infection. The fact that the immune system reacts vigorously to hookworm infection and fails to harm the parasite not only hampers identification of target vaccine molecules for antigen discovery (about which much has been writtenCitation3-Citation5,Citation28,Citation39), but also provides challenges for development of potential vaccine antigens.
Hookworm in the Laboratory
The life cycle of many viral and bacterial pathogens are easy to maintain in vitro and permissive animal models exist for them as well, making the testing of vaccines targeting these organisms for efficacy, immunogenicity, and potency relatively straightforward. However, like many other Neglected Tropical Diseases (NTDs), the life cycle of hookworms is difficult to maintain in vitro or as laboratory strains within animal models. In some cases, only a single stage of the life cycle can be consistently maintained in the laboratory. Moreover, like many NTDs, the laboratory animals that permit hookworm testing are large, expensive, and require extra care to maintain (e.g., canines). There also remains considerable scientific debate as to whether these animal models reproduce the infection as it occurs in the human host (see Fujiwara et al.Citation40 for review)
The current models used in the testing of experimental hookworm vaccines are canines and hamsters. Canines can be experimentally infected with A. caninum, which closely resembles human N. hookworm infection.Citation40 The hamster Mesocricetus auratus can be infected with either A. ceylanicum or N. americanus. These animal models are of limited benefit for the following reasons:
While initially permissive to infection by third-stage infective larvae (L3), both models become refractory to the infection over time.Citation41,Citation42 For example, canines develop natural resistance to hookworm infection after 20 weeks of infection, after which time the parasites are expelled from the host.Citation41,Citation42 This makes the immunization and challenge model difficult to mount and a long-term pathological endpoint such as anemia difficult to induce given such a short time span of infection.
Neither canines nor hamsters easily reproduce the clinical endpoints seen with human hookworm disease–specifically, development of IDA is uncommon. The relationship between worm burden and intestinal blood loss is not straightforward in either model, probably due to the method of infection (a single bolus challenge of infective larvae), which has to be given over a short time frame to permissively infect a large group of animals.
Finally, the hamster model, especially for N. americanus, has the further limitation that even under optimal conditions, less than 20% of infective larvae develop into adult hookworms in the gastrointestinal tract during challenge infectionsCitation43
Over the last few decades there have been several attempts to create alternative animal models for hookworm infection.Citation40 The need for alternative experimental models has been driven by the need to develop new anthelminthic therapies as well as a vaccine for this parasite. Hookworm infections have been attempted in species such as rabbits, chickens, and chimpanzees.Citation40 Mice have also been challenged with Ancylostoma spp. and N. americanus. However, none of these are permissive hosts for the human hookworms; in many cases, their infective larvae fail to mature into blood-feeding adult hookworms. Consequently, mice challenged with hookworm larvae do not go on to experience intestinal blood loss.
The First Hookworm Vaccine
A detailed history of the advances leading to canine and human vaccines can be found in . Briefly, in 1964, MillerCitation44 showed that Ancylostoma caninum larvae could be attenuated using 40,000 röntgens of X-ray. As the amount of radiation increased, the larval infectivity decreased and the pathogenicity was also reduced. He also observed that female larvae irradiated at 40,000 röntgens or more were consistently sterile. In a single subcutaneous vaccination in dogs 3–4 mo old using 1,000 A. caninum larvae exposed to 40,000 röntgens of X-ray, a single subcutaneous vaccination resulted in a high degree of resistance to the challenge burden of normal worms in terms of hematological, clinical and coprological changes. Miller then conducted a series of experiments to investigate the effect of double vaccination with irradiated A. caninum larvae (irL3) and if the determine the route of vaccine administration (subcutaneous (s.c.) or oral) had any effect on the immunogenic efficiency.Citation44,Citation45 He concluded that double oral vaccination was not as effective as double s.c. vaccination in dogs that were 3–4 mo of age, by measuring the establishment of adult hookworms after challenge with infective larvae. Both routes of vaccination seemed equally effective in terms of resistance to the pathogenic effects of hookworm infection, involving hematologic, coprologic (eggs per gram of feces), and clinical observations after challenge with infection with L3. The arrest of irL3 in somatic locations along their migratory route (primarily the lungs) after subcutaneous inoculation generated a more intense immunogenic stimulation of the canine host.Citation44,Citation45 Consequently, Miller favored subcutaneous vaccination for the commercialized version of the irL3 vaccine.Citation42,Citation46
Figure 2. A century of parasitology that has led to the successful industrial-scale development of a canine hookworm vaccine and the experimental testing of a human hookworm vaccine.
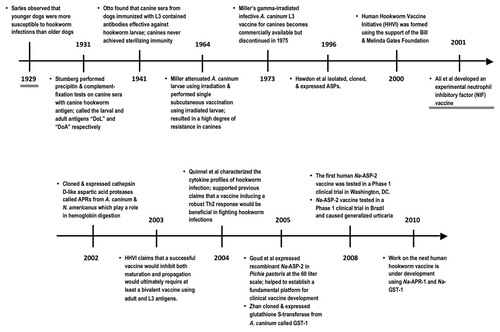
As mentioned above, Miller believed that the presence or absence of somatic migration (L3 migrating to the lungs) was a primary moderator in the increased efficacy observed when irradiated larvae were administered subcutaneously rather than orally.Citation45,Citation46 This hypothesis was established on the observed basis that nearly 75% of irL3 became “arrested” before or died prior to reaching the intestines, the location where the hookworm cause substantial host blood loss. Miller believed that the larvae’s journey to the intestines concluded in the lungs, which was the case in a hypothesis put forth for an experimental irradiated schistosome vaccineCitation47 and for irradiated Nippostrongylus brasiliensis larvae.Citation48 Moreover, Miller showed that subsequent challenge infections of vaccinated dogs was due to the administration of irradiation to third stage infective larvae (L3), which attenuated the L3. Three characteristics of attenuation were observed in irL3:Citation44
a reduction in larval infectivity;
a reduction in the pathogenicity of the worms that eventually reached the small intestine; and
(3) a sterilizing effect on female worm’s fecundity as measured by eggs per gram of feces (epg).
Until this point in time, successful passive immunization for helminth parasites exhibited variable and contradictory results primarily because of experimental techniques. A few years after establishing his “characteristics of attenuation,” Miller conducted a series of experiments revealing that the immunity of double vaccinated dogs with X-irradiated A. caninum larvae could be passively and adoptively transferred to susceptible pups using serum and/or lymphoid cells.Citation49 Despite a lack of immunological reagents at this time (e.g., monoclonal antibodies, cell surface markers), these findings on passive immunization enabled Miller to conclude that the mechanism of resistance induced to irL3 was the result of an immunological phenomenon rather than a mechanical event (the attenuated L3 being trapped in the lung).
Miller used these “characteristics of attenuation” for the, product development, industrial manufacture, and USDA licensing of the 1st hookworm vaccine, which consisted of gamma-irradiated infective A. caninum L3 vaccine for canine.Citation42 The vaccine was field tested by approximately 1,500 practicing veterinarians across the United States.Citation41,Citation42 While the vaccine proved to be safe for the dogs and even effective (90%) for canine, it had a considerably short shelf-life (6 mo) and a unique set of storage condition. The vaccine was discontinued in 1975 due to commercial failure; most veterinarians were unwilling to incorporate it into their vaccination programs because of difficulties in storing the vaccine, the short shelf life, the lack of sterilizing immunity, and economics–it prevented routine de-worming of canines, a routine and rather lucrative source of income for veterinarians.Citation42
A Chance for Learning
Although the irL3 vaccine failed commercially, it provide compelling evidence that the possible existence of human hookworm vaccine and the expectations (see Target Product Profile in ) of such a vaccine.Citation39 First, dog owners complained about the appearance of eggs in feces following vaccination. Miller countered that it was an “unrealistic expectation for successful banishing of all hookworm infection: and that sterilizing immunity to such macro-parasite was probably not option; a reducing in the number of worms and not complete elimination of the worms was sufficient to have an important clinical effect”Citation42.Indeed, such expectation on the part of the dog-owners was not unreasonable. The majority of human vaccines are indeed sterilizing in nature, eliciting a rapid and specific immune response that kills the pathogen–a characteristic vital to combating viral and bacterial pathogens that reproduce asexually, such as varicella and measles or the effect of toxins such as from tetanus. Additionally, the success of human vaccines against viral and bacterial pathogens are measured in terms of the decreasing the incidence of disease in populations receiving the vaccination and also generate “herd immunity.” However, given the size and the life cycle of this blood-feeding parasitic nematodes, a human hookworm (or any other helminth vaccine for that matter) is unlikely have a sterilizing effect (see MaizelsCitation28,Citation50 for classic and still timely reviews of the challenges facing helminth vaccinologists) (Box 1). Hence, an important lesson learned from Miller is to product profile of a human hookworm vaccine (see ), the indication of which would be to reduce the total worm burden, resulting in an associated decrease in the morbidity from hookworm diseases ().Citation39 For example, Miller claimed that the irradiated canine hookworm vaccine was discontinued due to “the failure of veterinarians to differentially diagnose hookworm infection from hookworm disease”Citation42. In other words, it failed to induce a sterilizing immunity in canines. However, as Miller goes on to argue, the degree of pathology generated from hookworm is directly related to the number of worms residing within the host and simply infection alone; i.e., there is a critical difference between “hookworm infection” and “hookworm disease,” and it is based on the intensity of infection. The more hookworms infect the human or canine host the greater the chance for hookworm disease; hence, a human hookworm vaccine should target “hookworm disease” and not “target hookworm infection.” More specifically, a human hookworm vaccine should diminish the risk for moderate and heavy worm burdens and possible associated blood loss and clinical sequelae.Citation3,Citation39,Citation51,Citation52
Table 1. The optimal and acceptable profiles of a human hookworm vaccine given
Box 1. The Major Hurdle
The major hurdle in the development of a hookworm vaccine, as well as vaccines against other intestinal parasites, is that protection against these helminths has never been demonstrated in humans–a crucial impendent to the making of a prophylactic vaccine (for a classic review of these challenges see Citation28)
A critical characteristic of the irradiated canine hookworm vaccine developed by Miller was that the efficacy of the vaccine depended on the viability of the larvae.Citation39 More specifically, the attenuated larvae in the canine vaccine were able to live and generate antigens that would be secreted upon entry in the host.Citation39,Citation42 This viability factor has proven to be extremely informative for the development of a recombinant human hookworm vaccine. While immunizing canines with live attenuated hookworm larvae appears to have a low risk-benefit ratio, immunizing children with live attenuated hookworm larvae is both unpractical for hookworm control. Consequently, the observation dealing with larval viability coupled with findings that larval-secreted antigens induce protective immunityCitation39,Citation42 inspired era of successful antigen discovery 30 y later (Box 2).
Box 2. Limitations of field epidemiological of the immune response to hookworm and the impact on vaccine development
• Only a snapshot of a failed immunological situation can be taken, in which generations of the parasite have already successfully colonized its human host for months, years or possibly, even decades. While hookworms are though to live for 5 to 7 y in their human host 21, individuals are constantly becoming re-infected, substituting “old” infections with “new” infection and in many cases accumulating infections. In fact, the prevalence and intensity of hookworm infection increases with age, such that the elderly often have the greatest number of parasites in endemic areas.Citation86,Citation87
• It is difficult to classify humans into the different phases of hookwrom infection: (1) exposure, (2) pre-patency (larvae), patency (estbalsihed blood feeding infection), and post-patency (cured) as most individuals resident in endemic areas have already been infected for several years. Indeed, given the chronicity (5 to 7 y per worm) and the prevalence (up to 70% in some communities], it is often difficult to find hookworm näive individuals to follow longitudinally in order to elucidate the evolution of an immune response to pathogen from initial exposure to acute infection to chronic infection.
• Moreover, it is not ethically acceptable to follow hookworm-infected individuals for long periods of time without offering treatment, thus hampering longitudinal studies, on the evolution of chronic infection.
• The most common study design of the immune response to hookworm infection comes from the rather “unnatural” situation of the treatment-reinfection study design conducted in high-transmission areas. In this study design, patients are treated for their established hookworm infections with one of the usually very effective BMZ class of anthelminthic drugs and their immune response monitored until they become re-infected. usually within 12 mo. A comparison is then amde betwen individuals who become reinfected and those who do not. See Quinnell et al.Citation88 for an elegant example of this method.
A Model of Human Hookworm Infection
During the early 1980s, Carroll and Grove started doing work with the hookworm Ancylostoma ceylanicum.Citation53-Citation58 This species of hookworm could be a model of human hookworm due to its ability to infect humans. In 1984, they began a series of experiments to observe the parasitological, hematologic and immunologic responses to these hookworms in dogs.Citation53-Citation58 They successfully stimulated transitory lymphocytes using adult A. ceylanicum antigens. Carroll and Grove further observed that IgG antibodies to the antigens persisted for an indefinite period of time while specific IgM antibodies only lasted temporarily. Carroll and Grove later discovered that two immunizing doses of 1 mg of protein from A. ceylanicum suspended in Freund’s complete adjuvant significantly reduced fecal egg output and total intestinal adult hookworm numbers.Citation53-Citation58 They concluded that injection of soluble adult hookworm antigens preceding an infection would establish specific protective immunity. Simultaneously, Hotez and Cerami proposed developing vaccines comprised of recombinant soluble enzymes.Citation59,Citation60 These observations further improved the outlook for human hookworm vaccine development using soluble molecules rather than entire parasites that have previously been irradiated. Using such soluble molecules completely eliminated the chance of accidental infection that persisted with vaccines constructed from whole hookworm larvae. These findings proved to revolutionize the search for a successful human hookworm vaccine during the two decades to follow.Citation39
The Human Hookworm Vaccine
shows the optimal and acceptable profiles of a human hookworm vaccine, including the proposed indication, target population, and route of administration, as developed by the Human Hookworm Vaccine Initiative, the only group currently working on vaccines targeting this parasite. Ancylostoma Secreted Protein-2 of N. americanus (Na-ASP-2) is a 21 kDa protein that is secreted by infective hookworm larvae upon entry into the host.Citation61-Citation64 Vaccination of hamsters and dogs with recombinant ASP-2 results in reduced worm burdens and fecundity following challenge infection.Citation35 Furthermore, sera from vaccinated animals inhibit the migration of infective hookworm larvae through tissue in vitro. Studies of populations living in hookworm endemic areas have also shown that antibodies to ASP-2 are associated with a reduced risk of developing heavy hookworm infections.Citation35 Based on these results, Na-ASP-2 was chosen as a lead hookworm vaccine candidate.Citation39 In a phase 1 study in hookworm-naïve adults living in the United States, Na-ASP-2 adjuvanted with Alhydrogel® was well-tolerated and immunogenic.Citation65 However, a phase 1 safety and immunogenicity trial of this vaccine in healthy adults from a hookworm endemic area in rural Brazil has to be halted when 3 participants developed immediate, generalized urticarial reactions following a single injection of 10 ug of recombinant Na-ASP-2. Subsequent analysis showed that the urticarial reactions were associated with elevated levels of IgE antibodies specific for Na-ASP-2, which were present before immunization, most likely as a result of previous infection with hookworm.Citation66 A survey of adults and children from the same hookworm-endemic area revealed that a significant proportion had elevated levels of IgE to Na-ASP-2, therefore halting further development of the vaccine. These findings had a major implication for the development of vaccines against not only hookworm, but all helminths given their common propensity to induce strong T helper cell type 2 (Th2) immune responses and elevated levels of IgE to both parasite and bystander antigens.
Systemic allergic reactions are rare for licensed vaccines, averaging 0.65 cases per million doses.Citation67 Few of these reactions implicate the immunizing agent itself, as was the case with Na-ASP-2. More common are reports of allergic reactions to incipient components such as animal protein (gelatin), antibiotics (neomyocin), or latex (vial stoppers).Citation68 However, a comparable situation was reported for the clinical testing of the circumsporozoite Multiple Antigen Meptide (MAP) vaccine, an experimental vaccine against Plasmodium falciparum.Citation69 During phase 1 trials in malaria-unexposed individuals, vaccinees developed Iimediate-type hypersensitivity (ITH) reactions associated with elevated levels of IgE to MAP.Citation69 However, in contrast to the data presented here, vaccinees developed MAP-specific IgE only after repeated vaccinations and were not sensitized by a natural parasite infection such as was the case with Na-ASP-2. The mechanism behind these ITH reactions was hypothesized to be the presence of recombinant malarial proteins that were unbound to adjuvant, which cross-linked IgE attached to mast cells to stimulate release of histamine and other mast cell mediators involved in the pathogenesis of acute urticaria.Citation69 We propose a similar hypothesis whereby Na-ASP-2 cross-linked the IgE on mast cells armed during years of chronic infection, thus triggering a substantial histamine release.
It is possible that intrinsic structural or biological properties of the Na-ASP-2 molecule were responsible for the ITH reactions in previously infected individuals.Citation70 Few molecules have the necessary properties to bind and crosslink IgECitation71 (Traidl-Hoffmann 2009). An intriguing possibility is that humans have targeted a restricted range of antigens from metazoan parasites, such as helminths, to enable host defense without inducing self-harm or tolerance.Citation72 In this context, Na-ASP-2 would be both an attractive target for antibody-mediated defense against hookworm infection and a target for IgE. During penetration of host tissue, infective L3 of N. americanus encounter host-specific signals that induce a programmed chain of developmental events that result in the successful establishment of the parasitic relationship. Among the most abundant proteins released by hookworm larvae during this transition to parasitism are ASP-1 and ASP-2 65, both of which belong to the Pathogensis Related Protein (PRP) superfamily.Citation61-Citation64 Recombinant Na-ASP-2, expressed in Pichia pastoris, has shown significant protection in laboratory animal models, with sera from animals immunized with ASP-2 inhibiting the migration of infective larvae in vitro through host tissue.Citation35,Citation73 The latter observation suggests that antibodies against Na-ASP-2 might attenuate larvae during tissue migration and prevent them from reaching the host intestine and therefore developing into healthy adult hookworms.Citation35,Citation39
New Generation Hookworm Vaccines
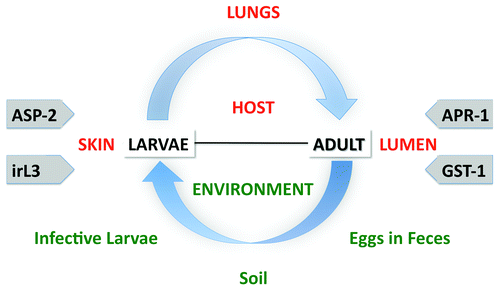
A new generation of hookworm vaccines have been described in detail elsewhereCitation3-Citation5 and are shown in in regards to their place in the hookworm life cycle. In brief, from more than 20 potential target proteins involved in the hookworm blood feeding process,Citation51,Citation74,Citation75 two lead proteins have emerged as promising candidate antigens for further clinical development, based on preclinical efficacy testing and reduced concern of hypersensitivity reactions as supported by data from human immunoepidemiology studies: Na-GST-1 and Na-APR-1. Each vaccine antigen is an enzyme (or modified enzyme) required for parasite blood feeding. When used as a vaccine, we hypothesize that they will induce neutralizing antibodies that will interfere with parasite blood-feeding and cause parasite death and reduced worm fecundity (). N. americanus hookworms depend on host hemoglobin for survival.Citation75 Following hemolysis, adult N. americanus use an ordered cascade of hemoglobinases beginning with the cleavage of intact hemoglobin by the aspartic protease Na-APR-1.Citation74-Citation77 The freed heme generates toxic oxygen radicals that can be bound and detoxified by homodimeric glutathione S-transferases (Na-GSTs).Citation78
Figure 3. The strategy for a human hookworm vaccine. Our previous strategy focused on excretory/secretory factors from the infective larval stage (L3). A newer strategy focuses on inhibit the consumption and detoxification of heme by the adult hookworm.
Na-glutathione S-transferase 1 (Na-GST-1) is a 24-kDa protein comprised of 205 amino acids and one N-glycosylation site.Citation79 Recombinant Na-GST-1 has been expressed in P. pastoris at high yield (> 0.2 g/L) and purified by three chromatographic steps. The X-ray crystal structure of this protein has been solved.Citation79 In dogs challenged with infective larvae, vaccination with Ac-GST-1 (the canine hookworm ortholog) induced high levels of antibody and resulted in substantially lower worm burdens (40%) and fecal egg counts compared with controls.Citation77 Using the hamster model of hookworm infection, approximately 57% protection (as measured by worm burden) was achieved by immunization with either Ac-GST-1 or Na-GST-1.Citation79,Citation80 Additionally, recent immunoepidemiological studies indicate that individuals living in hookworm endemic areas of Brazil who acquire anti-Na-GST-1 IgG1 antibodies have a significantly reduced risk of having heavy hookworm infections (unpublished data).
N. americanus-Aspartic Protease-1 (Na-APR-1) is a 45-kDa recombinant protein consisting of 407 amino acids with a C-terminal histidine tag.Citation77,Citation81 For stability and safety reasons, this aspartic protease has been inactivated by the mutation of the two canonical aspartic acid residues to alanines.Citation76 The recombinant protein has been expressed in multiple systems, with tobacco plants (Nicotania benthamiana) producing the highest yields with the greatest stability and solubility being achieved in the presence of zwitterionic detergents (unpublished data). In animals models, immunization with recombinant Na-APR-1 or its canine ortholog Ac-APR-1 substantially diminished blood loss, adult hookworm burden, and fecal egg counts, as well as reduced gut pathology compared with controls animals immunized with adjuvant alone.Citation74,Citation76
Both Na-GST-1 and Na-APR-1 have been formulated with the aluminum salt adjuvant Alhydrogel®. However, in addition to Alhydrogel®, it may be advantageous to co-administer these formulated antigens with one of the new generation of immunostimulants such as the Toll-like receptor agonist GLA-AF (an aqueous formulation of synthetic glucopyranosyl lipid A) as described inCitation82-Citation85 to improve not only the quantity of the antibody response (more antibody), but also the quality of this response (better affinity and the develop or greater memory B-cells production) (Box 3).
Box 3. Technical challenges for the development of a human hookworm vaccine
1. The difficulty of maintaining human hookworms in animal models and the cost of maintaining the hookworm in laboratory-canine model.
2. The absence of a laboratory animal that is permissive to human hookworms and can accurately reproduce human disease (anemia).
3. Paucity of in vitro functional tests to determine the effectiveness of the immune response induced by an experimental hookworm vaccine
4. The lack of a protective immune response in humans and the consequent absence of Correlates of Protection (CoP) that can guide the discovery of vaccine antigens and be used to assess their effectiveness in preclinical and clinical trials
5. No model of an effective immune response in humans to determine the biological effect of the vaccine in humans.
Current Plans
By simultaneously targeting two enzymes in the blood digestion and detoxification pathway, we hypothesize that the bivalent human hookworm vaccine will elicit neutralizing antibodies that will have a substantial impact on host worm burden (as measured by quantitative fecal egg counts) and blood loss. It is anticipated that vaccination with both recombinant proteins will be needed to disrupt this complex system of hemoglobin digestion, and thereby reduce parasite fecundity and survival at a more rapid rate. Based on previous animal trials conducted during preclinical efficacy testing of each antigen individually, it is anticipated that the impact of the HHV on human populations will prevent the establishment of adult hookworms in the range of 40–50%. By preventing the establishment (or re-establishment with reinfection) of adult hookworms, most (≥ 80%) moderate and heavy infections would be prevented along with the associated IDA).
Acknowledgments
The Human Hookworm Vaccine Initiative is supported by grants from the Bill and Melinda Gates Foundation, the Dutch Ministry of Foreign Affairs, and the Brazilian Ministry of Health. DJD and JMB are also supported by pilot funding from the Clinical and Translational Science Award (CTSA), from the Children’s National Medical Center and the George Washington University Medical Center
References
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol 2004; 58:197 - 288; http://dx.doi.org/10.1016/S0065-308X(04)58004-1; PMID: 15603764
- Thompson RC, Reynoldson JA, Garrow SC, McCarthy JS, Behnke JM. Towards the eradication of hookworm in an isolated Australian community. Lancet 2001; 357:770 - 1; http://dx.doi.org/10.1016/S0140-6736(00)04162-3; PMID: 11253974
- Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, Loukas A, et al. Vaccines to combat the neglected tropical diseases. Immunol Rev 2011; 239:237 - 70; http://dx.doi.org/10.1111/j.1600-065X.2010.00976.x; PMID: 21198676
- Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol 2010; 8:814 - 26; http://dx.doi.org/10.1038/nrmicro2438; PMID: 20948553
- Hotez PJ, Bethony JM, Oliveira SC, Brindley PJ, Loukas A. Multivalent anthelminthic vaccine to prevent hookworm and schistosomiasis. Expert Rev Vaccines 2008; 7:745 - 52; http://dx.doi.org/10.1586/14760584.7.6.745; PMID: 18665774
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA 2008; 299:1937 - 48; http://dx.doi.org/10.1001/jama.299.16.1937; PMID: 18430913
- Gonçalves ML, Araujo A, Ferreira LF. Human intestinal parasites in the past: new findings and a review. Mem Inst Oswaldo Cruz 2003; 98:Suppl 1 103 - 18; http://dx.doi.org/10.1590/S0074-02762003000900016; PMID: 12687769
- Dock G, Bass C. Hookworm Disease. St Louis: Mosby Co, 1910.
- Stiles CW. A new species of hookworm (Uncinaria americana) parasite in man. American Medicine 1902; 3:777 - 8
- Stiles CW. Frequency ofhookworm disease or ground itch anemia among public school children in Southern Florida. Public Health Rep 1910; 25:351 - 4; http://dx.doi.org/10.2307/4564592; PMID: 19314191
- Farley J. To Cast Out Disease: A History of the International Health Division of Rockefeller Foundation [1913-1951], Oxford University Press 2004.
- Stiles CW, Leonard G. Hookworm Diseases: number of treatments and number of doses of thymol adminisered in 61 hospital and 22 home-cured cases of hookworm infection. Public Health Rep 1913; 28:119 - 24; http://dx.doi.org/10.2307/4569192
- Stiles CW, Miller H. Observatioins on the viability of the eggs of hookworms (Necator americanus) and of eelworms (Ascaris lumbricoides) in feces allowed to decompose in water. Public Health Rep 1911; 26:1565 - 1600; http://dx.doi.org/10.2307/4566877; PMID: 19314272
- Stoll NR. This wormy world. J Parasitol 1947; 33:1 - 18; PMID: 20284977
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 2003; 19:547 - 51; http://dx.doi.org/10.1016/j.pt.2003.10.002; PMID: 14642761
- Nawalinski T, Schad G. Historical Introduction. In: Gilles H, Ball P, eds. Human parasitic dieases volume 4: Hookwrom Infection, 1991:1-4.
- Bundy D. Is hookworm just another geohelminth? In: Schad GA, Warren K, eds. Hookworm Diseases–current status and new directions. London: Taylor and Francis, 1990:146-64.
- Stiles CW. Is It “Fair to Say That Hookworm Disease Has Almost Disappeared from the United States?”. Science 1933; 77:237 - 9; http://dx.doi.org/10.1126/science.77.1992.237; PMID: 17773059
- Ministry of Health, PRC, National Institute of Parasitic Diseases. Report on the national survey of the current situation of major human parasitic diseases in China. Beijing: Ministry of Health, 2005.
- Ehrenberg JP, Ault SK. Neglected diseases of neglected populations: thinking to reshape the determinants of health in Latin America and the Caribbean. BMC Public Health 2005; 5:119; http://dx.doi.org/10.1186/1471-2458-5-119; PMID: 16283932
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 2006; 367:1521 - 32; http://dx.doi.org/10.1016/S0140-6736(06)68653-4; PMID: 16679166
- Crompton DW. The public health importance of hookworm disease. Parasitology 2000; 121:Suppl S39 - 50; http://dx.doi.org/10.1017/S0031182000006454; PMID: 11386690
- Loukas A, Constant SL, Bethony JM. Immunobiology of hookworm infection. FEMS Immunol Med Microbiol 2005; 43:115 - 24; http://dx.doi.org/10.1016/j.femsim.2004.11.006; PMID: 15681140
- Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J 2001; 20:63 - 75; http://dx.doi.org/10.1097/00006454-200101000-00013; PMID: 11176570
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055 - 65; http://dx.doi.org/10.1128/CVI.00131-10; PMID: 20463105
- Geiger SM, Caldas IR, Mc Glone BE, Campi-Azevedo AC, De Oliveira LM, Brooker S, et al. Stage-specific immune responses in human Necator americanus infection. Parasite Immunol 2007; 29:347 - 58; http://dx.doi.org/10.1111/j.1365-3024.2007.00950.x; PMID: 17576364
- Geiger SM, Fujiwara RT, Santiago H, Correa-Oliveira R, Bethony JM. Early stage-specific immune responses in primary experimental human hookworm infection. Microbes Infect 2008; 10:1524 - 35; http://dx.doi.org/10.1016/j.micinf.2008.09.003; PMID: 18848637
- Maizels RM, Holland MJ, Falcone FH, Zang XX, Yazdanbakhsh M. Vaccination against helminth parasites–the ultimate challenge for vaccinologists?. Immunol Rev 1999; 171:125 - 47; http://dx.doi.org/10.1111/j.1600-065X.1999.tb01345.x; PMID: 10582168
- Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med 2009; 206:2059 - 66; http://dx.doi.org/10.1084/jem.20091903; PMID: 19770272
- Maizels RM, Yazdanbakhsh M. T-cell regulation in helminth parasite infections: implications for inflammatory diseases. Chem Immunol Allergy 2008; 94:112 - 23; http://dx.doi.org/10.1159/000154944; PMID: 18802342
- Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 2003; 3:733 - 44; http://dx.doi.org/10.1038/nri1183; PMID: 12949497
- Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol 2001; 22:372 - 7; http://dx.doi.org/10.1016/S1471-4906(01)01958-5; PMID: 11429321
- Hagan P. IgE and protective immunity to helminth infections. Parasite Immunol 1993; 15:1 - 4; http://dx.doi.org/10.1111/j.1365-3024.1993.tb00565.x; PMID: 8433850
- Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium.. Nature 1991; 349:243 - 5; http://dx.doi.org/10.1038/349243a0; PMID: 1898985
- Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J 2005; 19:1743 - 5; PMID: 16037096
- Erb KJ. Helminths, allergic disorders and IgE-mediated immune responses: where do we stand?. Eur J Immunol 2007; 37:1170 - 3; http://dx.doi.org/10.1002/eji.200737314; PMID: 17447233
- Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med 2006; 174:514 - 23; http://dx.doi.org/10.1164/rccm.200603-331OC; PMID: 16778161
- Qin L, Gilbert PB, Corey L, McElrath MJ, Self SG. A framework for assessing immunological correlates of protection in vaccine trials. J Infect Dis 2007; 196:1304 - 12; http://dx.doi.org/10.1086/522428; PMID: 17922394
- Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis 2006; 6:733 - 41; http://dx.doi.org/10.1016/S1473-3099(06)70630-2; PMID: 17067922
- Fujiwara RT, Geiger SM, Bethony J, Mendez S. Comparative immunology of human and animal models of hookworm infection. Parasite Immunol 2006; 28:285 - 93; http://dx.doi.org/10.1111/j.1365-3024.2006.00821.x; PMID: 16842265
- Miller TA. Vaccination against the canine hookworm diseases. Adv Parasitol 1971; 9:153 - 83; http://dx.doi.org/10.1016/S0065-308X(08)60161-X; PMID: 4932829
- Miller TA. Industrial development and field use of the canine hookworm vaccine. Adv Parasitol 1978; 16:333 - 42; http://dx.doi.org/10.1016/S0065-308X(08)60577-1; PMID: 364958
- Xiao S, Zhan B, Xue J, Goud GN, Loukas A, Liu Y, et al. The evaluation of recombinant hookworm antigens as vaccines in hamsters (Mesocricetus auratus) challenged with human hookworm, Necator americanus.. Exp Parasitol 2008; 118:32 - 40; http://dx.doi.org/10.1016/j.exppara.2007.05.010; PMID: 17645877
- Miller TA. Effect of X-irradiation upon the infective larvae of Ancylostoma caninum and the immunogenic effect in dogs of a single infection with 40 Kr-Irradiated larvae. J Parasitol 1964; 50:735 - 42; http://dx.doi.org/10.2307/3276194; PMID: 14244804
- Miller TA. Effect of age of the dog on immunogenic efficiency of double vaccination with x-irradiated Ancylostoma caninum larvae. Am J Vet Res 1965; 26:1383 - 90; PMID: 5882555
- Miller TA. Effect of route of administration of vaccine and challenge on the immunogenic efficiency of double vaccination with irradiated Ancylostoma Caninum Larvae. J Parasitol 1965; 51:200 - 6; http://dx.doi.org/10.2307/3276081; PMID: 14275207
- Wilson RA, Coulson PS, Mountford AP. Immune responses to the radiation-attenuated schistosome vaccine: what can we learn from knock-out mice?. Immunol Lett 1999; 65:117 - 23; http://dx.doi.org/10.1016/S0165-2478(98)00134-5; PMID: 10065637
- Jennings FW, Mulligan W, Urquhart GM. Variables in X-ray “inactivation” of Nippostrongylus Brasiliensis larvae. Exp Parasitol 1963; 13:367 - 73; http://dx.doi.org/10.1016/0014-4894(63)90087-0
- Miller TA. Transfer of immunity to Ancylostoma caninum infection in pups by serum and lymphoid cells. Immunology 1967; 12:231 - 41; PMID: 6020124
- Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites–masters of regulation. Immunol Rev 2004; 201:89 - 116; http://dx.doi.org/10.1111/j.0105-2896.2004.00191.x; PMID: 15361235
- Bethony JM, Loukas A, Hotez PJ, Knox DP. Vaccines against blood-feeding nematodes of humans and livestock. Parasitology 2006; 133:Suppl S63 - 79; http://dx.doi.org/10.1017/S0031182006001818; PMID: 17274849
- Diemert DJ, Bethony JM, Hotez PJ. Hookworm vaccines. Clin Infect Dis 2008; 46:282 - 8; http://dx.doi.org/10.1086/524070; PMID: 18171264
- Carroll SM, Grove DI, Heenan PJ. Kinetics of cells in the intestinal mucosa of mice following oral infection with Ancylostoma ceylanicum.. Int Arch Allergy Appl Immunol 1986; 79:26 - 32; http://dx.doi.org/10.1159/000233937; PMID: 3941009
- Carroll SM, Howse DJ, Grove DI. The anticoagulant effects of the hookworm, Ancylostoma ceylanicum: observations on human and dog blood in vitro and infected dogs in vivo. Thromb Haemost 1984; 51:222 - 7; PMID: 6740554
- Carroll SM, Karthigasu KT, Grove DI. Serodiagnosis of human strongyloidiasis by an enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg 1981; 75:706 - 9; http://dx.doi.org/10.1016/0035-9203(81)90156-5; PMID: 7036430
- Carroll SM, Mayrhofer G, Dawkins HJ, Grove DI. Kinetics of intestinal lamina propria mast cells, globule leucocytes, intraepithelial lymphocytes, goblet cells and eosinophils in murine strongyloidiasis. Int Arch Allergy Appl Immunol 1984; 74:311 - 7; http://dx.doi.org/10.1159/000233566; PMID: 6735488
- Carroll SM, Pryor J, Kennett DW, Grove DI. Investigation of the haemolytic effects of Ancylostoma ceylanicum: observations on infected dogs in vivo and human and dog blood in vitro. Southeast Asian J Trop Med Public Health 1984; 15:129 - 34; PMID: 6740375
- Carroll SM, Robertson TA, Papadimitriou JM, Grove DI. Transmission electron microscopical studies of the site of attachment of Ancylostoma ceylanicum to the small bowel mucosa of the dog. J Helminthol 1984; 58:313 - 20; http://dx.doi.org/10.1017/S0022149X00025189; PMID: 6520374
- Hotez PJ, Le Trang N, Cerami A. Hookworm antigens: the potential for vaccination. Parasitol Today 1987; 3:247 - 9; http://dx.doi.org/10.1016/0169-4758(87)90148-7; PMID: 15462967
- Hotez PJ, Trang NL, McKerrow JH, Cerami A. Isolation and characterization of a proteolytic enzyme from the adult hookworm Ancylostoma caninum.. J Biol Chem 1985; 260:7343 - 8; PMID: 3888998
- Asojo OA, Homma K, Sedlacek M, Ngamelue M, Goud GN, Zhan B, et al. X-ray structures of Na-GST-1 and Na-GST-2 two glutathione S-transferase from the human hookworm Necator americanus.. BMC Struct Biol 2007; 7:42; http://dx.doi.org/10.1186/1472-6807-7-42; PMID: 17594497
- Hawdon JM, Jones BF, Hoffman DR, Hotez PJ. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem 1996; 271:6672 - 8; PMID: 8636085
- Hawdon JM, Narasimhan S, Hotez PJ. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum.. Mol Biochem Parasitol 1999; 99:149 - 65; http://dx.doi.org/10.1016/S0166-6851(99)00011-0; PMID: 10340481
- Parkinson J, Mitreva M, Whitton C, Thomson M, Daub J, Martin J, et al. A transcriptomic analysis of the phylum Nematoda. Nat Genet 2004; 36:1259 - 67; http://dx.doi.org/10.1038/ng1472; PMID: 15543149
- Bethony JM, Simon G, Diemert DJ, Parenti D, Desrosiers A, Schuck S, et al. Randomized, placebo-controlled, double-blind trial of the Na-ASP-2 hookworm vaccine in unexposed adults. Vaccine 2008; 26:2408 - 17; http://dx.doi.org/10.1016/j.vaccine.2008.02.049; PMID: 18396361
- Diemert DJ, Pinto AG, Freire J, Jariwala A, Hamilton R, Periago MV, et al. Generalized Urticaria Induced by the Na-ASP-2 hookworm vaccine – implications for the development of vaccines against helminths. J Allergy Clin Immunol 2011; submitted
- Bohlke K, Davis RL, Marcy SM, Braun MM, DeStefano F, Black SB, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics 2003; 112:815 - 20; http://dx.doi.org/10.1542/peds.112.4.815; PMID: 14523172
- Madaan A, Maddox DE. Vaccine allergy: diagnosis and management. Immunol Allergy Clin North Am 2003; 23:555 - 88; http://dx.doi.org/10.1016/S0889-8561(03)00099-7; PMID: 14753381
- Edelman R, Wasserman SS, Kublin JG, Bodison SA, Nardin EH, Oliveira GA, et al. Immediate-type hypersensitivity and other clinical reactions in volunteers immunized with a synthetic multi-antigen peptide vaccine (PfCS-MAP1NYU) against Plasmodium falciparum sporozoites. Vaccine 2002; 21:269 - 80; http://dx.doi.org/10.1016/S0264-410X(02)00468-1; PMID: 12450702
- Chapman MD, Pomes A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol 2007; 119:414 - 20; http://dx.doi.org/10.1016/j.jaci.2006.11.001; PMID: 17166572
- Traidl-Hoffmann C, Jakob T, Behrendt H. Determinants of allergenicity. J Allergy Clin Immunol 2009; 123:558 - 66; http://dx.doi.org/10.1016/j.jaci.2008.12.003; PMID: 19152966
- Fitzsimmons CM, Dunne DW. Survival of the fittest: allergology or parasitology?. Trends Parasitol 2009; 25:447 - 51; http://dx.doi.org/10.1016/j.pt.2009.07.004; PMID: 19744885
- Goud GN, Bottazzi ME, Zhan B, Mendez S, Deumic V, Plieskatt J, et al. Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials. Vaccine 2005; 23:4754 - 64; http://dx.doi.org/10.1016/j.vaccine.2005.04.040; PMID: 16054275
- Williamson AL, Lecchi P, Turk BE, Choe Y, Hotez PJ, McKerrow JH, et al. A multi-enzyme cascade of hemoglobin proteolysis in the intestine of blood-feeding hookworms. J Biol Chem 2004; 279:35950 - 7; http://dx.doi.org/10.1074/jbc.M405842200; PMID: 15199048
- Ranjit N, Zhan B, Hamilton B, Stenzel D, Lowther J, Pearson M, et al. Proteolytic degradation of hemoglobin in the intestine of the human hookworm Necator americanus.. J Infect Dis 2009; 199:904 - 12; http://dx.doi.org/10.1086/597048; PMID: 19434933
- Loukas A, Bethony JM, Mendez S, Fujiwara RT, Goud GN, Ranjit N, et al. Vaccination with recombinant aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in dogs. PLoS Med 2005; 2:e295; http://dx.doi.org/10.1371/journal.pmed.0020295; PMID: 16231975
- Pearson MS, Bethony JM, Pickering DA, de Oliveira LM, Jariwala A, Santiago H, et al. An enzymatically inactivated hemoglobinase from Necator americanus induces neutralizing antibodies against multiple hookworm species and protects dogs against heterologous hookworm infection. FASEB J 2009; 23:3007 - 19; http://dx.doi.org/10.1096/fj.09-131433; PMID: 19380510
- Perally S, Lacourse EJ, Campbell AM, Brophy PM. Heme transport and detoxification in nematodes: subproteomics evidence of differential role of glutathione transferases. J Proteome Res 2008; 7:4557 - 65; http://dx.doi.org/10.1021/pr800395x; PMID: 18720983
- Zhan B, Liu S, Perally S, Xue J, Fujiwara R, Brophy P, et al. Biochemical characterization and vaccine potential of a heme-binding glutathione transferase from the adult hookworm Ancylostoma caninum.. Infect Immun 2005; 73:6903 - 11; http://dx.doi.org/10.1128/IAI.73.10.6903-6911.2005; PMID: 16177370
- Zhan B, Perally S, Brophy PM, Xue J, Goud G, Liu S, et al. Molecular cloning, biochemical characterization, and partial protective immunity of the heme-binding glutathione S-transferases from the human hookworm Necator americanus. Infect Immun 2010; 78:1552 - 63; http://dx.doi.org/10.1128/IAI.00848-09; PMID: 20145100
- Pearson MS, Pickering DA, Tribolet L, Cooper L, Mulvenna J, Oliveira LM, et al. Neutralizing antibodies to the hookworm hemoglobinase Na-APR-1: implications for a multivalent vaccine against hookworm infection and schistosomiasis. J Infect Dis 2010; 201:1561 - 9; http://dx.doi.org/10.1086/651953; PMID: 20367477
- Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE 2011; 6:e16333; http://dx.doi.org/10.1371/journal.pone.0016333; PMID: 21298114
- Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS ONE 2010; 5:e13677; http://dx.doi.org/10.1371/journal.pone.0013677; PMID: 21060869
- Coler RN, Carter D, Friede M, Reed SG. Adjuvants for malaria vaccines. Parasite Immunol 2009; 31:520 - 8; http://dx.doi.org/10.1111/j.1365-3024.2009.01142.x; PMID: 19691556
- Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol 2009; 30:23 - 32; http://dx.doi.org/10.1016/j.it.2008.09.006; PMID: 19059004
- Bethony J, Chen J, Lin S, Xiao S, Zhan B, Li S, et al. Emerging patterns of hookworm infection: influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin Infect Dis 2002; 35:1336 - 44; http://dx.doi.org/10.1086/344268; PMID: 12439796
- Gandhi NS, Jizhang C, Khoshnood K, Fuying X, Shanwen L, Yaoruo L, et al. Epidemiology of Necator americanus hookworm infections in Xiulongkan Village, Hainan Province, China: high prevalence and intensity among middle-aged and elderly residents. J Parasitol 2001; 87:739 - 43; PMID: 11534635
- Quinnell RJ, Pullan RL, Breitling LP, Geiger SM, Cundill B, Correa-Oliveira R, et al. Genetic and household determinants of predisposition to human hookworm infection in a Brazilian community. J Infect Dis 2010; 202:954 - 61; http://dx.doi.org/10.1086/655813; PMID: 20681887