Abstract
The immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine (Cervarix®, GlaxoSmithKline Biologicals) administered according to its licensed vaccination schedule (3-dose, 3D) and formulation (20 μg of each HPV antigen; 20/20F) has previously been demonstrated. This partially-blind, controlled, randomized trial (NCT00541970) evaluated 2-dose (2D) schedules using the licensed 20/20F or an alternative formulation containing 40 μg of each antigen (40/40F), compared with the licensed 3D schedule. Healthy females stratified by age (9-14, 15-19, 20-25 years) were randomized to receive 2 doses of 20/20F at Months (M) 0,6 (n=240), 40/40F at M0,6 (n=241) or 40/40F at M0,2 (n=240), or 3 doses of 20/20F at M0,1,6 (licensed schedule/formulation, n=239). One month after the last dose, the 3D schedule was not immunologically superior to 2D schedules except in the 40/40F M0,2 group for HPV-16 (lower limit of 95% CI geometric mean antibody titer (GMT) ratio [2D/3D] <0.5). For both HPV-16 and HPV-18, the 2D schedules in girls 9-14 years were immunologically non-inferior to the 3D schedule in women 15-25 years (the age group in which efficacy has been demonstrated) (upper limit of 95% CI for GMT ratio [3D/2D] <2) one month after the last dose. At Month 24, non-inferiority was maintained for the 2D M0,6 schedules in girls 9-14 years versus the 3D schedule in women 15-25 years. All formulations had acceptable reactogenicity and safety profiles. These results indicate that the HPV-16/18 vaccine on a 2D M0,6 schedule is immunogenic and generally well tolerated in girls 9-14 years and that the 2D schedule is likely adequate for younger females.
GlaxoSmithKline Biologicals has developed a prophylactic human papillomavirus (HPV) vaccine (Cervarix®) containing L1 proteins of HPV-16 and HPV-18 formulated with AS04 (comprised of aluminum hydroxide and 3-O-desacyl-4′-monophosphoryl lipid A). In clinical trials, the vaccine has been shown to have a clinically acceptable safety profile,Citation1 to elicit antibody titers that are well above those induced by natural HPV infection,Citation2 and to be efficacious against persistent infections and high grade cervical lesions associated with HPV-16 and/or HPV-18,Citation3,Citation4 as well as certain non-vaccine oncogenic types (i.e., HPV-31, 33 and 45).Citation4 The vaccine was first approved in 2007 and is currently licensed in over 100 countries worldwide, including the US, Canada and countries in the European Union. The licensed schedule for the HPV-16/18 AS04-adjuvanted vaccine consists of three doses to be given at months (M) 0,1,6, with each dose formulated to contain 20 µg of HPV-16 L1 protein virus-like particles (VLP) and 20 µg of HPV-18 L1 VLP.
There is no accepted immunological correlate of protection against persistent HPV infections or lesions. Antibody titers are generally used as a surrogate marker of protection in clinical trials, if it is not feasible to evaluate efficacy. For example, as efficacy studies cannot be conducted in young girls, the licensure of the HPV-16/18 AS04-adjuvanted vaccine in preteens/adolescents was based on immunological bridging studies with data from young women aged 15–25 y, as efficacy has previously been demonstrated in this age group.Citation2–Citation6 These immunobridging studies showed that three doses of the HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls aged 10–14 y induced geometric mean antibody titers (GMTs), measured by ELISA, that were approximately 2-fold higher than those elicited in women aged 15–25 y.Citation7
The higher immunogenicity of the vaccine in young adolescents, along with logistical issues linked to the completion of a 3-dose vaccination course in this population, as well as potential cost savings, has prompted discussions about the evaluation of a 2-dose HPV vaccine schedule. The current trial was therefore designed to evaluate options for a 2-dose schedule, using either the currently licensed vaccine formulation [HPV-16/18 AS04-adjuvanted formulation containing 20 µg of each HPV antigen (referred to as 20/20F)], or an increased antigen dosage formulation that contained twice the amount of each HPV VLP (40 µg, referred to as 40/40F). The higher dosage formulation was investigated as an alternative to the licensed vaccine formulation, should the latter not induce an acceptable immune response on a 2-dose schedule. Both the licensed 20/20F and the alternative 40/40F were evaluated on a 2-dose schedule at M0,6; the 40/40F was also evaluated on a 2-dose schedule at M0,2.
The co-primary objectives of this trial were to evaluate the immunogenicity and reactogenicity of each 2-dose schedule compared with the licensed 3-dose regimen. Secondary objectives were to assess non-inferiority of GMTs for different age strata (9–14 y, 15–19 y and 20–25 y) for each 2-dose schedule, compared with the licensed 3-dose schedule in subjects aged 15–25 y (the age group in which efficacy was previously demonstrated in ref. Citation2–Citation6); to evaluate the kinetics of antibody responses; and to evaluate safety.
Results
Trial population.
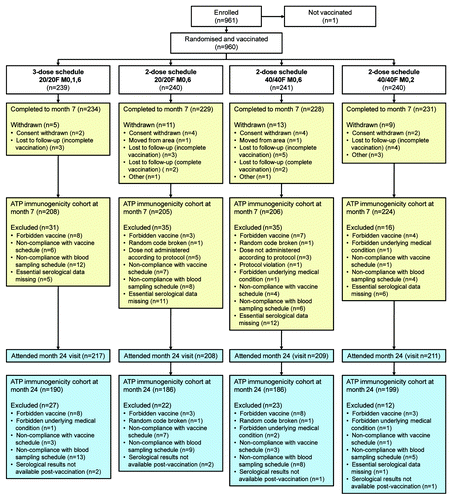
A total of 961 girls and young women were enrolled of whom 960 received at least one vaccine dose, representing the total vaccinated cohort (1 subject was excluded as she was not vaccinated) (). In total, 922 (96%) subjects completed the trial to Month 7 and 845 (88%) completed the Month 24 visit. No subject withdrew from the trial due to an adverse event (AE) or serious adverse event (SAE). The according-to-protocol (ATP) immunogenicity cohort included 843 (88%) subjects at Month 7 and 761 (79%) subjects at Month 24. Reasons for exclusion from ATP immunogenicity cohorts are shown in .
The four groups were comparable in terms of baseline demographics (). Overall, the mean age of vaccinated participants was 17.2 y with approximately one-third of subjects in each age stratum, and 97% of subjects were of Caucasian/European ethnic heritage.
Overall, 730 (87%) and 734 (87%) subjects in the ATP immunogenicity cohort were seronegative at baseline for HPV-16 and HPV-18 antibodies, respectively (Table S1). Baseline serostatus by age stratum is also shown in Table S1.
Immunogenicity.
All subjects who were initially seronegative for HPV-16 and HPV-18 antibodies had seroconverted when assessed one month after the last vaccine dose (i.e., Month 3 in the 40/40F M0,2 group and Month 7 in the other groups). All subjects evaluated at Month 24 were still seropositive.
The primary immunogenicity objective was to evaluate the immune response to each 2-dose schedule compared with the licensed 3-dose regimen. The 3-dose regimen was considered to be superior to a 2-dose schedule if there was more than a 2-fold difference in the ratio of GMTs (i.e., the lower limit of the 95% confidence interval (CI) around the GMT ratio was <0.5). Differences in HPV-16 and -18 GMTs between the four vaccine groups were evaluated for subjects who were seronegative at baseline using a 2-way analysis of variance (ANOVA) model, for each antibody separately. The group-by-age interaction term in this model was not significant at 10% for either antibody; therefore, comparisons of the licensed 3-dose schedule vs. each 2-dose schedule were made across all age strata combined. One month after the last vaccine dose and for both HPV-16 and HPV-18, the licensed 3-dose 20/20F M0,1,6 schedule was not superior to the 2-dose schedules, except for HPV-16 where it was superior to the 2-dose 40/40F M0,2 schedule (). GMTs elicited by each schedule/formulation, by age stratum, are shown in for subjects who were seronegative at baseline.
A secondary objective was to assess sequentially for each age stratum (9–14 y, 15–19 y and 20–25 y) the non-inferiority of the 2-dose schedule groups, compared with the licensed 3-dose schedule in subjects aged 15–25 y in terms of HPV-16 and HPV- 18 GMTs. Non-inferiority was demonstrated if the upper limit of the 95% CI for the GMT ratio between the licensed 3-dose schedule in subjects 15–25 y of age over the 2-dose schedule in each year age stratum was below 2. For the 9–14 y age stratum, non-inferiority was demonstrated one month after the last vaccine dose for both HPV-16 and HPV-18 GMTs for each of the 2-dose schedules, when compared with the licensed 3-dose schedule in subjects aged 15–25 y (). For the 15–19 y age stratum, and for both HPV-16 and HPV-18, non-inferiority was demonstrated one month after the last vaccine dose for each of the 2-dose schedules when compared with the licensed 3-dose schedule in subjects aged 15–25 y, except for the 40/40F M0,2 schedule for HPV-16 (). For the 20–25 y age stratum, non-inferiority was demonstrated one month after the last vaccine dose when compared with the licensed 3-dose schedule in subjects aged 15–25 y for the 40/40F M0,6 group but not the 20/20F M0,6 or 40/40F M0,2 groups for HPV-16 and for the 20/20F M0,6 and 40/40F M0,6 groups but not the 40/40F M0,2 group for HPV-18 ().
For the 9–14 y age stratum, non-inferiority was also demonstrated at Month 24 for both HPV-16 and HPV-18 GMTs for the 20/20F M0,6 and 40/40F M0,6 groups, but not for the 40/40F M0,2 group, when compared with the licensed 3-dose schedule in subjects aged 15–25 y (). The kinetics of antibodies against both HPV-16 and HPV-18 in the 2-dose 20/20F M0,6 and 40/40F M0,6 vaccine groups for girls aged 9–14 y followed a similar pattern to those observed in the licensed 3-dose vaccine group for young women aged 15–25 y, i.e., after a peak response at Month 7, a parallel decline in antibody titers toward a plateau was observed (). In the 40/40F M0,2 group, GMTs peaked at Month 3 and antibody titers from Month 7 through Month 24 appeared lower than in other groups.
Reactogenicity and safety.
Pain was the most frequently reported local solicited symptom in the 7-d post-vaccination period, reported after 81% to 86% of all doses across groups (). Redness was reported after 33% to 41% of doses and swelling after 24% to 31% of doses. No urticaria or rash was observed within 30 min after any vaccine dose in the four groups. Headache, fatigue and myalgia were the most frequently reported general solicited symptoms in the 7-d post-vaccination period (). Headache was reported after 29% to 33% of doses, fatigue after 29% to 32% of doses and myalgia after 25% to 33% of doses.
The percentage of subjects reporting at least one unsolicited symptom was 45%, 32%, 35% and 35% in the 20/20F M0,1,6, 20/20F M0,6, 40/40F M0,6 and 40/40F M0,2 groups, respectively. The percentage of subjects reporting at least one grade 3 unsolicited symptom ranged from 3% to 6% across groups. The three most common unsolicited symptoms reported were nasopharyngitis (ranging from 4% to 6% across groups), headache (2% to 4%) and pharyngolaryngeal pain (1% to 4%). No deaths were reported (). A total of 34 subjects reported 43 non-fatal SAEs through Month 24; none of the reported SAEs were considered related to vaccination by the investigator. No AEs or SAEs led to the premature discontinuation of study vaccine and/or withdrawal from the trial.
Medically-significant AEs and new onset autoimmune diseases were reported at a similar frequency across groups (). Reported new onset autoimmune diseases consisted of thyroid disorders and one case each of diabetes mellitus, celiac disease and reactive arthritis.
During the entire trial period through Month 24, 36 pregnancies were reported: 23 resulted in birth of a normal infant, 10 ended by elective termination, 2 resulted in a premature birth and the outcome of 1 was unknown.
Discussion
This is the first publication of the HPV-16/18 AS04-adjuvanted vaccine given as a 2-dose schedule. We showed that 2 doses of the HPV-16/18 AS04-adjuvanted vaccine administered to girls aged 9–14 y (the main population for organized HPV vaccination programs) elicited HPV-16 and -18 immune responses that were non-inferior to those elicited by the same vaccine given as a 3-dose schedule in young women aged 15–25 y (the age group in which efficacy has previously been demonstrated in clinical studies in ref. Citation2–Citation6) one month after the last vaccine dose. Non-inferiority was also shown at Month 24. All schedules and formulations tested had a clinically acceptable safety profile through Month 24, which was similar to that previously reported for this vaccine in other clinical trials.Citation1
Our trial also tested a formulation of the HPV-16/18 AS04-adjuvanted vaccine that contained a higher dosage of each HPV antigen (40/40 formulation). This increased antigen content formulation given over a 6-mo dosing interval (40/40F M0,6) induced similar antibody titers to the licensed 3-dose 20/20F M0,1,6 schedule and in girls aged 9–14 y, HPV-16 and -18 immune responses were non-inferior to those induced by the licensed 3-dose 20/20F M0,1,6 schedule in young women aged 15–25 y. The use of a shorter 2-mo dosing interval (40/40F M0,2), however, induced lower antibody titers than the other formulations. This 40/40F M0,2 schedule was non-inferior one month after the last vaccine dose when given to girls aged 9–14 y compared with the licensed 3-dose schedule in women aged 15–25 y, but did not achieve non-inferiority at Month 24. This suggests that the interval between vaccine doses is likely to be an important factor for the induction of immune response with a 2-dose formulation, as previously demonstrated for other vaccines such as hepatitis B.Citation8 Therefore, further development of the 40/40 formulation is currently not justified, as the 20/20F M0,6 formulation elicits an immune response in adolescents that is non-inferior to the 3-dose schedule in young women.
When antibody titers were considered within each age stratum, we observed that for 9–14 y-old girls, the licensed 3-dose schedule elicited an immune response that was generally higher than the one elicited with the 20/20F M0,6 schedule for HPV-16, but not for HPV-18. The clinical relevance of this observation is unknown, as titers elicited after 2 doses in girls aged 9–14 y were comparable to those elicited after 3 doses in young women, and these levels have been determined to be sufficient to confer protection. The observed differences in antibody titers could potentially affect the longevity of immune responses for 2-dose schedules, and long-term assessment of subjects will help to more fully elucidate the impact of this finding. This trial has now been extended to a follow-up period of 5 y following the start of the vaccination series to assess longevity.
A randomized trial comparing a 2-dose M0,6 and 3-dose M0,2,6 schedule of the HPV-6/11/16/18 vaccine (Gardasil®, Merck and Co., Inc.,) also showed that HPV-16 and -18 antibody responses to the 2-dose schedule in adolescent girls were non-inferior to the 3-dose schedule in young women one month after the last vaccine dose, and at Month 24.Citation9
In the absence of an identified correlate of protection, and to put the immunogenicity results observed in this trial into context, the observed GMTs in the current study were retrospectively compared with other immunogenicity benchmarks, i.e., the GMTs achieved in subjects who were able to clear a natural infection and mount an immune response,Citation3 and the GMTs achieved in vaccinated subjects in whom sustained protection has been shown up to 6.4 y after first vaccination ().Citation2 In all vaccine groups, HPV-16 and HPV-18 antibody titers at Month 24 remained well above these benchmarks.
A limitation of our trial is that HPV-16/18 immune responses were assessed using ELISA, whereas preclinical data suggest that neutralizing antibodies are probably the primary mediator of protection.Citation10–Citation15 An excellent correlation between ELISA and the pseudovirion based neutralization assay was previously demonstrated during the clinical development of the HPV-16/18 vaccine, supporting the use of ELISA as the primary read-out in clinical trials.Citation16 Ultimately, measurement of neutralizing antibodies and their persistence over time would be valuable to better characterize the immune response induced by 2-dose schedules. Another limitation of our trial was the relatively small number of participants evaluated; the trial was designed to evaluate if it would be feasible to use a 2-dose schedule rather than the licensed 3-dose regimen, but findings require further evaluation in confirmatory trials. Finally, our trial only evaluated the 2-dose schedules in terms of immunogenicity and safety, and therefore efficacy can only be inferred. The efficacy of the HPV-16/18 AS04-adjuvanted vaccine has previously been evaluated in women who did not complete the 3-dose vaccination schedule in a large vaccine trial, and preliminary results showed the vaccine was still efficacious against one-year persistent HPV-16/18 infections in these women.Citation17
Experience from existing organized HPV vaccination programs has shown that administration of the 3-dose vaccination course to adolescents is challenging in countries that have not implemented school-based vaccination programs. For example, vaccination coverage data for 2010 from the US Centers for Disease Control and Prevention indicated that only half of surveyed females aged 13–17 y received at least one dose of HPV vaccine, and 30% of the teenagers who commenced the 3-dose series did not complete it.Citation18 There are a number of factors that might play an important role in HPV vaccine coverage, including the fact that it is a 3-dose series. Thus, an effective 2-dose schedule for adolescents could have important public health implications.
In summary, the licensed vaccination schedule of the HPV-16/18 AS04-adjuvanted vaccine includes administration of 3 doses to be given at months 0, 1 and 6. Results from our trial indicate that a 2-dose schedule may be feasible in 9–14-y-olds as a potential alternative vaccination regimen. Further evaluation of a 2-dose schedule is needed, including confirmatory immunological non-inferiority studies in young adolescents.
Participants and Methods
Trial design and participants.
This was a phase I/II, partially-blind, controlled, randomized, age-stratified, parallel group trial designed to evaluate the immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule. The trial was conducted at 21 centers in Canada and Germany between October 2007 and May 2010. The trial was approved by the appropriate Independent Ethics Committee for each center and was conducted according to the Declaration of Helsinki and Good Clinical Practice. The trial is registered with ClinicalTrials.gov (registration number NCT00541970). Participants below the legal age of consent signed and dated a written informed assent and written informed consent was obtained from their parents or legally acceptable representative prior to the performance of any trial specific procedures. Participants above the legal age of consent provided written informed consent themselves.
Healthy girls and young women aged 9 to 25 y at the time of first vaccination were eligible for the trial. Subjects of child-bearing potential had to have used adequate contraception for 30 d prior to vaccination, have a negative pregnancy test, and continue contraceptive precautions for 2 mo after completion of the vaccination series. Subjects were excluded if they were pregnant or breast-feeding, had autoimmune disease or immunodeficiency, had received more than 14 d of immunosuppressants or immune-modifying drugs within 6 mo prior to the first vaccine dose, or had previously received HPV vaccine, AS04 adjuvant or 3-O-desacyl-4′-monophosphoryl lipid A.
Eligible subjects were randomized (1:1:1:1) and stratified by age (9–14, 15–19, 20–25 y) to one of four groups to receive: three doses of HPV-16/18 (20 µg/20 µg) vaccine at Months 0, 1 and 6 (i.e., the licensed vaccine formulation and schedule) (Group 20/20F M0,1,6), two doses of HPV-16/18 (20 µg/20 µg) vaccine at Months 0 and 6 (Group 20/20F M0,6), two doses of HPV- 16/18 (40 µg/40 µg) vaccine at Months 0 and 6 (Group 40/40F M0,6), or two doses of HPV-16/18 (40 µg/40 µg) vaccine at Months 0 and 2 (Group 40/40F M0,2). The randomization list was computer-generated at GlaxoSmithKline Biologicals. Treatment allocation at the investigator site was performed using a central randomization call-in system on the internet; the randomization algorithm used a minimization procedure accounting for center and age (9–14, 15–19 and 20–25 y).
The trial was partially blinded within the 2-dose schedule groups (observers were blinded to group assignment) and open in the 3-dose schedule group. In the 2-dose schedule groups, a placebo was administered at Month 2 (Groups 20/20F M0,6 and 40/40F M0,6) or at Month 6 (Group 40/40F M0,2) to maintain blinding. Due to differences in the visual appearance of the two HPV formulations and the placebo, syringes were prepared and administered by qualified medical personnel not otherwise involved in the conduct of the trial or in the assessment of symptoms. Subjects were followed for 24 mo after first vaccination.
Vaccines.
All vaccines used in this trial were developed and manufactured by GlaxoSmithKline Biologicals, Rixensart, Belgium. Each 0.5 mL dose of the licensed formulation of the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®) contained 20 µg HPV-16 L1 VLP and 20 µg HPV-18 L1 VLP in AS04 adjuvant (50 µg 3-O-desacyl-4′-monophosphoryl lipid A and 500 µg aluminum hydroxide [Al(OH)3]). Each 0.5 mL dose of the increased antigen content formulation of the vaccine contained 40 µg HPV-16 L1 VLP and 40 µg HPV-18 L1 VLP in the same AS04 adjuvant as the licensed formulation. Each dose of placebo contained 500 µg Al(OH)3, 150 mM NaCl, 8 mM NaH2PO4.2H2O and 0.5 mL water for injection. Vaccines were administered intramuscularly into the deltoid of the non-dominant arm according to the relevant dosing schedule.
Serologic evaluation.
Blood samples for serologic evaluation were drawn prior to first vaccination (Month 0), at Month 3 (2-dose groups only), and at Months 7, 12, 18 and 24. Antibodies to HPV-16 and HPV-18 were measured by ELISA, as described previously in reference Citation16. Antibody concentrations greater than or equal to the lower limit of detection for each assay were prespecified to indicate seropositivity (≥8 ELISA units [EU]/mL for HPV-16 antibodies and ≥7 EU/mL for HPV-18 antibodies).
Safety evaluation.
Solicited local symptoms (pain, redness or swelling at injection site) and general symptoms (fever, headache, fatigue, gastrointestinal symptoms, arthralgia, myalgia, rash or urticaria) occurring within 7 d after each vaccination were recorded by the subject or her parent/legally acceptable representative using a diary card. Investigators documented the presence or absence of urticaria/rash within 30 min after each vaccine dose. Unsolicited AEs occurring within one month of each vaccine dose were documented by the investigator.
SAEs, other medically significant conditions (i.e., AEs prompting emergency room or physician visits that were not related to common diseases), new onset chronic diseases including new onset autoimmune diseases and pregnancies occurring through Month 24 were documented. Pregnancies were followed until delivery. New onset chronic diseases and new onset autoimmune diseases (potential autoimmune events, which excluded allergy-related events or isolated signs and symptoms) were identified by comparing all reported AEs with a pre-defined list of potential chronic diseases derived from the Medical Dictionary for Regulatory Activities, as described previously in reference Citation19.
Outcomes.
The co-primary objectives were to evaluate the immunogenicity of 2-dose schedules of the HPV-16/18 AS04-adjuvanted vaccine one month after the last active dose when administered at different dosages (20 or 40 µg of each HPV antigen) and on different schedules (0, 2- or 0, 6 mo) compared with the licensed 3-dose schedule, and to evaluate reactogenicity with respect to solicited local and general symptoms reported within 7 d after each and any vaccination.
Secondary objectives were to demonstrate non-inferiority of antibody responses to each 2-dose schedule in 9–14, 15–19 and 20–25 y age strata compared with the licensed 3-dose schedule in subjects aged 15–25 y (the age group in which efficacy was previously demonstrated in ref. Citation2–Citation6), one month after the last active dose of vaccine; to evaluate the kinetics of the antibody response to all dose schedules and dosages of the vaccine in each age stratum; and to evaluate the safety of the vaccine when administered at different dosages and on different schedules through Month 24.
An exploratory objective was to demonstrate non-inferiority of antibody responses at Month 24 to each 2-dose schedule of the HPV-16/18 vaccine in the 9–14 y age stratum as compared with the licensed 3-dose schedule in subjects aged 15–25 y.
Statistical methods.
The total vaccinated cohort, for analysis of safety, included all subjects who received at least one dose of vaccine. The ATP cohort for analysis of immunogenicity included all evaluable subjects (i.e., those meeting all eligibility criteria, complying with the procedures defined in the protocol, with no elimination criteria during the trial) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one vaccine antigen component after vaccination. Analyses of HPV-16 and -18 immune responses were stratified by serostatus for the corresponding antigen at baseline.
For the primary immunogenicity objective, it was estimated that a target sample size of approximately 960 enrolled subjects (768 evaluable subjects assuming a non-evaluable rate of 20%) would allow the detection of a 2-fold difference between the four groups in terms of GMTs in initially seronegative subjects one month after the last active dose with at least 90% power (α = 0.025) for both HPV-16 and 18.
For the primary immunogenicity objective, a two-way ANOVA model was applied for HPV-16 and HPV-18 separately, using titers (log10) as the response variable. The model contained age, group and group-by-age interactions as fixed factors. The interaction term (group-by-age) was tested at 10%. It was pre-specified that statistical analyses of pairwise comparisons for the licensed 3-dose schedule vs. each 2-dose schedule by age stratum would only be done if the group-by-age interaction term was significant at 10%. The group-by-age interaction term was not significant at 10% for either HPV-16 or HPV-18 responses; therefore, further estimations were drawn across all age strata combined, for initially seronegative subjects, using Dunnett's multiple comparisons for each 2-dose schedule compared with the licensed 3-dose schedule. The licensed 3-dose schedule was considered to be superior to a 2-dose schedule if the lower limit of the 95% CI for the ratio of GMTs (2-dose divided by 3-dose) was less than 0.5 (2-fold difference).
For secondary and exploratory immunogenicity objectives, for initially seronegative subjects, non-inferiority was demonstrated if the upper limit of the 95% CI for the GMT ratio between the licensed 3-dose schedule in subjects 15–25 y of age over the 2-dose schedule in each year age stratum was below 2. Comparisons were made sequentially starting with the 9–14 y age stratum, followed by the 15–19 y age stratum then the 20–25 y age stratum.
The percentage of subjects with solicited or unsolicited symptoms after each vaccine dose and overall was calculated with exact 95% CI.
Conflict of Interest
The submitted work received financial support from GlaxoSmithKline Biologicals.
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: Barbara Romanowski (B. Ro), L.M.F. and P.H. received support for travel to meetings for the study. B.Ro., L.M.F. and P.H. received grants for their institutions. B.Ro. and T.F.S. received financial support for board membership. B.Ro., L.M.F., P.H. and T.F.S. received financial support for consultancy. B.Ro., T.F.S. and L.M.F. received payment for lectures including service on speaker bureaus. B.Ro. and P.H. received payment for expert testimony. G.C., A.S., K.D. and D.D. are GlaxoSmithKline employees. A.S., K.D. and D.D. have GlaxoSmithKline stock options. K.S., Brian Ramjattan (B. Ra), K.P. and M.D. declare no conflict of interest.
Abbreviations
| 20/20F | = | formulation containing 20 µg of HPV-16 and 20 µg of HPV-18 L1 protein virus-like particles |
| 40/40F | = | formulation containing 40 µg of HPV-16 and 40 µg of HPV-18 L1 protein virus-like particles |
| 2D | = | 2-dose |
| 3D | = | 3-dose |
| AE | = | adverse event |
| ANOVA | = | analysis of variance |
| AS04 | = | GlaxoSmithKline proprietary adjuvant system consisting of aluminum hydroxide plus 3-O-desacyl-4′-monophosphoryl lipid A |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| ELISA | = | enzyme-linked immunosorbent assay |
| EU | = | ELISA unit |
| GMT | = | geometric mean antibody titer |
| HPV | = | human papillomavirus |
| M | = | month |
| SAE | = | serious adverse event |
| VLP | = | virus-like particle |
Figures and Tables
Figure 2 Kinetics of antibody responses against HPV-16 and HPV-18 (according to protocol immunogenicity cohort, initially seronegative subjects). CI, confidence interval; EU, ELISA unit; GMT, geometric mean titer. Natural infection, GMT in subjects who had cleared a natural infection (i.e., 29.8 [28.5 to 31.1] EU/mL for HPV-16 and 22.7 [21.7 to 23.7] EU/mL for HPV-18).Citation3 Plateau, GMT at the plateau level (Month 45–50) in a study in which sustained protection with the HPV-16/18 AS 04-adjuvanted vaccine was shown up to 6.4 y after first vaccination (i.e., 397.8 [344.7 to 459.1] EU/mL for HPV-16 and 297.3 [258.2 to 342.2] EU/mL for HPV-18).Citation2
![Figure 2 Kinetics of antibody responses against HPV-16 and HPV-18 (according to protocol immunogenicity cohort, initially seronegative subjects). CI, confidence interval; EU, ELISA unit; GMT, geometric mean titer. Natural infection, GMT in subjects who had cleared a natural infection (i.e., 29.8 [28.5 to 31.1] EU/mL for HPV-16 and 22.7 [21.7 to 23.7] EU/mL for HPV-18).Citation3 Plateau, GMT at the plateau level (Month 45–50) in a study in which sustained protection with the HPV-16/18 AS 04-adjuvanted vaccine was shown up to 6.4 y after first vaccination (i.e., 397.8 [344.7 to 459.1] EU/mL for HPV-16 and 297.3 [258.2 to 342.2] EU/mL for HPV-18).Citation2](/cms/asset/e086fe6f-3b1c-47a0-8c8e-3b0638f6f174/khvi_a_10918322_f0002.gif)
Figure 3 Incidence of solicited local symptoms reported during the 7-d post-vaccination period following any dose, overall per dose (total vaccinated cohort). CI, confidence interval; Grade 3, pain that prevented normal activity or diameter of redness or swelling >50 mm.
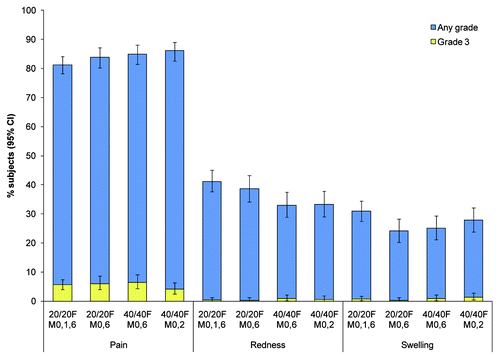
Figure 4 Incidence of solicited general symptoms reported during the 7-d post-vaccination period following any dose, overall per dose (total vaccinated cohort). CI, confidence interval; GI, gastrointestinal; Grade 3, symptom that prevented normal activity or fever >39°C.
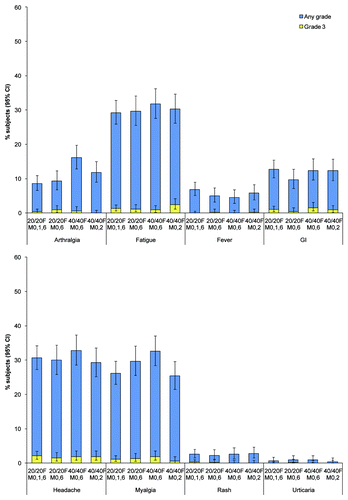
Table 1 Demographic characteristics (total vaccinated cohort)
Table 2 Pairwise comparisons of GMTs between each 2-dose schedule and the licensed 3-dose schedule one month after the last active vaccine dose (ATP immunogenicity cohort, initially seronegative subjects)
Table 3 Immune response elicited by the 2-dose schedules and by the licensed 3-dose schedule, by age stratum, one month after the last vaccine dose and at Month 24 (ATP immunogenicity cohort, initially seronegative subjects)
Table 4 Non-inferiority assessment for each 2-dose schedule in each age stratum compared to the licensed 3-dose schedule in subjects aged 15–25 years (ATP immunogenicity cohort, initially seronegative subjects)
Table 5 Summary of safety through month 24 (total vaccinated cohort)
Additional material
Download Zip (61.7 KB)Acknowledgments
We would like to thank the following members of the trial group, who are not named as authors:
Investigators: Canada: Rizwan Somani; Germany: Ulrich Behre, Karl-Heinz Belling, Rolf Ebert, Per Kristian Gildberg, Wolf-D. Höpker, Klaus Kindler, Ulrich Kohoutek, Siegfried Schönian.
Statistical support: Dorothée Meric (GlaxoSmithKline Biologicals, Belgium).
Clinical trial support: Florence Thomas-Jooris (GlaxoSmithKline Biologicals, Belgium) and Marjan Hezareh (Chiltern International, Belgium, on behalf of GlaxoSmithKline Biologicals).
Medical writing assistance: Julie Taylor (Peak Biomedical Ltd., UK, on behalf of GlaxoSmithKline Biologicals).
Manuscript co-ordination: Dirk Saerens (Keyrus Biopharma, Belgium, on behalf of GlaxoSmithKline Biologicals, Belgium).
References
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin 2009; 5:332 - 340; PMID: 19221517; http://dx.doi.org/10.4161/hv.5.5.7211
- The GlaxoSmithKline Vaccine HPV-007 Study Group. Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975 - 1985; PMID: 19962185; http://dx.doi.org/10.1016/S0140-6736(09)61567-1
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161 - 2170; PMID: 17602732; http://dx.doi.org/10.1016/S0140-6736(07)60946-5
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 314; PMID: 19586656; http://dx.doi.org/10.1016/S0140-6736(09)61248-4
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757 - 1765; PMID: 15541448; http://dx.doi.org/10.1016/S0140-6736(04)17398-4
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247 - 1255; PMID: 16631880; http://dx.doi.org/10.1016/S0140-6736(06)68439-0
- Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40:564 - 571; PMID: 17531764; http://dx.doi.org/10.1016/j.jadohealth.2007.02.015
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety and immunologic memory. Pediatrics 2001; 107:626 - 631; PMID: 11335734; http://dx.doi.org/10.1542/peds.107.4.626
- Dobson S, Dawar M, Kollmann T, McNeil S, Halperin S, Langley J, et al. A two-dose HPV vaccine schedule in girls: immunogenicity at 24 months 26th International Papillomavirus Conference July 3–8, 2010 Montreal 690
- Lin YL, Borenstein LA, Selvakumar R, Ahmed R, Wettstein FO. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 1992; 187:612 - 619; PMID: 1312271; http://dx.doi.org/10.1016/0042-6822(92)90463-Y
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol 1995; 69:3959 - 3963; PMID: 7745754
- Jansen KU, Rosolowsky M, Schultz LD, Markus HZ, Cook JC, Donnelly JJ, et al. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine 1995; 13:1509 - 1514; PMID: 8578834; http://dx.doi.org/10.1016/0264-410X(95)00103-8
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA 1995; 92:11553 - 11557; PMID: 8524802; http://dx.doi.org/10.1073/pnas.92.25.11553
- Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with virus like particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol 1996; 70:960 - 965; PMID: 8551636
- Kirnbauer R, Chandrachud LM, O'Neil BW, Wagner ER, Grindlay GJ, Armstrong A, et al. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 1996; 219:37 - 44; PMID: 8623552; http://dx.doi.org/10.1006/viro.1996.0220
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425 - 434; PMID: 18948732; http://dx.doi.org/10.4161/hv.4.6.6912
- Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103:1444 - 1451; PMID: 21908768; http://dx.doi.org/10.1093/jnci/djr319
- Centers for Disease Control and Prevention (CDC). National, state and local area vaccination coverage among adolescents aged 13–17 years United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117 - 1123; PMID: 21866084
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin 2009; 5:705 - 719; PMID: 19684472; http://dx.doi.org/10.4161/hv.5.10.9518