Abstract
Antibody based products are not widely available to address multiple global health challenges due to high costs, limited manufacturing capacity, and long manufacturing lead times. Nicotiana-based manufacturing of antibody products may now begin to address these challenges as a result of revolutionary advances in transient expression and altered glycosylation pathways. This review provides examples of emerging antibody-based products (mucosal and systemic) that could be competitive and commercially viable when the attributes of Nicotiana-based manufacturing (large scale, versatile, rapid, low cost) are utilized.
Introduction
Monoclonal antibody (mAb) based products have been therapeutically and financially successful in developed markets, but are not widely available to address multiple global health challenges due to high costs, limited manufacturing capacity and long manufacturing lead times. Now, rapid and versatile platform technologies, like Nicotiana-based manufacturing, may begin to address these challenges. Nicotiana-based manufacturing of antibody products has been revolutionized by two major technological advances: (a) viral based transient expression allowing high accumulation of antibody in days; (b) transgenic plants with altered glycosylation pathways that can produce antibody with mammalian glycoforms.
The abundant production of mAbs in plants has recently been reviewed,Citation1 but success is in the products and a major criticism addressed to plant biotechnologists is “after 20 years, where are the products?”Citation2 This review provides examples of emerging antibody-based products (mucosal and systemic) that could be competitive and commercially viable when the attributes (large scale, versatile, rapid, low cost, customizable glycosylation) of Nicotiana-based manufacturing are utilized.
Manufacturing mAbs in Nicotiana benthamiana
Transient expression.
Proof of concept studies with plant-made mAbsCitation3,Citation4 and antigensCitation5 stimulated significant interest in transgenic plant production. However, low expression levels, extended manufacturing timelines associated with generating stable transgenics, safety concerns regarding plant glycosylation and environmental concerns, hindered rapid development of products. Now, transient expression systems for Nicotiana, e.g., magnICON,Citation6 Geneware,Citation7 Gemini,Citation8 pEAQ,Citation9 and plastocyaninbasedCitation10 have been developed that alleviate many of these hurdles.
The first industrialized system to demonstrate the ability to produce antibodies “on demand” in large quantities, at low cost was magnICON.Citation6,Citation7 The technology is in essence an en masse infiltration of whole mature plants with a highly dilute Agrobacterium suspension carrying t-DNAs encoding viral replicons. The result is a high copy number of RNA molecules that encode the desired antibody, reducing the time needed to produce the protein to 6–8 days. The technique appears to be the most rapid route from genes to full-length, assembled mAb (i.e., 8 days from DNA delivery via Agrobacterium tumifaciens to harvested cells expressing mAbs). Moreover, increasing the volume of mAb-containing biomass does not require changes in growing conditions, infection procedures and is directly scalable. Unlike stable transgenic approaches, no genes are incorporated into the plant genome, and as a result, there is no risk of propagation of the transgene from pollen, seeds or other routes. Further, no intact and replication-competent virus is produced, eliminating the risk of virus-mediated spreading of the recombinant genes. Finally, the entire production system can be performed indoors in enclosed growth rooms, providing an additional layer of environmental security and quality control.
Until recently, most production of mAbs in plants had been at small scale in academic laboratories. Now however, a number of institutions are actively manufacturing molecules in Nicotiana for clinical studies under Good Manufacturing Practices (GMP). Contract GMP manufacturing in Nicotiana is currently offered by Kentucky BioProcessing (Owensboro, KY); Texas A&M (College Station, TX) has recently begun construction of a contract GMP manufacturing facility for Nicotiana. Icon Genetics (Bayer; Halle, Germany), Fraunhofer (Newark, DE) and Medicago (Quebec, Canada) are reported to have their own GMP facilities for Nicotiana production.
Glycan modification.
Wild-type N. benthamiana glycosylates proteins differently than mammalian expression systems.Citation11 N. benthamiana, like other plants, produces the same core glycan as found in mammals, but uses xylose (which generally is not found in mammals) and fucose in a non-mammalian linkage (alpha 1,2). Because of the potential for the novel plant glycans to affect pharmacokinetics as well as immunogenicity in humans, it is highly desirable to produce mAbs in plants that have been modified to generate mammalian-like glycans. Now, with the development of transgenic strains of N. benthamiana with fucosyl- and xylosyl-transferase knocked down by RNAi,Citation12,Citation13 plants can produce mAbs with glycoforms that are essentially mammalian.
The resulting glycoforms in the double “knockout” (ΔXF, ) are more homogeneous than FDA-approved mAbs produced in mammalian cell culture (top two rows); obtaining a consistent glycoform profile in production is desirable from a quality and regulatory perspective. Of particular note for the development of mAbs where Antibody Dependent Cellular Cytotoxicity (ADCC) is an important mechanism of action (e.g., anti-cancer antigen mAbs), the predominant glycoform is one that is ideal for ADCC activity—elimination of core fucose has been shown to increase ADCC activity dramatically.Citation14
Knock-in strategies are now being used for mAbs that require galactosylated and sialylated N-glycans;Citation10,Citation11,Citation15,Citation16 current manufacturing methods based on mammalian cell culture allow only limited control of this important posttranslational modification.Citation17 Galactosylated and sialylated HIV mAbs have been produced in Nicotiana.Citation15,Citation16
Multipurpose Microbicidesfor Sexual and Reproductive Health
Unsafe sex is the second most important risk factor for disability and death in the world's poorest communities and the ninth most important in developed countries.Citation18 Every year, more than 120 million couples have an unmet need for contraception (80 million women have an unintended pregnancy, half a million die from complications associated with pregnancy, childbirth and the postpartum period) and 340 million acquire a sexually transmitted infection (STI).
Improved reproductive health outcomes—lower fertility rates, improved pregnancy outcomes and lower sexually-transmitted infections (STIs)—have broad individual, family, societal and environmental benefits.Citation19–Citation22
Although there are products for preventing pregnancy (e.g., the pill, IUDs, diaphragms) and STIs (male and female condoms), the epidemic incidence rates of both unintended pregnancy and STIs clearly illustrate the need for multipurpose prevention technologies with improved acceptability and access.Citation23 mAbs are highly specific, but in combination could yield a multipurpose microbicide active against a variety of STI pathogens with or without contraceptive activity.
HIV antibodies 2F5, 2G12, 4E10 combined as mAbGel are currently in early clinical trials as microbicides.Citation24 mAb 2G12 has been produced in transgenic maize plants,Citation25,Citation26 and the HIV-neutralization capability of the antibody is equal to or superior to that of the same antibody produced in CHO cells. The HIV mAb 2G12 has been produced in the ΔXF knockout Nicotiana line and was found to contain a relatively homogeneous N-glycan species without detectable xylose or α-1,3-fucose residues.Citation13 Plant-derived mAbs were indistinguishable from Chinese hamster ovary (CHO)-derived 2G12 with respect to electrophoretic properties and exhibited functional properties (i.e., antigen binding and HIV neutralization activity) at least equivalent to those of the CHO counterpart. Fully galactosylated 4E10 and 2G12 were reported to be several fold higher in neutralization potency than CHO produced mAbs.Citation15 Sialylated 2G12 exhibits similar in vitro HIV neutralization potency to other glycoforms derived from plants and CHO cells.Citation16
The 4E10 mAb has been produced in a transgenic tobacco rhizosection system.Citation27 A fusion protein of the HIV mAb b12 and cyanovirin produced in transgenic tobacco increased HIV potency compared to b12 or cyanovirin alone.Citation28 Several non-antibody microbicides, e.g., griffithsin and actinohivin have been transiently expressed in Nicotiana.Citation29,Citation30
An immune infertile diagnosis can be determined by the presence of anti-sperm antibodies that agglutinate and trap sperm in cervical mucus (reviewed in ref. Citation31), suggesting that an anti-sperm mAb may add contraceptive activity to a microbicide. Indeed, an anti-rabbit sperm mAb has been shown to provide contraceptive activity in a rabbit model.Citation32 A mAb (HC4) against a surface glycolipid present on human sperm has been produced in the ΔXF Nicotiana line and shown to co-agglutinate 100% of sperm and other seminal cells in less than thirty seconds at 100 ug/ml (). Testing the mucus trapping ability of this version of HC4 as well as aglycosylated, galactosylated and sialylated HC4 mAb is planned; the importance of mAb glycans for mucosal trapping is currently unknown—mAbs can trap sperm in mucus by making them “mucophilic”, i.e., the antibodies form adhesive interactions with the mucus gel that stops all forward motility (the “shaking phenomenon”); this is a primary mechanism that mediates immune infertility and appears to be associated with the Fc regions of antibodies.Citation33 A similar mechanism occurs with mucosal pathogens,Citation34 i.e., a sufficient number of low-affinity cross-linkages trap the pathogen in the mucus gel, thereby reducing the flux of pathogen that reach target cells.
A multipurpose microbicide that includes HSV and HIV mAbs ( and ) has been produced under Good Manufacturing Practices (GMP) in Nicotiana.Citation7 The cost and capacity challenges for an antibody-based microbicide are significant—a 10 mg multipurpose microbicide if used twice a week by ten million women would require ∼10,000 kg of mAb per year. Coupled with conventional unit operations for very large scale mAb purification,Citation35 the price/dose could be less than one US dollar for Nicotiana-based manufacturing.
RSV Antibodies for Neonates and the Elderly
Globally, respiratory syncytial virus (RSV) is the most common cause of childhood acute lower respiratory infection (19.3–46.2 million new episodes per year), a major cause of admission to hospitals (2.8–4.3 million per year) and 66,000 to 199,000 deaths (99% in developing countries).Citation36 The impact of RSV in adults is less well characterized; however, it is estimated that approximately 2–9% of all community acquired pneumonia hospitalizations may be associated with RSV infection.Citation37 Additionally, 5–10% of elderly patients in long-term care facilities develop RSV infections per year with rates of pneumonia and death of 10–20% and 2–5% respectively.Citation38
A number of analyses have been performed on the pharmacoeconomics of RSV immunoprophylaxis. Although Synagis, an anti-RSV mAb administered prophylactically to high-risk infants, is an effective drug, most studies find that RSV immunoprophylaxis is not cost-effective. Indeed, some regions have begun to recommend more restrictive indications for the use of Synagis.Citation39 If the costs of the drug were lowered, the patient populations in which Synagis would be cost-effective would be expanded.Citation40,Citation41 A second generation, affinity-improved version of Synagis, Motavizumab, was recently rejected by an FDA advisory panel due to safety considerations.
Since the quasispecies nature of RNA viruses such as RSV allows for rapid adaptation and selection of antiviral resistance, it is desirable to develop a mAb to a different epitope than Synagis as a second-defense if Synagis-resistant RSV strains become widespread. Production in plants offers a low-cost manufacturing option capable of significantly reducing costs and thereby enabling broader cost-effective use of an RSV mAb product for at-risk infant and adult populations. RSV25, a mAb to a different epitope on glycoprotein F has been transiently expressed in Nicotiana and has been evaluated for binding affinity (affinity for glycoprotein F as determined by surface plasmon resonance: Synagis = 2.2 × 10−10; Nicotiana derived mAb = 5.5 × 10−11 M) and neutralization ().
Antibody Products to Reduce Inflammation Associated with Autoimmunity
High doses of human immunoglobulin G administered intravenously (IVIG) is capable of suppressing inflammation associated with a variety of autoimmune diseases.Citation42,Citation43 The precise mechanism by which IVIG alleviated autoimmune disease-related inflammation in humans remains unresolved. The drawbacks of IVIG treatment involve both the cost and the availability;Citation44 IVIG costs about $100 per gram and a single treatment can require 20–30 g. For autoimmune diseases requiring high doses, treatment may involve up to five infusions.
The recent observation that IVIG activity in mouse models of autoimmune disease is caused by a limiting concentration of a sialic acid-bearing IgG glycoformCitation45–Citation47 provides the rationale that a fully recombinant product composed of hypersialylated IgG constant region could be a potent anti-inflammatory agent for use in autoimmune diseases in humans. It has recently been shown that the anti-inflammatory activity of IVIG in the K/BxN murine model of inflammatory arthritis can be attributed to a minor species of IgGs that is modified with terminal sialic acids on their Fc-linked glycans.Citation48,Citation49 Since this molecule does not involve antigen binding, it was demonstrated that a functional, recombinant substitute for IVIG could be provided by a sialylated IgG Fc constant region.Citation50 Although these results have yet to be extended to human autoimmune inflammation, there exists the possibility that a resolution to the problem of IVIG shortage and cost might ultimately be provided by a recombinant production system capable of producing completely human sialo-glycan mAbs. At the moment, only Nicotiana manufacturing has the capability of this type of mAb engineering coupled with scale-up capability to address demand of this magnitude.
A sialylated mAb was recently achieved by the introduction of an entire mammalian biosynthetic pathway into N. benthamiana, comprising the coordinated expression of the genes for (1) biosynthesis, (2) activation, (3) transport and (4) transfer of Neu5Ac to terminal galactose.Citation16 The transient overexpression and functional integrity of six mammalian proteins that act at various stages of the biosynthetic pathway and correct subcellular localization was demonstrated. Sialylation was at great uniformity when glycosylation mutants that lack plant-specific N-glycan residues were used as expression hosts. Further, efficient neutralization activity of the sialylated monoclonal antibody was demonstrated, indicating full functional integrity. A hypersialylated mAb to a pro-inflammatory cytokine (e.g., TNF or IL-6) may result in superior autoimmune or anti-inflammatory products (see Biobetters section).
Antibody Products for Alzheimer Disease
There are currently four mAbs and two IVIG preparations being tested in human clinical trials: bapineuzumab (Wyeth), solanezumab (Eli Lilly), PF-04360365 (Pfizer), GSK933776 (GlaxSmithKline), Gammagard (Baxter) and Octagam (Octapharma). The mAbs are all directed against the prevalent amyloid peptide, Aβ, the accumulation of which in the brain is a hallmark of Alzheimer disease (AD). The IVIG preparations also contain antibodies against Aβ and the contribution of these polyclonal anti-Aβ antibodies to IVIG efficacy is a matter of continuing research since, as noted above, IVIG can function by a variety of mechanisms. Moreover, there are many additional mAbs that are in various stages of pre-clinical development and testing. The multiple unknowns of AD present challenges in mAb development and production. First, the specific epitope(s) on Aβ that is optimal for antibody binding resulting in clinical benefit has yet to be defined. Simple, high affinity binding of Aβ is not sufficient to elicit cognitive benefit in mouse models of AD (Mapp, unpublished observations). The epitopes that do result in cognitive amelioration are difficult to define since the Aβ amyloid peptide is continually aggregating until it is insoluble.Citation51–Citation53 Consequently, anti-Aβ mAb discovery is a highly iterative process requiring evaluation of a large number of mAbs coupled with further optimization of isotype, serum half-life and, probably, glycan structure. Second, it has become increasingly clear that clinical intervention in AD needs to occur as early as possible. Where the results have been made available of the clinical trials mentioned above, significant cognitive benefit has been marginal. It may be that the inclusion criteria (generally mild-to-moderate AD) results in a clinical population that already has significant irreversible neuronal damage. The continuing validation of very early stage biomarkers for AD during the next decade will hopefully allow for clinical intervention in advance of extensive neuronal damage.Citation54 These factors will push passive immunotherapy farther into the realm of prophylaxis. Third, a vaccine for preventing AD is problematic in view of the complicating issues such as immune senescence in the elderly, inflammation resulting from auto-antigen immunization, and control of Th2 versus Th1 immune responses. The single vaccine clinical trial published to date (AN1792) failed for just these reasons resulting in a significant occurrence of meningoencephalitis.Citation55
As a result of these characteristics of AD, passive immunotherapy requires a production system that is highly versatile. A short expression cycle from DNA to protein as well as an ease of scalability to very large production runs is essential. If passive immunotherapy for AD is to find clinical application in the very early stages of the disease, the cost and production capacity will be critical elements of success.
Anti-amyloid mAbs have been transiently expressed in Nicotiana (Mapp, unpublished data) and are being evaluated in a transgenic mouse AD model.
Antibody Products for Biodefenseand Emerging Infections
Although some biodefense agents have been stockpiled, there is a need for rapid, versatile, low cost and large capacity manufacturing systems with “surge” capability to rapidly address a bioterror event as well as newly emergent and re-emergent pathogens. The surge capability of transient expression in Nicotiana was recently demonstrated with a project that went from gene to purified VLP vaccine in less than a month,Citation56 and with individualized full IgG idiotype vaccines in less than four months.Citation57 Cost and capacity is another vital component: one US funder has targeted GMP manufacturing of three million doses of vaccine (50 ug dose) at <$1/dose and antibody (400 mg dose) at <$10/dose in one to three months (DARPA BAA 06-31).
For anthrax therapy, a recombinant fusion protein comprised of a fusion of CMG2 (a human receptor for anthrax toxins) and the Fc of human IgG1, for long circulating half-life and immune effector cell interaction, has been expressed in stably transformed Nicotiana at high levels and processed to a high level of purity (Wycoff, PBVA 2009). A Nicotiana-based mAb has been shown to bind a highly conserved epitope on the envelope protein of the West Nile virus and protects mice in challenge studies.Citation58
Pathogen specific sialylated mAbs may also have additional therapeutic efficacy if they reduce inflammation (as described above), a common feature in the pathogenesis and morbidity of many pathogens (e.g., via a cytokine storm).
Although recent successes with an Ebola vaccine and siRNA technology are encouraging, because of the excellent safety and efficacy profile mAbs are being evaluated as Ebola therapeutics. Three mAbs (individually and in combination) have been shown to provide significant prophylactic and therapeutic protection in mice.Citation59 Humanized versions of these mAbs have been expressed in N. benthamiana (), tested in mice and GMP Nicotiana manufacturing is underway to provide supply for evaluation in primate efficacy studies. Similar earlier stage projects are underway for Marburg and Lujo viruses, as well as a set of potential biowarfare toxins (C. perfringens epsilon toxin, ricin, Staphylococcal enterotoxin B).
Antibodies and Vaccines
There are a number of synergies between recombinant mAbs and vaccines: (a) blood from vaccinees can be used as a source for mAb discovery; (b) mAbs can be used in challenge models to predict the neutralizing titers required for protection by vaccine candidates; (c) mAbs can be used as a method of purifying antigens; (d) mAbs can be used as anti-idiotype vaccines. Further, for some diseases, the use of vaccines coupled with passive immunotherapy is routine (e.g., Hepatitis B, rabies).
Purification of antigens.
The large-scale production of scFv in transgenic Nicotiana and its use for immunopurification of Hepatitis B surface antigen for a vaccine has been described.Citation60 Lab scale purification of Nictotiana-derived HSV glycoprotein D (gD)—a potential vaccine antigen—has been used with transiently expressed full length anti-gD mAb from Nicotiana (personal communication, Hugh Mason). Although economically desirable, the use of mAbs for industrial purification of target antigens is not currently routine.
IgG idiotype vaccines.
Individualized vaccines require very small scale manufacturing runs but are highly dependent on rapid manufacturing capability. The cost, production time and availability of tailor-made, patient- and tumor-specific antigens (idiotypes) have been significant hurdles to previous developments in this field. An scFv idiotype vaccine for non-Hodgkins Lymphoma (NHL) has been transiently expressed in Nicotiana and successfully completed a phase I clinical study for safety and immunogenicity in NHL patients.Citation61 To improve expression and streamline purification (via Protein A), whole IgG idiotype NHL vaccines were transiently expressed (0.5 to 4.8 g/kg of leaf biomass) in Nicotiana;Citation57 clinical trials with this strategy are expected to yield early results in 2011.
IgG idiotype vaccines can utilize standard mAb purification technology, including protein A. Downstream processing of mAbs are complex and costly (accounting for as much as 50–80% of total manufacturing costs); the use of a Nicotiana-derived immunoabsorbent (based on a tobamovirus displaying protein A) may be an especially attractive tool for the purification of Nicotiana-made mAbs,Citation62 including anti-idiotype vaccines.
Although subunit,Citation63,Citation64 VLP,Citation56 and full IgG idiotypeCitation57 vaccines have been expressed in Nicotiana, a unique opportunity with anti-idiotype mAbs is the use of recombinant technology as mimics of vaccine antigens that are difficult to manufacture (e.g., carbohydrate antigens).
Overall, the worldwide vaccine market has registered revenue of US $21 Billion in 2008. However, new vaccines are often not globally accessible due to cost.Citation65 In addition, new subunit vaccines may require significantly larger quantities if used without adjuvant, are mucosally boosted or have multiple antigens. Several scenarios for subunits and the required manufacturing capacity are shown in .
Biobetter Antibodies
A significant number of mAb products will be coming off patent over the next five years. To achieve sales in the biosimilars sector, companies will have to invest significant capital while risking product failure during development stages. Due to recent data exclusivity laws in the US, some developers may be better off pursuing a BLA with a superior product and better pricing. The barrier of entry to this market is substantial, but may be mitigated by the low capital costs and the speed to phase 1 cGMP supply with transient Nicotiana manufacturing ().
Inflammatory diseases.
TNF is a major pro-inflammatory cytokine involved in multiple inflammatory diseases (e.g., rheumatoid arthritis, Crohn's disease, psoriasis, septic shock). Single-domain antibodies to TNF have been produced in Nicotiana and prevented septic shock in mice.Citation66 For commercially available TNF antibodies, the cost to a US consumer is ∼$50,000/year.
Cancer.
If long-term (years) cancer therapy with mAbs becomes routine, the pharmacoeconomics and supply requirements may change substantially. For example the mAb Herceptin (Genentech) can cost a breast cancer patient more than $37,000 for a year of treatment. A HER-2 mAb has been transiently expressed, purified from N. benthamiana and evaluated in a mouse cancer model.Citation67
Infectious disease.
Specific immunoglobulin products are commercially available as therapeutics and prophylactics for a broad range of pathogens (Tetanus, Varicella/Zoster, Hepatitis B, Rabies, Rubella, Vaccinia, Staphylococcus, Cytomegalovirus, Hepatitis A, Pertussis). At present, mAbs against RSV, HIV, HSV, HBV, C. difficile, have been transiently expressed in Nicotiana.
Summary
A new generation of mAb-based products have the potential to alter the pharmacoeconomic landscape due to Nicotiana manufacturing that is versatile, rapid, scalable, lower in cost and glycan modifiable. These Nicotiana manufacturing attributes () may have a significant impact on the development of mucosal and systemic antibody products that require one of the attributes for commercial viability. With the growing number of GMP Nicotiana manufacturers available to facilitate development of quality products at commercial scale, the plant biotechnology field may soon be able to answer the question, “where are the products?”Citation2
Abbreviations
| IVIG | = | intravenous immunoglobulin |
| Mab | = | monoclonal antibody |
| HIV | = | human immunodeficiency virus |
| HSV | = | herpes simplex virus |
| RSV | = | respiratory syncytial virus |
| ΔXF | = | xylose and fucose knockout |
| CHO | = | chinese hamster ovary |
| gD | = | glycoprotein D |
Figures and Tables
Figure 1 Distribution (%) of N-glycosylation patterns of two FDA approved Mabs produced in CHO (Rituxan) and NS0 (Synagis) compared to reported glycan patterns of anti-HIV mAb 2G12 produced in wild-type and transgenic N. benthamiana (Strasser et al. 2008). wt, wild-type N. benthamiana; ΔXF, transgenic N. benthamiana with native fucosyland xylosyl-transferase knocked-out via RNA i. Glycoforms representing less than 5% are not included in the table.
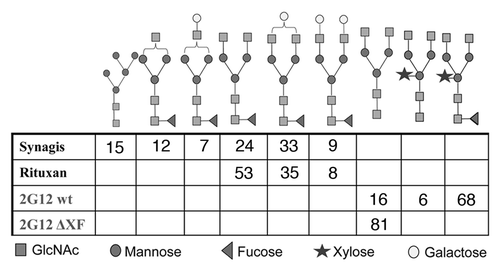
Figure 2 Agglutination of human sperm with mAb HC4 produced in N. benthamiana. Purified mAb was added to undiluted human semen and observed within 30 seconds via light microscopy.
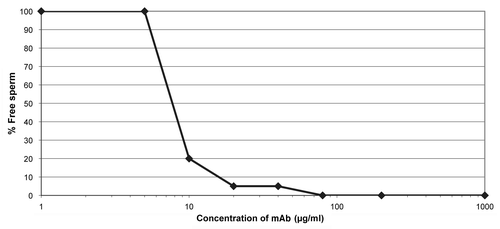
Figure 3 HSV neutralization testing of an anti-HSV mAb (αHSVgD) produced in N. benthamiana. Serial dilutions of acyclovir or αHSVgD were added to ME-180 cell monolayers followed by 2.5 × 103 pfu/ml of Herpes Simplex Virus (HSV)-2. After 5 days, the cytopathic effect was assessed. (Data courtesy of C. Lackman-Smith, Southern Research Institute, Fredrick, MD).
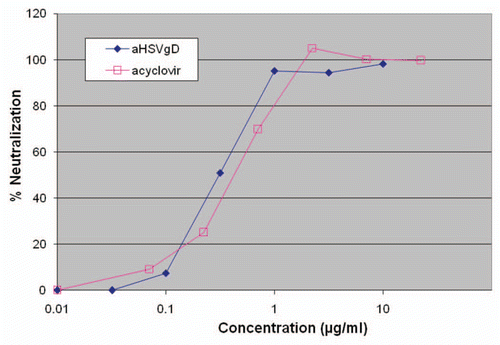
Figure 4 RSV neutralization assay comparing Synagis (palivizumab; MedImmune) with mAb RSV25 produced in wild type (wt) or ΔXF (deltaXF) N. benthamiana (Synagis). The neutralizing activity of the Mabs was measured by a plaque reduction assay using HEp-2 cell culture and the A2 strain of RS V. Plaques for each Ab dilution were counted, triplicates averaged and Ab dilution vs. plaque number plotted. This study was performed by Dr. James Crowe (Vanderbilt University, Nashville, TN).
Figure 5 Antigen binding ELISA with anti-Ebola mAb 13F6 produced in NS0 cells and in N. benthamiana. ELISA plates were coated with inactivated Ebola Zaire, serial dilutions of mAb were added and bound mAb detected with labeled anti-human lambda polyclonal antibody.
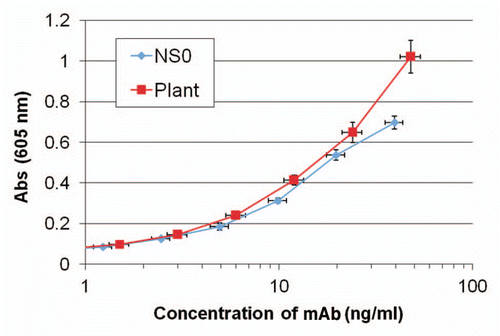
Table 1 Neutralization with anti-CCR 5 Mab produced in N. benthamiana
Table 2 Manufacturing capacity requirements: scenarios for subunit vaccines*
Table 3 Cost and time comparisons for mAb manufacturing systems
Table 4 Attributes of Nicotiana-manufacturing and the importanceTable Footnote* for each attribute to successfully commercialize the product
Acknowledgements
The projects described were supported by Grant Numbers AI62150, AI061270, AI063681, AG025641 from NIAID and NIA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- De Muynck B, Navarre C, Boutry M. Production of antibodies in plants: status after twenty years. Plant Biotech J 2010; 529 - 563
- Faye l, Gomord V. Success stories in molecular farming—a brief overview. Plant Biotech J 2010; 8:525 - 528
- Hiatt AH, Caffertey R, Bowdish K. Production of antibodies in transgenic plants. Nature 1989; 342:76 - 78
- Ma Jk, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, et al. Generation and assembly of secretory antibodies in plants. Science 1995; 268:716 - 719
- Mason HS, Lam DMK, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. PNAS 1992; 89:11745 - 11749
- Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. PNAS 2006; 103:14701 - 14706
- Pogue GP, Vojdani F, Palmer KE, Hiatt E, Hume S, Phelps J, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotech J 2010; 8:638 - 654
- Huang Z, Phoolcharoen W, Lai H, Piensook K, Cardineau G, Zeitlin L, et al. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng 2010; 106:9 - 17
- Sainsbury F, Lomonossoff GP. Extremely high-level and rapid protein production in plants without the use of viral replication. Plant Physiology 2008; 148:1212 - 1218
- Vezina LP, Faye L, Lerouge P, D'Aoust MA, Marquet-Blouin E, Burel C, et al. Transient co-expression for fast and high-yield production of antibodies with human-like N-glycans in plants. Plant Biotech J 2009; 7:442 - 455
- Gomord V, Fitchette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, Faye L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotech J 2010; 8:564 - 587
- Schahs M, Strasser R, Stadlmann J, Kunert R, Rademacher T, Steinkellner H. Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotech J 2007; 5:657 - 663
- Strasser R, Stadlmann J, Schahs M, Stiegler G, Quendler H, Mach L, et al. Generation of glycoengineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotech J 2008; 6:392 - 402
- Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. mAbs 2009; 1:230 - 236
- Strasser R, Castilho A, Stadlmann J, Kunert R, Quendler H, Gattinger P, et al. Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous beta1,4-galactosylated N-glycan profile. J Biol Chem 2009; 284:20479 - 20485
- Castilho A, Strasser R, Stadlmann J, Grass J, Jez J, Gattinger P, et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J Biol Chem 2010; 285:15923 - 15930
- Hossler P, Khattak SF, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 2009; 19:936 - 949
- Glasier A, Gülmezoglu AM, Schmid GP, Moreno CG, Van Look PF. Sexual and reproductive health: a matter of life and death. Lancet 2006; 368:1595 - 1607
- Singh S, Darroch JE, Vlassoff M, Nadeau J. Adding it up: the Benefits of Investing in Sexual and Reproductive Health Care 2004; New York UNFPA/Alan Guttmacher Institute
- Greene ME, Merrick TW. Poverty Reduction: Does Reproductive Health Matter?. HNP Discussion Paper Series 2005; Washington, DC World Bank
- The World Bank's Reproductive Health Action Plan 2010–2015 2010; World Bank
- Cates W. Family planning: the essential link to achieving all eight Millennium Development Goals. Contraception 2010; 81:460 - 461
- PATH and the Coalition Advancing Multipurpose Innovations (CAMI). Saving Lives With Multipurpose Prevention Technologies: Turning Ideas Into Solutions for Sexual and Reproductive Health 2010; Seattle PATH
- Morris G, Chindove S, Woodhall S, Wiggins R, Vcelar B, Lacey C. A prospective randomized double blind placebo-controlled phase 1 pharmacokinetic and safety study of a vaginal microbicide gel containing three potent broadly neutralizing monoclonal antibodies (2F5, 2G12, 4E10) (MabGel). Microbicides 2010; Abstract LB1
- Rademacher T, Sack M, Arcalis E, Stadlmann J, Balzer S, Altmann F, et al. Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly sincle GlcNAc N-glycans. Plant Biotech J 2008; 6:189 - 201
- Ramessar K, Rademacher T, Sack M, Stadlmann J, Platis D, Stiegler G, et al. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. PNAS 105:3727 - 3732
- Drake PMW, Barbi T, Sexton A, McGowan E, Stadlmann J, Navarre C, et al. Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. FASEB J 2009; 23:3581 - 3589
- Sexton A, Harman S, Shattock RJ, Ma JK. Design, expression and characterization of a multivalent, combination HIV microbicide. FASEB J 2009; 23:3590 - 3600
- O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. PNAS 2009; 106:6099 - 6104
- Matoba N, Husk AS, Barnettt BW, Pickel MM, Arntzen CJ, Montefiori DC, et al. HIV-1 neutralization profile and plant-based recombinant expression of Actinohivin and Env glycan-specific lectin temoid of T-cell mitogenic activity. PLoS One 2010; 5:11143
- Cone RA, Whaley KJ. Monoclonal antibodies for reproductive health: Preventing sexual transmission of disease and pregnancy with topically applied antibodies. Am J Reprod Immunol 1994; 32:114 - 131
- Castle PE, Whaley KJ, Hoen TE, Moench TR, Cone RA. Contraceptive effect of sperm-agglutinating monoclonal antibodies in rabbits. Biol Reprod 1997; 56:153 - 159
- Olmsted SS, Pdgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human ⇑cervical mucus. Biophys J 2001; 81:1930 - 1937
- Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonett PJ, Corthesy B. Secretory component a new roled in secretory IgA-mediataed immune exclusion in vivo. Immunity 2002; 17:107 - 115
- Kelley B. Very large scale monoclonal antibody purification: the case for conventional unit operations. Biotechnol Prog 2007; 23:995 - 1008
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and metaanalysis. Lancet 2010; 375:1545 - 1555
- Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis 1999; 179:25 - 30
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000; 13:371 - 384
- Vogel AM, McKinlay MJ, Ashton T, Lennon DR, Harding JE, Pinnock R, Graham D, et al. Cost-effectiveness of palivizumab in New Zealand. J Paediatr Child Health 2002; 38:352 - 357
- Joffe S, Ray GT, Escobar GJ, Black SB, Lieu TA. Cost-effectiveness of respiratory syncytial virus prophylaxis among preterm infants. Pediatrics 1999; 104:419 - 427
- Roeckl-Wiedmann I, Liese JG, Grill E, Fischer B, Carr D, Belohradsky BH. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr 2003; 162:237 - 244
- Nimmerjahn F, Ravetch JV. The anti-inflammatory activity of IgG: the intravenous IgG paradox. J Exp Med 2007; 204:11 - 15
- Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 2008; 26:513 - 533
- Bayry J, Kazatchkin MD, Kaveri SV. Shortage of human intravenous immunoglobulin—reasons and possible solutions. Nat Clin Pract Neurol 2007; 3:120 - 121
- Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody induced autoimmune disease. Immunity 2003; 18:573 - 581
- Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med 2006; 203:789 - 797
- Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001; 291:484 - 486
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005; 310:1510 - 1512
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVI. Proc Natl Acad Sci USA 2008; 105:19571 - 19578
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008; 320:373 - 376
- Wong KT, Allen IV, McQuaid S, McConnell R. An immunohistochemical study of neurofibrillary tangle formation in post-encephalitic Parkinsonism. Clin Neuropathol 1996; 15:22 - 25
- Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003; 300:486 - 489
- Hartley DM, et al. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 1999; 19:8876 - 8884
- Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, et al. Biological markers of amyloid β-related mechanisms in Alzheimer's disease. Exp Neurol 2010; 223:334 - 346
- Tabira T. Immunization therapy for Alzheimer disease: a comprehensive review of active immunization strategies. Tohoku J Exp Med 2010; 220:95 - 106
- D'Aoust MA, Couture MMJ, Charland N, Trepanier S, Landry N, Ors F, Vezina LP. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficicient and safe response to pandemic influenza. Plant Biotech J 2010; 8:607 - 619
- Bendandi M, Marillonnnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, et al. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin's lymphoma. Ann Oncol 2010; 21:2420 - 2427
- Lai H, Engle M, Fuchs A, Keller T, Johnson S, Gorlatov S, et al. Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. PNAS 2010; 107:2419 - 2424
- Wilson JA, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn AL, Hart MK. Epitopes involved in antibody-mediated protection from Ebola virus. Science 2000; 287:1664 - 1666
- Pujol M, Ramierez NI, Ayala, Gavilondo JV, Valdes R, Rodriguez M, et al. An integral approach towards a practical application of a plant-made monoclonal in vaccine purification. Vaccine 2005; 23:1833 - 1837
- McCormick AA, Reddy S, Reinl SJ, Cameron TI, Czerwinkski DK, Vojdani F, et al. Plant-produced idiotype vaccines for the treatment of non-Hodgkin's lymphoma: safety and immunogenicity in a phase 1 clinical study. PNAS 2008; 105:101031 - 101036
- Werner S, Marillonnet S, Hause G, Klimyuk V, Gleba Y. Immunoabsorbent nanoparticles based on a tobamorvirus displaying protein A. PNAS 2006; 103:17678 - 17683
- Glovokin M, Spitsin S, Andrianov V, Smirnov Y, Xiao Y, Pogrebnyak N, et al. Smallpox subunit vaccine produced in planta confers protection in mice. PNAS 2007; 104:6864 - 6869
- Brodzik R, Spitsin S, Pogrebnyah K, Banurska K, Portocarrero C, Andryszak K, et al. Generation of plant-derived recombinant DTP subunit vaccine. Vaccine 2009; 27:3730 - 3734
- Oxfam/Medecins Sans Fronteries. Giving developing countries the best shot: An overview of vaccine access and R&D 2010;
- Conrad U, Plagmann I, Malchow S, Sack M, Floss DM, Kruglov AA, et al. ELPylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock. Plant Biotechnol J 2011; 9:22 - 31
- Grohs BM, Niu Y, Veldhuis L, Trabelsi S, Garabagi F, Hassell JA, et al. Plant-produced Trastuzumab inhibits the growth of HER2 positive cancer cells in vitro. Plant Biotech J 2010; 58:10056 - 10063