Abstract
The HPV-16/18 AS04-adjuvanted vaccine (Cervarix®, GlaxoSmithKline Biologicals) has been shown to induce a robust immune response in women aged 15-55 years (103514/NCT00196937). This follow-up study is the first report of persistence of immune response and safety profile through 48 months after vaccination in women aged 15-55 years. In this open-label, age-stratified Phase III study in Germany and Poland (105882/NCT00196937), healthy women aged 15-55 years received 3 doses of HPV-16/18 AS04-adjuvanted vaccine at 0, 1, and 6 months. Anti-HPV-16/18 seropositivity rates and geometric mean antibody titers (GMTs) were assessed by enzyme-linked immunosorbent assay (ELISA) in women aged 15-25 (n=168), 26-45 (n=186) and 46-55 years (n=177) from the time of first vaccination through 48 months. At Month 48, all subjects were seropositive for anti-HPV-16 antibodies and 99.4% were seropositive for anti-HPV-18. Antibody kinetics were as previously reported, with peak response at Month 7 followed by a gradual decline tending towards a plateau in all age groups. Anti-HPV-16/18 GMTs were sustained at Month 48 in all age groups, including women aged 46-55 years in whom GMTs were respectively 11-fold and 5-fold higher than natural infection levels. The vaccine exhibited a clinically acceptable safety profile in all age groups. In summary, the HPV-16/18 AS04-adjuvanted vaccine induces high and sustained immune responses in women aged 15-55 years, with antibody levels remaining several-fold higher than natural infection levels for at least 4 years after the first vaccine dose.
Introduction
Persistent infection with an oncogenic human papillomavirus (HPV) type is responsible for the development of cervical cancer,Citation1–Citation3 the second most common cause of cancer death among women worldwide.Citation3,Citation4 Of the 15 HPV types currently classified as oncogenic, HPV-16 and HPV-18 are the most common, cumulatively accounting for over 70% of all cases of invasive cervical cancer.Citation5–Citation7 The HPV-16/18 AS04-adjuvanted vaccine (Cervarix®) has been shown to be highly effective for the prevention of HPV-16 and HPV-18 infections and related precancerous cervical lesions in randomized controlled clinical trials.Citation8–Citation12 The vaccine has also been shown to provide protection against precancerous cervical lesions associated with other non-vaccine oncogenic HPV types, such as HPV-31, HPV-33 and HPV-45.Citation11
The HPV-16/18 AS04-adjuvanted vaccine is now licensed in more than 100 countries worldwide, and national and regional immunization programmes aimed at young adolescent girls have been widely implemented.Citation13 As women are at risk of infection with an oncogenic HPV type throughout their sexually active life,Citation14–Citation16 vaccination should induce long-term protection. Efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine has been shown to be sustained for at least 6.4 years post-vaccination in women aged 15–25 years.Citation12 We have previously shown that the HPV-16/18 AS04-adjuvanted vaccine was immunogenic and generally well-tolerated in women aged 15–55 years, inducing a robust immune response that was sustained for 24 months.Citation17 This paper describes persistence of immune response and safety profile through 48 months after administration of the first vaccine dose.
Results
Study population.
Of the 667 women enrolled in the primary vaccination study, 666 received at least one dose of HPV-16/18 AS04-adjuvanted vaccine. As shown in , the total vaccinated cohort at Month 48 comprised a total of 541 women, of whom 531 were included in the according to protocol (ATP) cohort for analysis of immunogenicity at Month 48. Almost all subjects (99.8%) were White/Caucasian.
Immunogenicity.
All subjects had seroconverted for anti-HPV-16 and anti-HPV-18 antibodies at Month 7 (i.e., one month after completion of the 3-dose vaccination course). At Month 48, all subjects were still seropositive for anti-HPV-16 antibodies and all but one subject in the 46–55 years age group (99.4%) remained seropositive for anti-HPV-18 antibodies. Geometric mean titers (GMTs) of anti-HPV-16/18 antibodies peaked at Month 7, then gradually declined in all age groups (). The decrease in antibody levels between Months 36 and 48 was lower than that observed between previous time points, with antibody response curves appearing to plateau over this period of time.
An age-dependent decrease in anti-HPV-16/18 antibody GMTs was observed, as indicated by the non-overlapping 95% CIs between the three different age groups at all time points (). At Month 48, GMTs for anti-HPV-16 antibodies in initially seronegative subjects were 1382.7 ELISA units (EL.U)/ml in the 15–25 years age group, 524.2 EL.U/ml in the 26–45 years age group, and 324.0 EL.U/ml in the 46–55 years age group. Anti-HPV-16 antibody GMTs at Month 48 were 46-fold higher in women aged 15–25 years, 18-fold higher in women aged 26–45 years, and 11-fold higher in women aged 46–55 years than anti-HPV-16 antibody levels achieved in subjects who cleared a natural infection and mounted a natural immune response in the Phase III PATRICIA trial (29.8 EL.U/ml).Citation11 GMTs for anti-HPV-18 antibodies at Month 48 were 475.5, 189.0 and 122.9 EL.U/ml in the three age groups, respectively. Anti-HPV-18 antibody GMTs at Month 48 were 21-fold, 8-fold and 5-fold higher than levels after a natural infection (22.7 EL.U/ml) in the three age groups, respectively.
Safety.
The HPV-16/18 AS04-adjuvanted vaccine had a clinically acceptable safety profile in all age groups through 48 months after administration of the first vaccine dose ().
During the entire follow-up period, a total of 139 medically significant adverse events (AEs) were reported by 96 subjects (110 medically significant AEs were reported by 79 subjects from Month 0 to Month 24, as previously reported in ref. Citation17). The percentage of subjects reporting medically significant AEs was similar in all age groups (). The most frequently reported medically significant AEs were bronchitis which was reported by 9 subjects (3 in each age group), hypertension which was reported by 8 subjects (3 in the 26–45 years age group and 5 in the 46–55 years age group), and depression which was reported by 6 subjects (1 in the 15–25 years age group, 2 in the 26–45 years age group and 3 in the 46–55 years age group).
A total of 29 serious adverse events (SAEs) were reported by 25 subjects (15 SAEs were reported by 14 subjects from Month 0 to Month 24, as previously described in ref. Citation17). No clustering of SAEs was noted at any time during the 48 months of follow-up. As previously reported in reference Citation17, only 1 SAE was considered by the investigator as possibly related to vaccination: optic neuritis in 1 subject in the 26–45 years age group, which developed in the left eye 9 days after the first vaccine dose. The subject received no further vaccine doses and was treated with high-dose steroids and was free from symptoms four-and-a-half months later. Two deaths occurred during the 48 months of follow-up, both of which happened during the fourth year (one suicide in a subject with a history of bipolar disorder and one road traffic accident). Neither event was considered by the investigator to be related to vaccination.
Twelve subjects reported 14 events that were classified as potential new onset of chronic diseases (NOCDs) (4 events were reported by 4 subjects from Month 0 to Month 24, as previously described in ref. Citation17). Two NOCDs were considered as potentially causally related to vaccination by the investigator (the SAE of optic neuritis and a case of urticaria occurring 7 days post-dose 2 in 1 subject in the 15–25 years age group).
A total of 53 pregnancies were reported over the 48 months of follow-up (15 pregnancies were reported from Month 0 to Month 24, all of which occurred after Month 7) (). Of these, 43 ended in normal births, 2 in premature births (1 normal infant, 1 infant with a congenital heart abnormality), 1 in elective termination and 7 in spontaneous abortion.
Discussion
Prophylactic vaccines against HPV infection are expected to offer a major advance in the prevention of cervical cancer. Such vaccines must provide long-term protection, as the risk of acquiring an infection starts at sexual debut and women remain vulnerable to new infection and development of HPV-related lesions throughout their sexually active life.Citation14–Citation16 Although HPV infection is most prevalent in adolescent girls and young adult women,Citation14–Citation16 a second peak of infection with HPV is observed in women aged 45–50 years.Citation18–Citation20 It has been suggested that HPV infections may be more likely to become persistent in older women,Citation16,Citation19 presumably due to the general decline in immune function with advancing age; however, more recent data suggest that this may not actually be the case.Citation21 Nevertheless, older sexually active women may also have the potential to benefit from HPV vaccination as these women may be (re-)exposed to oncogenic HPV types and may not have already generated protective immunity. We have previously shown the HPV-16/18 AS04-adjuvanted vaccine to be immunogenic and generally well-tolerated in women aged 15–55 years, inducing high antibody levels which were well above natural infection levels in all age groups and sustained through 24 months after the first vaccine dose.Citation17 We report follow-up data on persistence of immune response and safety profile of the HPV-16/18 AS04-adjuvanted vaccine through 48 months post-vaccination.
The HPV-16/18 AS04-adjuvanted vaccine induced high and persistent immune responses in all age groups, with all but one subject remaining seropositive for both antigens through 48 months after administration of the first vaccine dose. Antibody kinetics were as previously reported for this vaccine,Citation9,Citation17 with peak response at Month 7 followed by a gradual decline in all age groups. Of note, the decrease in antibody levels between Months 36 and 48 was lower than that observed between previous time points, with antibody response curves appearing to plateau over this period of time. Consistent with the results of previous studies of the HPV-16/18 AS04-adjuvanted vaccine,Citation17,Citation22 highest anti-HPV-16/18 antibody levels were seen in the younger age group in this study. This finding is not unexpected, since immune response to vaccination is known to decline with advancing age.Citation23–Citation26
The HPV-16/18 AS04-adjuvanted vaccine has been shown to be highly effective for the prevention of HPV-16/18 infection and associated cervical intraepithelial neoplasia (CIN) 2+ lesions through 6.4 years. In the absence of an identified correlate of protection, we evaluated Month 48 antibody titers against the plateau level of antibody titers associated with sustained protection in this previous efficacy trial (Study HPV-007).Citation9,Citation12 Anti-HPV-16/18 antibody titers at Month 48 appeared higher in the 15–25 years age group in the present study than the plateau level achieved at Month 45–50 in Study HPV-007.Citation9 In the older age groups, anti-HPV-16 antibody titers at Month 48 appeared similar to the plateau level achieved in women aged 15–25 years in Study HPV-007.Citation9 Anti-HPV-18 antibody titers at Month 48 in women aged 26–45 and 46–55 years appeared lower than the plateau level achieved in women aged 15–25 years in Study HPV-007.Citation9 Nevertheless, GMTs at Month 48 remained substantially higher in all age groups than those induced by natural infection.Citation10 Mathematical modeling has predicted that antibody levels following vaccination with the HPV-16/18 AS04-adjuvanted vaccine will remain substantially higher than those associated with natural infection for at least 20 years for both antigens.Citation27 However, the relevant antibody titers for complete protection have not yet been established.
Although the mechanism by which prophylactic HPV vaccines induce protection has not been fully elucidated, preclinical studies suggest that protection against subsequent HPV infection is predominately mediated by vaccine-induced type-specific serum neutralizing antibodies.Citation28–Citation31 Transudation of vaccine-induced neutralizing antibodies across the cervical and vaginal epithelium to the site of HPV infection is believed to prevent virus particles from infecting the cervical basal cell layer at the transformation zone.Citation32–Citation34 We have previously reported high correlation between anti-HPV-16/18 antibody levels in serum and cervicovaginal secretions 24 months post-first vaccine dose in all age groups of women participating in this study, supporting transudation of vaccine-induced serum antibodies into the cervical mucosa.Citation17 Experience with many different types of vaccines has shown persistence of vaccine-induced antibodies to be closely related to the magnitude of the humoral response induced.Citation35–Citation40 Memory B-cells play an important role in replenishing the pool of plasma cells that maintain antibody levels in the absence of a pathogen and are known to be responsible for driving the rapid anamnestic antibody response that occurs after re-exposure to antigen in subjects who have lost detectable antibodies after vaccination.Citation39,Citation41,Citation42 Cell-mediated immune responses are also thought to play a role in the clearance of established HPV infection and associated lesions.Citation32
The AS04 Adjuvant System (AS) in the vaccine formulation is potentially a key factor in the sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine. The AS04 adjuvant system consists of monophosphoryl lipid A (MPL), a purified, detoxified derivative of the lipopolysaccharide molecule of the bacterial wall of Salmonella minnesota absorbed onto aluminum hydroxide.Citation43 MPL is a powerful stimulant of the immune system, which acts through binding on the Toll-like receptor 4 on antigen-presenting cells (APCs), stimulating the maturation of these cells and their migration to the lymph nodes. MPL-stimulated APCs express increased levels of costimulatory molecules and secrete cytokines, inducing strong humoral and cellular responses, depending on the antigen considered.Citation44,Citation45 The HPV-16/18 AS04-adjuvanted vaccine has previously been shown to induce higher antibody levels for up to 4 years after the first vaccine dose compared with the same HPV-16/18 antigens formulated with aluminum hydroxide alone.Citation46 The HPV-16/18 AS04-adjuvanted vaccine has also been shown to induce higher levels of anti-HPV-16/18 neutralizing antibodies and antigen-specific memory B-cells than a quadrivalent prophylactic HPV vaccine containing HPV-16 and HPV-18 virus-like particles formulated with amorphous aluminum hydroxyphosphate sulfate salt in women aged 18–45 years.Citation47 This quadrivalent prophylactic HPV vaccine has been shown to be effective for the prevention of cervical disease or infection caused by HPV-16 and 18 in women aged 24–45 years.Citation48
The safety profile of the HPV-16/18 AS04-adjuvanted vaccine over the 48 months of follow-up in this study was consistent with the results of other long-term studies.Citation11,Citation12,Citation49 Results of a pooled analysis of data from almost 30,000 women and girls participating in Phase II and III trials confirm that the HPV-16/18 AS04-adjuvanted vaccine has a clinically acceptable safety profile in women of all ages.Citation50
The main limitations of this study are the open design and lack of a direct control group. In addition, AEs occurring at very low frequency cannot be completely excluded, since this study was not specifically powered for this purpose.
In summary, women remain at risk of new oncogenic HPV infections and the development of cervical lesions and cancer throughout their sexually active life. Adult women may be (re-)exposed to oncogenic HPV types and may not have already generated protective immunity as natural infection does not reliably protect against (re-)infection. Results of this study demonstrate that the HPV-16/18 AS04-adjuvanted vaccine induces a sustained immune response in women aged 15–55 years, with antibody levels remaining several-fold higher than natural infection levels for at least 4 years after the first vaccine dose. This suggests that the HPV-16/18 AS04-adjuvanted HPV vaccine combined with continued screening has the potential to provide benefit in sexually active women aged over 25 years. Vaccination of a broader age range may also reduce overall HPV infection rates through herd immunity. Further studies are underway to extend our findings and to evaluate vaccine efficacy. Ongoing follow-up of the women enrolled in the present study is continuing to assess immunogenicity and safety through 10 years after the first vaccine dose (NCT00947115). A phase III, double-blind, randomized, controlled study to assess the efficacy of the HPV-16/18 AS04-adjuvanted vaccine for the prevention of persistent HPV-16/18 infection and associated precancerous cervical lesions in women aged 26 years and older is also ongoing (NCT00294047).
Methods
Study design.
The primary study (103514/NCT00196937) took place from October 2004 to July 2005 in 6 centers in Germany and Poland.
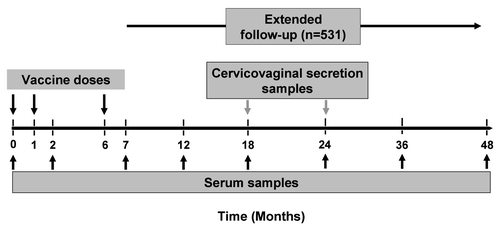
This was an extension study (105882/NCT00196937) conducted in Germany and Poland from July 2008 to February 2009 as an open-label, age-stratified, multicenter, follow-up study designed to evaluate the safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to Month 48 in women vaccinated at the age of 15–55 years. Study design is summarized in . Healthy women aged 15–55 years who participated in the primary study and received 3 doses of HPV-16/18 AS04-adjuvanted vaccine at 0, 1 and 6 months were eligible for inclusion in this follow-up study. Women included in the extension phase had to have completed the full primary vaccination course. Enrolment into the primary study was irrespective of HPV serological status. Women of childbearing potential were required to be abstinent from sexual activity or using adequate contraception from 30 days prior to vaccination to 2 months after the third vaccine dose, and to have a negative pregnancy test on the day of vaccination. Exclusion criteria included use of an investigational drug or vaccine within 30 days, chronic immune-modifying drugs within 6 months, immunoglobulins or blood products within 3 months or planned use of any of these during the study period; breastfeeding; or prior vaccination with HPV or AS04-based vaccines.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and the study protocol, informed consent/assent forms and recruitment materials were approved by the Institutional Review Board or equivalent at all participating centers. Written informed consent (or informed assent with written consent from a parent or legal representative if below the legal age of consent) was obtained from all participants.
Study vaccine.
Each dose of HPV-16/18 AS04-adjuvanted vaccine contained 20 µg each of HPV-16 and HPV-18 L1 virus-like particles formulated with a proprietary Adjuvant System comprising 50 µg MPL and 500 µg Al(OH)3, designated AS04. The vaccine was supplied in individual 0.5 ml pre-filled syringes and administered into the deltoid muscle.
Immunogenicity.
Serum samples were collected at Months 0, 2, 7, 12, 18, 24, 36 and 48 (). HPV-16 and HPV-18 antibody titers were measured by ELISA using type-specific VLPs as coating antigens (serum standardized protocol as previously published in ref. Citation8–Citation10, Citation17 and Citation51). This assay has been used in all clinical trials of the HPV-16/18 AS04-adjuvanted vaccine. Seropositivity was defined as an antibody titer greater than or equal to 8 EL.U/ml for HPV-16 and 7 EL.U/ml for HPV-18, as previously reported in reference Citation9, Citation10 and Citation17.
Safety.
Information was collected on medically significant AEs, SAEs, NOCDs and pregnancies during the entire study period from Month 0 to Month 48. An SAE was defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalization, resulted in disability or incapacity, was an important medical event or was a congenital anomaly/birth defect in the offspring of a study participant. Medically significant AEs were defined as AEs prompting emergency room or physician visits that were not related to common diseases or routine visits for physical examination or vaccination or SAEs not related to common diseases. NOCDs included autoimmune conditions, allergies and asthma.
Statistical analysis.
The primary population for analysis of immunogenicity was the ATP cohort. The ATP cohort for analysis of immunogenicity included all subjects who were included in the ATP immunogenicity analysis of the primary study, meeting all eligibility criteria, complying with the procedures and intervals defined in the protocol, with no elimination criteria during the study and for whom serology results were available for Month 48. Immunogenicity analyses were stratified according to three age groups (15–25, 26–45 and 46–55 years at first vaccination). Seropositivity rates and GMTs were calculated with 95% confidence intervals (95% CI) for each antigen in all age groups at Months 0, 2, 7, 12, 18, 24, 36 and 48.
In the absence of an accepted serological correlate of protection, descriptive comparisons were performed between anti-HPV-16/18 GMTs in this study and anti-HPV-16/18 GMTs in women aged 15–25 years who cleared a natural infection and mounted an immune response in the Phase III PATRICIA trial.Citation11
The primary population for analysis of safety was the total vaccinated cohort (TVC). The safety analysis was stratified by age group (15–25, 26–45 and 46–55 years at first vaccination). The percentage of subjects reporting at least one SAE, medically significant AEs or NOCDs was calculated with 95% CI. Pregnancies and their outcomes were described in detail.
All statistical analyses were performed using SAS 9.1 and Proc Statxact 7.0.
Conflict of Interest
T.F. Schwarz received honoraria from GlaxoSmithKline, Novartis Vaccines and Wyeth Pharma for conducting clinical trials, lecturing and travel accommodations as well as membership of advisory boards. M. Spaczynski received grants from GlaxoSmithKline Biologicals through his institution for conducting the study. He also obtained consulting fee, travel support for study meetings, expert testimony and payment for lectures from GlaxoSmithKline Biologicals. A. Schneider received consulting fee and payment for lectures from GlaxoSmithKline Biologicals. J. Wysocki obtained grants from GlaxoSmithKline Biologicals through his institution for conducting the study and received support to travel to investigator meeting and payment for lectures. K. Schulze received payment for lectures as well as fees for participation in review activities such as data monitoring boards from GlaxoSmithKline Biologicals. A. Galaj declared he has no conflict of interest. S. Poncelet, G. Catteau, F. Thomas and D. Descamps are GlaxoSmithKline Biologicals' employees. F. Thomas and D. Descamps own GlaxoSmithKline Biologicals stock options.
Financial Support
GlaxoSmithKline Biologicals.
Trademark
Cervarix is a registered trademark of the GlaxoSmithKline group of companies.
e-track/NCT
105882/NCT00196937.
Abbreviations
| AEs | = | adverse events |
| APC | = | antigen-presenting cell |
| AS | = | adjuvant system |
| ATP | = | according to protocol |
| CI | = | confidence interval |
| CIN | = | cervical intraepithelial neoplasia |
| ELISA | = | enzyme-linked immunosorbent assay |
| EL.U | = | ELISA units |
| GMT | = | geometric mean titer |
| HPV | = | human papillomavirus |
| MPL | = | monophosphoryl lipid A |
| NOCDs | = | new onset of chronic diseases |
| SAEs | = | serious adverse events |
| SD | = | standard deviation |
| TVC | = | total vaccinated cohort |
Figures and Tables
Figure 1 Subject disposition. n, number of subjects; 15–25 years, subjects 15–25 years of age at the time of first vaccine dose; 26–45 years, subjects 26–45 years of age at the time of first vaccine dose; 46–55 years, subjects 46–55 years of age at the time of first vaccine dose.
Figure 2 Antibody levels in women between 15 and 55 years of age initially seronegative for HPV-16 or HPV-18 antibodies (ATP immunogenicity cohort). GMT, geometric mean titer; Error bars, 95% confidence interval; 15–25 years, subjects 15–25 years of age at the time of first vaccine dose; 26–45 years, subjects 26–45 years of age at the time of first vaccine dose; 46–55 years, subjects 46–55 years of age at the time of first vaccine dose; Natural infection: GMTs of subjects from Study HPV-008 who were (a) HPV-16 or (b) HPV-18 DNA-negative and seropositive at baseline (i.e., who had cleared a natural infection. GMTs were (a) 29.8 EL.U/ml and (b) 22.7 EL.U/ml11; Plateau phase: GMTs of subjects from Study HPV-007 in women aged 15–25 years at Months 45–50 after the first vaccine dose (Total cohort). GMTs were (a) 397.8 EL.U/ml and (b) 297.3 EL.U/ml.Citation9
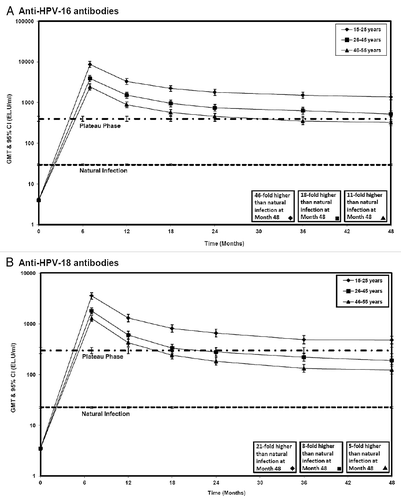
Table 1 Number of women reporting medically significant adverse events, serious adverse events, fatal serious adverse events or new onset of chronic diseases [classified by Medical Dictionary for Regulatory Activities (MedDRA) Primary System Organ Class and Preferred Term] and number of pregnancies and pregnancy outcomes over the 48 months of follow-up (total vaccinated cohort)
Acknowledgments
The authors gratefully acknowledge and thank the study participants and the staff members of the study centers that participated in this study: Jens Vollmar for his support as Principal Investigator in Germany for the study coordination; Günter Cichon for his support as study physician, Andreas Kaufmann for laboratory coordination and Ursula Kastner for technical support at the Charité Universitätsmedizin Berlin site in Germany.
We also would like to acknowledge the GlaxoSmithKline Biologicals Clinical Study network: Geneviève Meiers for the central study coordination; Anja-Natascha Straube, Andreas Cischek and Beata Wejer for the local study coordination; Isabelle Stainier, Rudy Crudenaire, Nathalie Durant for the clinical immunology; Catherine Bougelet and Valerie Xhenseval for performing the serology testing, Fatoumata D. Gassama for protocol development coordination; Bart Spiessens and Toufik Zahaf for statistical contributions.
We would also like to thank Jennifer Coward (freelance medical writer) for writing assistance, and Mélanie Muylaert (XPE Pharma & Science) and Denis Sohy (Business and Decision) for editorial assistance and manuscript coordination on behalf of GlaxoSmithKline Biologicals.
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12 - 19
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244 - 265
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890 - 907
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin 2005; 55:74 - 108
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518 - 527
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121:621 - 632
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; 26:1 - 16
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757 - 1765
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247 - 1255
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161 - 2170
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 314
- GlaxoSmithKline Vaccine HPV-007 Study Group. Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975 - 1985
- Schwarz TF. Clinical update of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine, Cervarix®. Adv Ther 2009; 26:983 - 998
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813 - 819
- Coupé VMH, Berkhof J, Bulkmans NWJ, Snijders PJF, Meijer CJLM. Age-dependent prevalence of 14 high-risk HPV types in the Netherlands: implications for prophylactic vaccination and screening. Br J Cancer 2008; 98:646 - 651
- Castellsagué X, Schneider A, Kaufman AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol 2009; 115:15 - 23
- Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine 2009; 22:581 - 587
- Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003; 89:101 - 105
- Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis 2005; 191:1808 - 1816
- de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007; 7:453 - 459
- Rodríguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315 - 324
- Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40:564 - 571
- Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis 2002; 35:1368 - 1375
- Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines 2008; 7:467 - 479
- Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol 2009; 30:351 - 359
- Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol 2009; 333:413 - 429
- David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, Van Damme P. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: Modeling of sustained antibody responses. Gynecol Oncol 2009; 115:1 - 116
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA 1995; 92:11553 - 11557
- Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol 1996; 70:960 - 965
- Bryan JT, Jansen KU, Lowe RS, Fife KH, McClowry T, Glass D, Brown DR. Human papillomavirus type 11 neutralization in the athymic mouse xenograft system: correlation with virus-like particle IgG concentration. J Med Virol 1997; 53:185 - 188
- Day PM, Kines RC, Thompson CD, Jaqu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260 - 270
- Stanley M, Lowy DR, Frazer I. Prophylactic HPV vaccines: Underlying mechanisms. Vaccine 2006; 24:106 - 113
- Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol 2008; 110:1 - 10
- Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol 2008; 109:15 - 21
- Jilg W, Schmidt M, Deinhardt F, Zachoval R. Hepatitis B vaccination: how long does protection last?. Lancet 1984; 2:458
- Jilg W, Schmidt M, Deinhardt F. Persistence of specific antibodies after hepatitis B vaccination. J Hepatol 1988; 6:201 - 207
- European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity?. Lancet 2000; 355:561 - 565
- Banatvala JE, Van Damme P. Hepatitis B vaccine—do we need boosters?. J Viral Hepat 2003; 10:1 - 6
- Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine 2003; 21:35 - 37
- Van Damme P, Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K, et al. Hepatitis A booster vaccination: is there a need?. Lancet 2003; 362:1065 - 1071
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity 1998; 8:363 - 372
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002; 298:2199 - 2202
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007; 6:723 - 739
- Ulrich JT, Myers KR. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol 1995; 6:495 - 524
- Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev Vaccines 2003; 2:219 - 229
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24:5937 - 5949
- Einstein MH, Baron M, Levin M, Chatterjee A, Edwards R, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin 2009; 5:705 - 719
- Muñoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity and efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet 2009; 373:1949 - 1957
- De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247 - 6255
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention. A pooled analysis of 11 clinical trials. Hum Vaccin 2009; 5:332 - 340
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425 - 434