Abstract
Influenza vaccines were developed in the 1930s and were shown in randomized clinical trials to prevent influenza in young healthy adults. The significant morbidity and mortality associated with influenza in adults, age 65 y and older, prompted the early recommendation for influenza vaccination in that age group, based on efficacy data in younger adults. Subsequently a number of studies have demonstrated vaccine effectiveness in older adults, but it appears to be lower than in younger adults. New vaccines are being developed with enhanced immunogenicity to improve the protection of older adults. In the meantime, the currently licensed influenza vaccines need to be administered annually to prevent the estimated 90,000 hospitalizations and 5,000 deaths attributed to influenza in adults ≥65 y of age each year.
Since 1960, US public health officials have recommended yearly influenza vaccination for all adults ≥65 y of age due to the increased morbidity and mortality seen in that age groupCitation1 These policies were based on the assumption that the influenza vaccines had been effective in preventing clinical illness during most epidemics and “would reduce the risk of death among the aged and chronically ill.”Citation1 However, randomized placebo-controlled efficacy trials of the vaccines had not been conducted in older adults prior to these recommendations. Subsequently, numerous observational studies have been performed to quantify the effectiveness of the trivalent influenza vaccine in older adults. The actual assessment of the impact of influenza vaccination on all-cause hospitalizations and deaths has been particularly challenging.
Influenza vaccines were first developed in the 1930s using whole inactivated influenza strains grown in embryonated chicken eggs.Citation2 Since that time influenza vaccines have been refined to reduce local and systemic reactions, but are still grown in embryonated eggs. Currently the inactivated vaccine is generally supplied as either split (whole virus treated with detergents or organic solvents) or subunit (created by separation of viral membrane proteins from the viral core) products.Citation3 Early vaccine efficacy studies were performed in the Army Specialized Training Program units at multiple universities where the overall attack rate for clinical influenza A was 2.2% in the vaccinated cohort and 7.1% in the unvaccinated participants.Citation4 However, this population was young and healthy, and it was unclear how these efficacy results extrapolated to older populations.
Vaccine effectiveness can be measured using several different endpoints. The most commonly used endpoints include influenza-like illness (ILI), viral culture, and serologic rises between pre and post season samples. Each endpoint has advantages and disadvantages. Classically influenza presents with the acute onset of fever, myalgia, and cough.Citation5 For research purposes, ILI has been defined by the Centers for Disease Control and Prevention (CDC) as fever with either cough or sore throat.Citation6 This CDC-ILI definition has high positive predictive value in young adults (86.8%)Citation7 during periods of high influenza activity. However, it is much lower in older adults since they often do not have fever and other manifestations of influenza.Citation8,Citation9 For example, in one prospective study of influenza in older patients with obstructive lung disease,Citation10 fever had only 26% sensitivity for the diagnosis of influenza, when compared with viral culture and serology as the gold standard. In another hospitalized cohort, only 51% of adults aged 15–99 y (median age 60 y) with laboratory-confirmed influenza had classic ILI symptoms.Citation11 In addition, ILI can be caused by other respiratory pathogens, not only influenza. Hence, ILI can both under and overestimate effectiveness of the influenza vaccine.
Traditionally, culture has been the gold-standard for the diagnosis of influenza. However, older adults generally have lower viral titers in their respiratory secretions than younger adults and children, decreasing the sensitivity of culture in this age group when compared with serology and polymerase chain reaction (PCR).Citation12 In a study of older adults with serologically confirmed influenza, culture only identified approximately half (22/43) of the infections.Citation13 Using PCR as the gold standard, the sensitivity of the culture has been reported to vary between 21–50%.Citation14,Citation15 Serology is another common endpoint for effectiveness studies. A positive case of influenza is usually defined as a ≥4-fold rise in antibody titers between the pre and post season serology. As shown above, serology will identify influenza cases in the absence of positive cultures.
Randomized, placebo-controlled influenza vaccine efficacy (VE) trials in older adults have been conducted, but these studies have been complicated by small sample sizes, early study termination due to changes in local vaccination practices, and the use of non-specific study endpoints such as influenza-like illness, rather than laboratory-confirmed influenza.Citation16-Citation18 Three randomized clinical trials (RCTs) evaluating the efficacy of trivalent inactivated influenza vaccines (TIV) have been conducted in elderly adults. Two of these studies have used both ILI and serologic conversion as endpoints. Govaert et al.Citation17 in the Netherlands randomized 1,838 community-living healthy elderly persons ≥60 y of age, with no additional underlying risk factors, to receive either TIV or placebo. The patients were followed for 5 mo post-vaccination for physician-diagnosed ILI, serologically-confirmed influenza, and with postal questionnaires. Using ILI as the endpoint, 2% of TIV recipients and 3% of placebo recipients were diagnosed with ILI, yielding a statistically significant vaccine efficacy of 33%. Using serologically-confirmed influenza, defined as a 4-fold rise in titer between the post-vaccination and post-influenza season serology, 4% of TIV recipients and 9% of placebo recipients qualified, yielding a VE of 56%. However, this study was inadequately powered to examine the efficacy of the vaccine in adults ≥70 y of age, although the trend in effectiveness was similar to those younger than 70 y of age. PraditswanCitation16 conducted another randomized, double blind, placebo-controlled study in 653 subjects aged over 60 y in Thailand. Using ILI as the endpoint, subjects in the vaccine group had a relative risk reduction of 56% when compared with the placebo group. Using serologic evidence of infection as the endpoint, the relative risk reduction was 65% (95% CI, 16–85%). However, there were no differences in the number of treatments received or the cost of treatments for ILI between the vaccine and placebo groups, likely reflecting ILI due to other non-influenza viruses.
Finally, another RCT conducted by Allsup et al. randomized 729 subjects aged 65 to 74 y, without underlying risk factors, to receive either influenza vaccine or placebo, in a 3:1 ratio, delivered in combination with the 23-valent pneumococcal polysaccharide vaccine. No statistically significant differences between the two groups were noted in physician diagnoses of ILI or pneumonia. Because the study was conducted in the UK and routine vaccination for everyone ≥65 y was recommended during the second year of the study, the study was terminated so that all participants could receive influenza vaccine.Citation18
Other than the three trials outlined above, cohort and case-control studies have been the primary mode to evaluate influenza vaccine effectiveness in older adults. A recent Cochrane review, which reported 75 studies including RCTs, quasi-RCTs, cohort, and case-control studies, assessed VE using laboratory-confirmed influenza or ILI and concluded that the protection afforded by the influenza vaccines in older community adults was modest.Citation19 According to the review, the “available evidence is of poor quality and provides no guidance regarding the safety, efficacy or effectiveness of influenza vaccines for people aged 65 years or older.”Citation20
In our assessment there are a number of fundamental problems with prior evaluations of the effectiveness of influenza vaccine: Each of these will be discussed individually.
Non-Specific Outcome Measures
Clinicians have traditionally used ILI, viral culture, or rapid antigen testing, to diagnosis influenza. Unfortunately, none of these are both specific and sensitive methods. For example, in a clinical trial evaluating the efficacy of influenza vaccine in healthy adults, three distinct end-points were used: laboratory-confirmed influenza (serology), febrile respiratory tract illness and upper respiratory infection (URI). VE varied enormously depending on the end-point: it was 86% for laboratory-confirmed influenza, 34% for febrile respiratory illnesses but only 10% for URI.Citation21 Using these end-points, influenza vaccine was highly effective in preventing laboratory-confirmed influenza in healthy adults, but understandably ineffective in preventing the less specific URIs, likely due to other non-influenza agents such as rhinovirus, respiratory syncytial virus, or human metapneumovirus. Thus, including non-influenza-associated illness in an assessment of influenza VE results in misclassification of the outcome and falsely low estimates of VE, as recently summarized in a report evaluating methodological issues surrounding observational studies of VE, which found that “laboratory confirmation of cases is critical.”Citation22
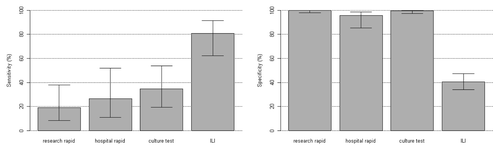
Because of the limitations of clinically available diagnostic tools for older adults, primary care providers often have difficulty identifying influenza. In one of our previously reported hospital-based two-year influenza surveillance studies, only 15% of patients with RT-PCR confirmed influenza were given the discharge diagnosis of influenza by their healthcare provider and during one of the study years, provider recognition was as low as 3%. In that study the sensitivity of the rapid antigen tests employed in the study hospitals was only 20%, when compared with RT-PCR.Citation23 ()
Figure 1. Comparison of the test characteristics of each diagnostic method used for older adult patients hospitalized with symptoms of respiratory illness or nonlocalizing fever during the 2006–2007 and 2007–2008 influenza seasons. Error bars represent confidence intervals. Hospital rapid, hospital rapid antigen test; ILI, diagnostic use of the clinical definition of influenza-like illness; Research rapid, research rapid antigen test. Copyright © (2010) by The Society for Healthcare Epidemiology of America. Reprinted with permission; Talbot et al.Citation23
We and others have used discharge codes to assess the impact of influenza vaccine on the number of excess cases of pneumonia and influenza or respiratory and circulatory admissions. This method allows the assessment of much larger databases to increase the sample size of the population studied, however a major limitation is that it is not possible to determine whether individual patients have laboratory-confirmed influenza. To highlight this point, in one of our recently published studies, we found that only 28.2% of the hospitalized individuals aged >50 y with RT-PCR confirmed influenza, were given discharge codes for Pneumonia and Influenza and another 69.2% were given other codes for respiratory and circulatory disorders. Similarly those who were RT-PCR negative, were given very similar discharge codes, with 28.3% receiving Pneumonia and Influenza codes and 67.2% given other respiratory and circulatory diagnostic codes.Citation24 Hence it was not possible to determine by discharge codes which patients had laboratory confirmed influenza and which ones did not, creating misclassification bias and overestimating vaccine effectiveness. (). Further, if laboratory-confirmed influenza was only responsible for between 2–20% of cardiopulmonary hospitalizations, then it would not be possible for influenza vaccine to prevent more than that number of hospitalizations. For the influenza vaccine to be 50% effective at preventing all cardiopulmonary hospitalizations,Citation14,Citation24 then the influenza vaccine would have to be at least 250% effective. In other words, it would have to prevent diseases not caused by influenza, which is unlikely to occur.
Figure 2. Propensity score–adjusted vaccine effectiveness [(1 – Odds Ratio)*100%] overall and stratified by year for hospitalization, age, sex, race, and smoking status in adults aged >50 y, Davidson County, Tennessee Overall VE is adjusted for age in years, sex, race, smoking status, home oxygen use, underlying medical conditions, immunosuppression, timing of admission relative to onset of influenza season, and specific influenza season (2006–2007, 2007–2008, or 2008–2009). Copyright © (2011). Published by Oxford University Press on behalf of the Infectious Diseases Society of America. Reprinted with permission; Talbot et al.Citation23
![Figure 2. Propensity score–adjusted vaccine effectiveness [(1 – Odds Ratio)*100%] overall and stratified by year for hospitalization, age, sex, race, and smoking status in adults aged >50 y, Davidson County, Tennessee Overall VE is adjusted for age in years, sex, race, smoking status, home oxygen use, underlying medical conditions, immunosuppression, timing of admission relative to onset of influenza season, and specific influenza season (2006–2007, 2007–2008, or 2008–2009). Copyright © (2011). Published by Oxford University Press on behalf of the Infectious Diseases Society of America. Reprinted with permission; Talbot et al.Citation23](/cms/asset/eae23962-6d3d-437c-8c35-298a29a50631/khvi_a_10918129_f0002.gif)
The Importance of Frailty Assessment
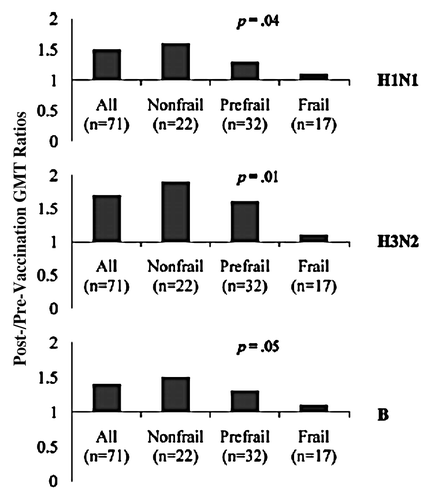
The uptake of influenza vaccine in the community varies greatly by race, socioeconomic status, and level of frailty. Although most studies of influenza vaccine effectiveness have controlled for race and socioeconomic status, the level of frailty has generally not been examined. Frailty is a multi-factorial syndrome that represents a reduction in physiological reserve and an inability to resist environmental stressors.Citation25,Citation26 Recently, frailty has been shown to be associated with increased susceptibility to influenza and decreased responsiveness to influenza vaccines. Yao et al. conducted a prospective observational study to evaluate the influence of frailty on the immune responses to seasonal influenza vaccines and rates of influenza-like illness in 70 community dwelling adults older than 70 y old. The authors showed that frailty was associated with significant impairment in strain-specific antibody titers and increased rates of ILI and laboratory-confirmed influenza infection.Citation27 () These findings suggest that frailty status is very important in the evaluation of the immunogenicity and effectiveness of influenza vaccines in the elderly. Frailty has been shown to predict vaccine response to the polysaccharide pneumococcal vaccine better than age,Citation28 and has been identified as a confounder in some influenza vaccine efficacy studies, being associated with both likelihood of vaccination and likelihood of hospitalization and/or death.Citation29,Citation30 In a retrospective population-based study the association between hospitalization and prior vaccination was evaluated in different seasons. Risk for hospitalization for pneumonia and influenza was associated with influenza vaccination even before influenza was circulating, so reduced mortality in the summer months, when influenza was not circulating, seen in those that have received influenza vaccine was likely due to other confounders, with frailty being one of them.Citation31
Figure 3. GMT ratios to H1N1, H3N2, and B strains in all study participants “All (n = 71),” nonfrail (n = 22), prefrail (n = 32), and frail (n = 17) groups. p Values were derived from linear regression analysis for stepwise trend of decrease in nonfrail, prefrail, to frail study groups, adjusted for age. Copyright © (2011) Elsevier Ltd. Reprinted with permission; Yao et al.Citation27
Vaccination in Nontraditional Sites
Previously most patients received vaccines either from their primary care provider or from public health clinics. However, now many different sources of influenza vaccine are available including; pharmacies, grocery stores, employers, and even large retail stores. Although expanded venues for vaccination enhance immunization rates, vaccinations delivered outside of the medical home are often not captured by administrative databases.Citation32 A recent survey completed by the CDC showed that approximately 24% of adults ≥65 y of age received vaccination from a nontraditional site, not from their primary care provider or the local health department.Citation33 Hence, large database studies using vaccine registries or insurance billing data may incorrectly categorize as many as 25% of the patients as unvaccinated, since retail stores are not required to report vaccination information to state registries in many states.
Recent Vaccine Effectiveness Study
One of our recently published studies,Citation24 sought to determine TIV vaccine effectiveness in older adults. Adults aged >50 y who were hospitalized with respiratory symptoms or non-localizing fever were prospectively tested for influenza using RT-PCR. Those individuals who were PCR positive were classified as cases and were compared with PCR negative individuals, who were classified as controls. Since both groups were hospitalized, it would be expected that the cases and controls would be similar. Vaccine verifications included patient medical providers but also other providers such as employers, retail pharmacies, and retail groceries. The estimated overall VE for two years was 61.2% (95% CI: 17.5%, 81.8%). ()
Immune Senescence
What makes older adults immunologically different from younger individuals? With aging, immune systems undergo multiple changes leading to alterations in all arms of the immune response. There is an overall increased inflammatory state, defects in antigen presentation, loss of naïve T cells due to thymic involution, restricted diversity of T cells, and defects in isotype switching and somatic hypermutation.Citation34 Not only are older adults at higher risk for complications due to viral respiratory diseases, but less likely to present with classical symptoms, and less likely to respond to vaccination. Studies of antibody response to influenza vaccination have shown consistently lower antibody responses in older adults.Citation35 A newly published study that assessed the levels of vaccine-specific plasmablasts one week after TIV in younger (18–51 y of age) and older adults (≥70 y of age) showed reduced plasmablasts and decreased antibody-secreting cells in the older adults, but similar production of secreted IgG per plasmablast and similar avidity of plasmablast-derived polyclonal and monoclonal antibodies between the two age groups. These findings show that the decreased serum antibody responses in elderly subjects are due to reduced quantities of influenza vaccine-specific antibodies, rather than a lack of antibody avidity or affinity.Citation36
New Vaccines
In an effort to overcome the effects of an aging immune system, several new approaches are being developed; the addition of potent new adjuvants and the administration of higher doses of vaccine. To detect a difference between the new and the standard vaccines, the immunogenicity of the new vaccines will need to be initially compared with standard TIV and further subjected to studies of vaccine effectiveness. A new high-dose (HD) influenza vaccine was recently licensed in the United States, containing four-times the usual amount of hemagglutinin (HA) protein for each of the three influenza vaccine strains (60 mcg of HA). A multicenter, randomized, double blind controlled study that included community dwelling adults ≥65 y compared the HD and standard dose influenza vaccine.Citation37 A statistically significant increase in the rates of seroconversion and mean hemagglutination inhibition titers at day 28 after vaccination for all three vaccine strains was noted in the subjects who received the HD formulation when compared with standard dose. Although local reactions were more frequent in the HD group, they were mild to moderate in intensity. A vaccine effectiveness study comparing these two vaccines is planned.
Oil in water emulsion-based adjuvants like MF-59 and AS03 have been shown to improve the immunogenicity of a number of vaccines in individuals of different ages. An influenza vaccine containing MF-59 has been licensed in Europe and has been shown to be safe when used in more than 45 million people.Citation38 A recent review of 64 clinical trials of MF-59 adjuvanted influenza vaccine included 27,998 individuals aged 6 mo to 100 y and revealed a good safety profile. Solicited local and systemic reactions 0–3 d after first vaccination were higher in subjects who received vaccine with adjuvant, but the reactions were mild and transient.Citation39
A recent multicenter open-label study conducted in Korea evaluated the immunogenicity and safety of H1N1 vaccine with and without MF-59 in both young adults and individuals aged ≥65 y using a two-dose regimen, administered 21 d apart. Three different vaccine doses were compared; 3.75 μg HA (MF-59 adjuvanted) vs. 7.5 μg HA (MF-59 adjuvanted) vs. 15 μg HA (nonadjuvanted) in young adults and 3.75 μg (MF-59 adjuvanted) vs. 7.5 μg (MF-59 adjuvanted) in the elderly. In young adults, all three vaccine regimens were immunogenic. In the elderly, on day 21 after the first dose, the rates of seroconversion were significantly higher for the 7.5-μg dose of MF-59 adjuvanted than for the 3.75-μg dose of adjuvanted vaccine [53.6% vs. 37.2% (p < 0.01), respectively]. After the second dose, the geometric mean titer (GMT) was less with the 15-μg dose of nonadjuvanted vaccine, but the GMT increased 2-fold with MF-59 adjuvanted vaccines. The adjuvanted vaccine was also well tolerated without serious adverse events.Citation40 Thus, in the elderly, a two-dose priming strategy with adjuvanted vaccine appears to be a promising option.
Another oil in water emulsion-based adjuvant (ASO3) when combined with influenza vaccines has also been shown to be highly immunogenic in adults and children.Citation41-Citation44 A recently published phase 2 open-label study that includes adults older than 61 y assessed the immune response elicited after two single or two double doses of ASO3 adjuvanted H5N1 vaccine administered 21 d apart compared with unadjuvanted influenza vaccine. Two injections of a single dose of 3.75µg H5N1 ASO3 adjuvanted vaccine were shown to elicit high levels of HA and neutralizing antibodies and cell mediated immunity was maintained for six months after vaccination. The single dose schedule, produced a lower immune response than the double dose, but still was adequate to meet licensing requirements.Citation45 ()
Table 1. Vaccination groups are as follows: 1 x H5N1-AS, single dose of the AS03A-adjuvanted vaccine; 1 x H5N1, single dose of the nonadjuvanted vaccine; 2 x H5N1-AS, double dose of the AS03A-adjuvanted vaccine; 2 x H5N1, double dose of the nonadjuvanted vaccine. ATP, according to protocol
Conclusion
Older adults are at increased risk for influenza and complications due to influenza infection. Routine vaccination has been recommended for years and older adults would likely benefit from newer, more immunogenic vaccines. Until these new vaccines become available, TIV should be administered to reduce the significant morbidity and mortality due to influenza in adults ≥65 y of age.
References
- Langmuir AD, Henderson DA, Serfling RE. The Epidemiological Basis for the Control of Influenza. Am J Public Health Nations Health 1964; 54:563 - 71; http://dx.doi.org/10.2105/AJPH.54.4.563; PMID: 14136320
- Francis T, Magill TP. The Antibody Response of Human Subjects Vaccinated with the Virus of Human Influenza. J Exp Med 1937; 65:251 - 9; http://dx.doi.org/10.1084/jem.65.2.251; PMID: 19870599
- Beyer WE, Palache AM, Osterhaus AD. Comparison of Serology and Reactogenicity between Influenza Subunit Vaccines and Whole Virus or Split Vaccines: A Review and Meta-Analysis of the Literature. Clin Drug Investig 1998; 15:1 - 12; http://dx.doi.org/10.2165/00044011-199815010-00001; PMID: 18370460
- Francis T Jr., Getting VA, et al. The present status of vaccination against influenza. Am J Public Health Nations Health 1947; 37:1109 - 12; http://dx.doi.org/10.2105/AJPH.37.9.1109
- Cate TR. Clinical manifestations and consequences of influenza. Am J Med 1987; 82:15 - 9; http://dx.doi.org/10.1016/0002-9343(87)90555-9; PMID: 3591813
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza?. JAMA 2005; 293:987 - 97; http://dx.doi.org/10.1001/jama.293.8.987; PMID: 15728170
- Boivin G, Hardy I, Tellier G, Maziade J. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis 2000; 31:1166 - 9; http://dx.doi.org/10.1086/317425; PMID: 11073747
- Govaert TM, Dinant GJ, Aretz K, Knottnerus JA. The predictive value of influenza symptomatology in elderly people. Fam Pract 1998; 15:16 - 22; http://dx.doi.org/10.1093/fampra/15.1.16; PMID: 9527293
- Matsuno O, Kataoka H, Takenaka R, Okubo F, Okamoto K, Masutomo K, et al. Influence of age on symptoms and laboratory findings at presentation in patients with influenza-associated pneumonia. Arch Gerontol Geriatr 2009; 49:322 - 5; http://dx.doi.org/10.1016/j.archger.2008.11.015; PMID: 19150140
- Neuzil KM, O'Connor TZ, Gorse GJ, Nichol KL. Recognizing influenza in older patients with chronic obstructive pulmonary disease who have received influenza vaccine. Clin Infect Dis 2003; 36:169 - 74; http://dx.doi.org/10.1086/345668; PMID: 12522748
- Babcock HM, Merz LR, Fraser VJ. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol 2006; 27:266 - 70; http://dx.doi.org/10.1086/501539; PMID: 16532414
- Walsh EE, Cox C, Falsey AR. Clinical features of influenza A virus infection in older hospitalized persons. J Am Geriatr Soc 2002; 50:1498 - 503; http://dx.doi.org/10.1046/j.1532-5415.2002.50404.x; PMID: 12383146
- Flamaing J, Engelmann I, Joosten E, Van Ranst M, Verhaegen J, Peetermans WE. Viral lower respiratory tract infection in the elderly: a prospective in-hospital study. Eur J Clin Microbiol Infect Dis 2003; 22:720 - 5; http://dx.doi.org/10.1007/s10096-003-1042-z; PMID: 14605944
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749 - 59; http://dx.doi.org/10.1056/NEJMoa043951; PMID: 15858184
- Talbot HK, Keitel W, Cate TR, Treanor J, Campbell J, Brady RC, et al. Immunogenicity, safety and consistency of new trivalent inactivated influenza vaccine. Vaccine 2008; 26:4057 - 61; http://dx.doi.org/10.1016/j.vaccine.2008.05.024; PMID: 18602726
- Praditsuwan R, Assantachai P, Wasi C, Puthavatana P, Kositanont U. The efficacy and effectiveness of influenza vaccination among Thai elderly persons living in the community. J Med Assoc Thai 2005; 88:256 - 64; PMID: 15962680
- Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA 1994; 272:1661 - 5; http://dx.doi.org/10.1001/jama.272.21.1661; PMID: 7966893
- Allsup S, Haycox A, Regan M, Gosney M. Is influenza vaccination cost effective for healthy people between ages 65 and 74 years? A randomised controlled trial. Vaccine 2004; 23:639 - 45; PMID: 15542184
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews. 2010.
- Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; 3:CD004876; PMID: 16856068
- Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA 2000; 284:1655 - 63; http://dx.doi.org/10.1001/jama.284.13.1655; PMID: 11015795
- Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol 2007; 36:623 - 31; http://dx.doi.org/10.1093/ije/dym021; PMID: 17403908
- Talbot HK, Williams JV, Zhu Y, Poehling KA, Griffin MR, Edwards KM. Failure of routine diagnostic methods to detect influenza in hospitalized older adults. Infect Control Hosp Epidemiol 2010; 31:683 - 8; http://dx.doi.org/10.1086/653202; PMID: 20470035
- Talbot HK, Griffin MR, Chen Q, Zhu Y, Williams JV, Edwards KM. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J Infect Dis 2011; 203:500 - 8; http://dx.doi.org/10.1093/infdis/jiq076; PMID: 21220776
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489 - 95; http://dx.doi.org/10.1503/cmaj.050051; PMID: 16129869
- Strandberg TE, Pitkala KH. Frailty in elderly people. Lancet 2007; 369:1328 - 9; http://dx.doi.org/10.1016/S0140-6736(07)60613-8; PMID: 17448804
- Yao X, Hamilton RG, Weng NP, Xue QL, Bream JH, Li H, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 2011; 29:5015 - 21; http://dx.doi.org/10.1016/j.vaccine.2011.04.077; PMID: 21565245
- Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine 2009; 27:1628 - 36; http://dx.doi.org/10.1016/j.vaccine.2008.11.098; PMID: 19100304
- Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006; 35:337 - 44; http://dx.doi.org/10.1093/ije/dyi274; PMID: 16368725
- Jackson LA, Nelson JC, Benson P, Neuzil KM, Reid RJ, Psaty BM, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol 2006; 35:345 - 52; http://dx.doi.org/10.1093/ije/dyi275; PMID: 16368724
- Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine 2010; 28:7267 - 72; http://dx.doi.org/10.1016/j.vaccine.2010.08.088; PMID: 20832494
- Greene SK, Shi P, Dutta-Linn MM, Shoup JA, Hinrichsen VL, Ray P, et al. Accuracy of data on influenza vaccination status at four Vaccine Safety Datalink sites. Am J Prev Med 2009; 37:552 - 5; http://dx.doi.org/10.1016/j.amepre.2009.08.022; PMID: 19944924
- Place of influenza vaccination among adults—United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:781 - 5; PMID: 21681175
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of Immune Responses to Vaccines in Elderly Persons. Clin Infect Dis 2008; 46:1078 - 84; http://dx.doi.org/10.1086/529197; PMID: 18444828
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159 - 69; http://dx.doi.org/10.1016/j.vaccine.2005.08.105; PMID: 16213065
- Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest 2011; 121:3109 - 19; http://dx.doi.org/10.1172/JCI57834; PMID: 21785218
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172 - 80; http://dx.doi.org/10.1086/599790; PMID: 19508159
- Rappuoli R, Del Giudice G, Nabel GJ, Osterhaus AD, Robinson R, Salisbury D, et al. Public health. Rethinking influenza. Science 2009; 326:50; http://dx.doi.org/10.1126/science.1179475; PMID: 19797644
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959 - 65; http://dx.doi.org/10.1016/j.vaccine.2009.08.101; PMID: 19751689
- Cheong HJ, Song JY, Heo JY, Noh JY, Choi WS, Park DW, et al. Immunogenicity and Safety of the Influenza A/H1N1 2009 Inactivated Split-Virus Vaccine in Young and Older Adults: MF59-Adjuvanted Vaccine versus Nonadjuvanted Vaccine. Clin Vaccine Immunol 2011; 18:1358 - 64; http://dx.doi.org/10.1128/CVI.05111-11; PMID: 21715575
- Carmona A, Omeñaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, et al. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6-35 months. Vaccine 2010; 28:5837 - 44; http://dx.doi.org/10.1016/j.vaccine.2010.06.065; PMID: 20600478
- Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis 2010; 201:1644 - 53; http://dx.doi.org/10.1086/652701; PMID: 20423222
- Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Drame´ M, et al. Priming with AS03A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: An open non-randomised extension of a double-blind randomised primary study. Vaccine 2010; 28:849 - 57; http://dx.doi.org/10.1016/j.vaccine.2009.10.017; PMID: 19835828
- Rubinstein E, Predy G, Sauve´ L, Hammond GW, Aoki F, Sikora C, et al. The responses of Aboriginal Canadians to adjuvanted pandemic (H1N1) 2009 influenza vaccine. CMAJ 2011; 183:E1033 - 7; http://dx.doi.org/10.1503/cmaj.110196; PMID: 21788422
- Heijmans S, De Meulemeester M, Reynders P, Giet D, Demanet E, Devresse PY, et al. Immunogenicity profile of a 3.75-mug hemagglutinin pandemic rH5N1 split virion AS03A-adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis 2011; 203:1054 - 62; http://dx.doi.org/10.1093/infdis/jiq174; PMID: 21450995