Abstract
Influenza virus infection contributes to substantial morbidity and mortality globally. Included in the list of groups at higher risk of either influenza infection or severe complications following influenza infection are pregnant women and their newborns. Influenza vaccination offers a safe and effective means to prevent or lessen the severity of influenza infections. Recent research has helped elucidate the impact of influenza infection and vaccination on pregnant women and their newborn children and young infants. This review summarizes recent findings in this area and identifies additional gaps in the evidence base that need to be addressed to appropriately inform vaccination policies worldwide, to protect pregnant women and their children from influenza and related complications.
Influenza has been responsible for four pandemics in the last hundred years with an additional global burden of seasonal influenza estimated to be between three and five million cases of severe illness and 250,000 to 500,000 deaths annually.Citation1
Burden of Influenza in Pregnancy
Pregnant women are among high risk groups for influenza. The incidence of influenza infection among pregnant women is similar compared with the incidence in non-pregnant women of similar age.Citation2,Citation3 However, pregnant women are at a higher risk of influenza complications.Citation3 Neuzil et al. evaluated the association between influenza and risk of hospitalizations or death from acute cardiopulmonary complications (e.g., pneumonia, influenza, heart failure, myocarditis) in pregnant women.Citation3 The risk of cardiopulmonary events was higher in pregnant women compared with both postpartum women and non-pregnant women of similar age. The odds ratios of complications increased with increases in gestational age. In an analysis of more than 6 million hospitalizations among pregnant women in the US, pregnant women who were hospitalized during the influenza season with a respiratory illness had longer hospital stay and higher likelihood of a complicated delivery compared with hospitalized pregnant women without respiratory illness.Citation4 The risk of adverse outcomes associated with influenza in pregnancy is higher for women with underlying co-morbidities such as diabetes, heart disease, immunosuppression, asthma, and chronic obstructive pulmonary disease.Citation3,Citation5-Citation7
Pregnant women are at high risk of influenza-related complications during pandemics. In a report of outcomes in 1,350 pregnant women during the 1918 pandemic, almost half were identified as having pneumonia and, of these women, more than half died, resulting in an overall case fatality rate of 27%.Citation8 A recent examination of birth records from the 1918 pandemic identified live birth rate decreases of approximately 5 to 15% below baseline expectations during the pandemic period, with the greatest depression in birth rate occurring 6 to 7 mo after the pandemic peak, indicating the possibility of increased first term miscarriages due to influenza infection.Citation9 The highest influenza-associated mortality in pregnant women was seen in the third trimester. During the 1957 pandemic, approximately 50% of all women of childbearing age who died were pregnant.Citation10,Citation11 Similarly, during the 2009 influenza A(H1N1) pandemic, pregnant women had a higher rate of hospital and intensive care unit admission than the general population and a high risk of complications—including death.Citation12-Citation14
Biology of Influenza in Pregnancy
The reasons for the higher risk of adverse consequences of influenza infection in pregnant women are not completely understood. However, several biological changes that occur in pregnancy may play a role. Pregnancy is associated with biochemical, mechanical, hemodynamic, and immunologic changes in the mother that become most pronounced by the third trimester. These changes include increased minute ventilation, reduced tidal volumes, and decreased functional residual capacity which leaves less reserve for stress on pulmonary function.Citation15 In pregnancy, humoral immunity remains generally intact with augmentation of the T-helper-type 2 (Th2) response.Citation16,Citation17 On the other hand, there is selective suppression of Th1 cell-mediated immunity. Suppression of cell-mediated immunity is likely meant to protect the developing fetus from maternal cytotoxic T-lymphocyte activity but, as a consequence, impairs maternal response to infection–including viral infections.Citation18-Citation20 This compromise of cell-mediated immunity continues throughout pregnancy.Citation21
Influenza Morbidity in Young Infants
Young infants (i.e., <6 mo) have a high risk of influenza infection and associated adverse outcomes.Citation22-Citation27 Although influenza viruses cause disease in all age groups, children and individuals 65 y or older have the highest incidence of seasonal influenza.Citation28-Citation31 Moreover, during most pandemics, children and the elderly have had high risk of severe influenza illness.Citation32,Citation33 Among children, the risk is highest in infants—particularly those younger than six months of age.Citation34,Citation35 In a population-based surveillance study conducted in 9 states (in the US), the incidence of laboratory confirmed influenza in infants <6 mo of age was more than double that observed in 6 to 23-mo-old children (311 vs. 118 per 100,000 population), with even lower rates in the older age groups.Citation35 In another study in Houston, the annual influenza incidence in infants younger than 6 mo of age was estimated to be 12%.Citation34 In a prospective, population-based active surveillance study conducted in children less than five years of age residing in three US counties, Poehling et al. documented an annual influenza associated hospitalization rate of 0.9 per 1,000 children.Citation27 The annual hospitalization rate was the highest in infants younger than six months of age with 4.5 hospitalizations per 1,000 children.Citation27 During the influenza A(H1N1)2009 pandemic, infants were considered a high risk group for acquiring and developing complications following infection with influenza A(H1N1)2009, with continued elevation of pediatric deaths after the initial 2009 pandemic waves.Citation28 Surveillance of pediatric influenza deaths continues to show a burden of influenza A(H1N1)2009 as well as influenza A(H3N2) as a cause of mortality in children.Citation36,Citation37
Respiratory Infections in Pregnancy and Fetal Growth and Development
Maternal respiratory infections have an impact on fetal outcomes,Citation11,Citation38 including preterm birth.Citation39 Respiratory infections, particularly pneumonia, have been associated with low birth weight and increased risk of preterm birth.Citation11,Citation38 In an examination of pregnancy hospitalizations over four influenza seasons, respiratory illness was associated with a 4-fold increase in odds of preterm delivery, 2.5-fold increase in odds of fetal distress, and nearly 4-fold increase in odds of Cesarean delivery.Citation4 In a 13 y population-based cohort study in Canada, pregnant women hospitalized with respiratory symptoms during the influenza season gave birth to newborns of lower mean birth weight and a higher likelihood of being small for gestational age (SGA).Citation40
Adverse fetal outcomes have been identified as an important consequence of influenza pandemics. In the 1918 influenza pandemic, high rates of premature births were reported among pregnant women admitted to Cook County Hospital in Chicago and among pregnant women treated by physicians—most of them in Maryland.Citation8,Citation41 In an analysis of data from the 1957–58 pandemic, infants born to women who had serological evidence of influenza during pregnancy were 50% more likely to be premature compared with infants of uninfected women.Citation38 In a national cohort study conducted in the UK, perinatal mortality and prematurity were higher in newborns of women who were hospitalized with confirmed influenza A(H1N1)2009 infection compared with the control group born during the pre-pandemic comparison period.Citation42
Previously, in a Chinese case-control study, neural-tube defects were associated with history of influenza-like illness, cold, and/or fever in pregnancy.Citation43 A large observational study from Hungary documented an association between history of influenza during the first trimester of pregnancy and cleft lip, neural-tube defects, and cardiovascular malformations.Citation44 However, a separate analysis of the same data did not find an association between maternal influenza-like illness and prematurity and low birth weight.Citation45 A few other observational studies have also not found an association between influenza infection and birth outcomes.Citation6,Citation46 The variability in observed effect of influenza on birth outcomes could be due to issues related to data sets based on medical records and administrative data, small and/or difficult to measure effect size, or a true lack of association. Evaluating the association between influenza in pregnancy and birth outcomes in the context of protective effect of maternal influenza immunization in a randomized controlled trial will help resolve the uncertainty around this question.
Since influenza virus rarely crosses the placenta,Citation2,Citation47,Citation48 the adverse effects of influenza infections on birth outcomes are likely to be associated with inflammation.Citation39,Citation49 Influenza virus infection induces gene expression of pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, interferon (IFN)-β, IFN-α and granulocyte macrophage colony-stimulating factor (GM-CSF).Citation50 Increased levels of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α and reduced levels of anti-inflammatory cytokines (e.g., IL-10) have been associated with `higher likelihood of preterm labor.Citation49,Citation51 The pro-inflammatory cytokines stimulate the amnion and the decidual tissue to produce prostaglandins which can induce labor and some cytokines can directly stimulate myometrial contractions.Citation50-Citation53
Immunogenicity, Efficacy and Effectiveness of Influenza Vaccine in Pregnant Women
Vaccination is the most effective tool for preventing morbidity and mortality due to influenza. The data on clinical efficacy of influenza vaccination—particularly in pregnant women (and their infants)—are limited. Immunogenicity studies of influenza vaccination in pregnancy indicate that the seroresponse in pregnant women is similar to of similar aged, non-pregnant women.Citation54-Citation61 Englund et al. evaluated the immunogenicity of the trivalent inactivated influenza vaccine in a small randomized controlled trial.Citation55 In this trial, 13 pregnant women received influenza vaccine and 13 women received the tetanus toxoid. All 13 women who received the influenza vaccine had 4-fold or higher increase in serum neutralizing antibody titer to at least one of the three influenza antigens included in the vaccine.Citation55 Four women had a 4-fold or higher response to all three antigens, 5 to two antigens, and 4 to only one antigen. There were no women in the tetanus toxoid group with 4-fold or higher increases in titer to any of the influenza antigens.Citation55 During the 1962 Asian influenza outbreak, pooled serologic analysis of 363 pregnant women and 138 non-pregnant women found little difference in immune response by pregnancy status.Citation56 Additionally, evaluations of the 1976 swine flu (influenza A/NJ/8/76) vaccine conducted through two small studies (n = 26 pregnant women and n = 56 pregnant women) found immune responses in pregnant women to be slightly lower, but not statistically significantly different from non-pregnant women and that maternal response and passive antibody transfer occurred in more than 50% of mother-infant pairs.Citation58,Citation60 More recently, clinical trial data from Bangladesh documented strong antibody responses in pregnant women exposed to trivalent seasonal influenza vaccine, with efficient antibody transfer to their babies, and similar proportion with seroprotective titers in mothers and infants at delivery.Citation59 During the 2009 influenza A(H1N1) pandemic, pregnant women who received monovalent influenza A(H1N1)2009 vaccine at doses of 25 μg and 49 μg mounted strong antibody responses, with seroprotective antibody levels in 89% and 97% of women, respectively.Citation57 However, these data have primarily looked at HAI responses and not cell-mediated immune responses, which may be important in responding to viral infection.
A limited number of observational studies have evaluated influenza vaccine’s clinical effectiveness among pregnant women.Citation56,Citation62 Munoz et al. conducted an analysis of an electronic database from a clinic in Houston.Citation62 In this observational study, 225 healthy pregnant vaccinated women were compared with 826 healthy pregnant unvaccinated women matched by type of insurance, age, and month of delivery.Citation62 Approximately 23% of vaccinated and 19% of unvaccinated women developed medically attended acute respiratory infection (p = 0.18). In an analysis of electronic data from Kaiser Permanente Northern California, Black et al., documented similar rates of outpatient visits due to febrile influenza-like illness among vaccinated vs. unvaccinated pregnant women (45.4 vs. 44.7 per 100,000; p = 0.09).Citation63
In the only randomized controlled trial of maternal immunization that measured clinical (in mother and infant) and virological (in infant) outcomes, the vaccine prevented approximately a third of influenza-like illness episodes in pregnant women.Citation61 However, this trial was conducted during a single influenza season in Bangladesh and needs to be replicated in other settings. Moreover, the Bangladesh trial did not assess laboratory confirmed influenza episodes in mothers.
Fetal Outcomes
There is recent evidence of protective effect of influenza vaccine in pregnancy on fetal outcomes. In a retrospective cohort study in Georgia (US), Omer et al. reported a reduced likelihood of prematurity among infant born during the influenza season to women who received TIV in pregnancy (adjusted odds ratio = 0.60; 95% CI, 0.38–0.94).Citation64 The impact of maternal influenza vaccine on reducing prematurity increased with the increase in influenza circulation in the study area, with the highest impact during the period of widespread activity (OR = 0.28; 95% CI, 0.11–0.74).Citation64 The protective effect of maternal influenza vaccination on SGA was limited to the period of widespread circulation in which there was approximately 70% reduction in the odds of being SGA among newborns of vaccinated women compared with the newborns of unvaccinated women (adjusted OR = 0.31; 95% CI, 0.13–0.75).Citation64 A long-term cohort study in Nova Scotia, Canada, over 13 y, reported increased levels of adverse fetal outcomes (i.e., SGA, low birth weight) among women who were hospitalized for respiratory infections while pregnant. Findings included a 66% increase in small for gestational age and mean birth weight 100 g less among children whose mothers were hospitalized for respiratory illness.Citation40 A recent examination of pregnant women infected with influenza A(H1N1)2009 in the UK identified increases in perinatal mortality, stillbirth, and preterm birth.Citation42 While Pierce et al. did not find significant decreases in birth weight, after adjusting for gestational age, additional analyses found a non-significant increase in small for gestational age.Citation42
Infant Outcomes
Infants, particularly those younger than 6 mo, are at a high risk of acquiring influenza and developing influenza related complications.Citation22-Citation27 However, there is no influenza vaccine currently approved for use in infants younger than 6 mo. A recent evaluation of an adjuvanted influenza vaccine in children aged 6 to less than 72 mo of age documented both increased immunogenicity and efficacy relative to standard trivalent inactivated influenza vaccine.Citation65 While this vaccine has not been evaluated in infants younger than 6 mo, the increased immune response shown in young children may indicate the possibility of provoking a sufficient immune response in children younger than 6 mo.
Maternal influenza immunization could potentially play a role in protecting newborns and young infants. There are limited data on immunological and clinical outcomes related to infant protection against influenza through immunization during pregnancy.
In the randomized, controlled immunogenicity trial by Englund et al., infants born to women in the influenza vaccine arm had significantly higher levels of vaccine specific IgG antibody both at birth and at two months of age than control women who received tetanus toxoid.Citation55 Sumaya et al. conducted a non-randomized observational study that included data from 40 mother-infant pairs.Citation60 In this study, evidence of protective levels of antibody titers was present in only 42% newborns.Citation60
In a retrospective matched cohort study using data from four managed care organizations, France et al. evaluated the association between maternal influenza immunization and acute respiratory illness in infants.Citation46 In this non-randomized study, there was no reduction in respiratory illness in infants (incidence rate ratio during peak influenza season, 0.96; 95% confidence interval, 0.86–1.07).Citation46 In the retrospective matched cohort study by Munoz et al., the rate of hospitalization for respiratory illness among infants of women who received influenza vaccine in pregnancy was similar to the hospitalization rate among infants of unvaccinated women.Citation62 In a retrospective cohort study by Black et al., infants of vaccinated and unvaccinated women had similar risk of influenza or pneumonia admissions and outpatient visits for influenza-like illness.Citation63 On the other hand, in a recent case-control study, maternal influenza immunization was 85% effective in preventing hospitalization due to laboratory confirmed influenza in infants younger than six months. Maternal influenza vaccine remained 79% effective in preventing influenza-related immunization through 12 mo of age.Citation66 Another case-control study of infants less than 6 mo of age, based on an existing active surveillance system in three US counties found that, compared with infants of unvaccinated mothers, those whose mothers received influenza vaccine were 45% to 48% less likely to have been hospitalized for influenza.Citation67 The randomized clinical trial in Bangladesh demonstrated that vaccination of pregnant women with the inactivated influenza vaccine was 63% effective in reducing laboratory-confirmed influenza in their infants, while also reducing all respiratory illness with fever by 29%.Citation61 While Eick et al. documented little difference in infant ILI incidence between mothers who received influenza vaccine and those who did not, they did identify a 41% reduction in laboratory-confirmed influenza infection and a 39% reduction in hospitalization for ILI among infants whose mothers were vaccinated.Citation68 The discrepancies between the observational studies argue for more randomized trials of maternal influenza immunization to assess the respiratory burden prevented in their infants.
An area of investigation that has to date received little attention is the potential benefit of maternal influenza immunization on the acquisition of bacterial pathogens during early infancy. Much of the respiratory burden of young infants is thought to be due to the synergistic lethality of influenza and other respiratory viruses with the bacteria acquired during infancy such as the pneumococcus.Citation69 Influenza may also play a role in the acquisition and transmission of the pneumococcus.Citation70 It is possible that among the potential benefits of maternal influenza vaccination may be a reduction in pneumococcal acquisition during exposure to influenza.
Maternal Influenza Immunization Recommendations
Pregnant women comprised approximately 50% of deaths in women of reproductive age during the 1956–58 influenza pandemic.Citation10 Following the pandemic, more than 100,000 women in the US were immunized against influenza.Citation71 However, in the early 1960s there was lack of data on serious complications of seasonal influenza in pregnant women. As a result, after 1965, the Advisory Committee on Immunization Practices (ACIP) discontinued the recommendation for immunizing pregnant women with the exception of women with certain co-morbidities.Citation72,Citation73 In the 1990s, the recommendations were expanded to include all women in their second and third trimester of pregnancy.Citation74 Since 2004, influenza immunization is recommended in all trimesters for women who are pregnant during the influenza season.Citation75,Citation76 More recently, pregnant women were included as a high priority group for receiving the pandemic influenza A(H1N1)2009 vaccine.Citation28 Starting with the 2010–2011 influenza season, influenza vaccination in the US is recommended for everyone 6 mo of age and older, and pregnant women remain in the high-priority list in the event of vaccine shortages.Citation77
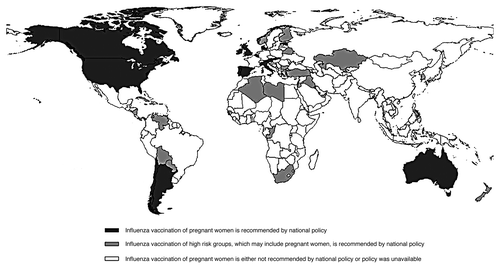
In 2005, the World Health Organization issued a position paper that included the recommendation that all pregnant women should be immunized during the influenza season.Citation78 Starting with the 2007–2008 season, Canada’s national advisory committee expanded its recommendations to include all pregnant women in all trimesters of pregnancy.Citation79 The Australian national guidelines recommend influenza vaccine for all women who will be in their second or third trimester of pregnancy during the influenza season -even if they are in the first trimester when they receive the vaccine.Citation80 In the UK, pregnant women with certain co-morbidities and high risk conditions are encouraged to vaccinate against influenza in all trimesters of pregnancy; however, the recommendations do not include routine influenza immunization for healthy pregnant women.Citation81 In addition to the US, Canada, Australia and the UK, we identified 24 other countries (Argentina, Austria, Bahamas, Belgium, Bermuda, Cayman Islands, Chile, Cyprus, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Malta, Netherlands, Norway, Portugal, Slovakia, Spain, Sweden Switzerland) that have recommendations directly calling for influenza vaccination of pregnant women, as reported for either seasonal influenza or the influenza A(H1N1)2009 pandemic. Additionally, 27 countries (Algeria, Bahrain, Barbados, Belarus, Bolivia, Bulgaria, Congo, Cuba, Iraq, Israel, Jamaica, Jordan, Kazakhstan, Kuwait, Libya, Marshall Islands, New Zealand, Nicaragua, Palau, Panama, Paraguay, South Africa, South Korea, St. Lucia, Turkey, United Arab Emirates, and Venezuela) include “high-risk” individuals in their influenza immunization recommendations, but do not specifically reference pregnant women as a high risk group. Despite the WHO recommendations, most countries do not have national recommendations for influenza vaccines in pregnancy (www.who.int/immunization_monitoring/en/globalsummary/countryprofileresult.cfm, apps.who.int//immunization_monitoring/en/globalsummary/scheduleselect.cfm) ().Citation82,Citation83 However, this list may be incomplete, based on the lack of availability of a systematic examination of policies on influenza vaccination of pregnant women in all countries. The authors request that representatives of any countries not properly identified here provide documentation of their country’s policies on influenza vaccination of pregnant women.
Figure 1. Countries with recommendations for provision of influenza vaccine to pregnant women.Citation82,Citation83 Additional data sources: (www.who.int/immunization_monitoring/en/globalsummary/countryprofileresult.cfm, apps.who.int/immunization_monitoring/en/globalsummary/scheduleselect.cfm.
Vaccine Coverage in Pregnancy
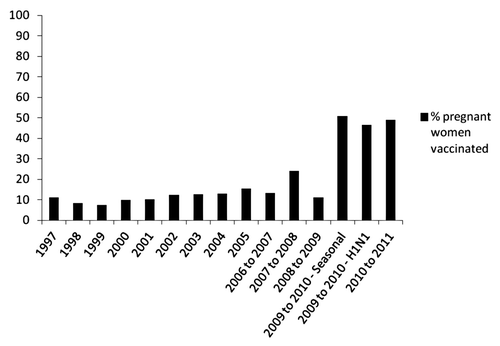
Despite ACIP’s strong recommendations for influenza immunization in pregnancy, the coverage of the seasonal vaccine in the US has been consistently low, though increases in coverage in recent years offer some hope that additional increases are possible. In 1997, the coverage of influenza vaccine in pregnant women was approximately 11% which only rose to 19% by 2008 (). Limited data from other countries where influenza vaccine is recommended in pregnancy (to at least some women e.g., with co-morbidities) suggests similar levels of vaccine uptake.Citation82,Citation84,Citation85 Following identification of the influenza A(H1N1)2009 virus and the related impact on pregnant women, there was increased outreach to pregnant women, encouraging influenza vaccination. This resulted in a substantial increase in maternal influenza immunization coverage during the 2009–2010 influenza season that was maintained through the 2010–2011 influenza season ().Citation77,Citation86,Citation87
Figure 2. Self reported influenza vaccine coverage in pregnant women, US 1997–2011. Note: earlier estimates (1997–2005) were based on calendar year surveys of influenza vaccine receipt in the past year. Starting with the 2006–2007 influenza season, more comprehensive seasonal estimates were available. Data sources: www.cdc.gov/flu/professionals/pdf/NHIS89_08fluvaxtrendtab.pdf; www.cdc.gov/mmwr/preview/mmwrhtml/mm5947a1.htm?s_cid-mm5947a1_w, Fiore MMWR Recomm Rep 2010, www.cdc.gov/mmwr/preview/mmwrhtml/mm6032a2.htm?s_cid=mm6032a2_w
Low influenza vaccine coverage in pregnancy is due to multiple individual and health system-related factors and a multifaceted approach will be required to significantly increase the coverage.Citation88 However, women’s and clinicians’ beliefs regarding maternal influenza vaccine’s impact on infants are among the factors that play a role in vaccine-related decisions.Citation89 In a survey conducted in Los Angeles, Silverman and Greif found that obstetricians who considered the maternal influenza vaccine to protective for infants were more likely to offer influenza vaccine to their patients.Citation89 Development of robust evidence base regarding infant and fetal protection due to maternal influenza vaccination will inform clinical decision-making and help increase vaccine acceptance.
Future of Maternal Influenza Immunization
From a programmatic and operational standpoint, the increase in maternal influenza immunization coverage in response to the H1N1 pandemic in the US was encouraging. While only approximately 50% of pregnant women were vaccinated with seasonal influenza vaccine and H1N1 influenza vaccine during the 2009–2010 influenza season, this was more than double the coverage seen in the US in prior years. It was reassuring to not see immunization coverage levels in pregnant women drop in the year following the pandemic, however they did not increase. In the US, to continue to improve influenza vaccination of pregnant women and ultimately reach the Healthy People 2020 goal of 80% influenza vaccination of pregnant women will take a concerted effort firmly grounded in science.
Recent clinical trials and observational studies have identified that influenza immunization of pregnant women is safe and effective in preventing influenza infections in both mother and infant. These have been bolstered by additional recent studies indicating other benefits, such as reductions in prematurity and low birth weight. It is important to note that thus far, the majority of the benefits of vaccinating pregnant women have been identified as benefits to the health of the mother. The recent focus on examining the impact among children too young to be vaccinated have produced promising results, but these studies currently consist of a minority of the literature in this area. Additional studies are needed to continue to develop this evidence base, including examinations of the benefit/cost ratio of maternal influenza vaccination, to ensure that the economics of this strategy are properly understood. While there are clinical trials of influenza vaccination of pregnant women currently scheduled or underway in South Africa (clinicaltrials.gov/ct2/show/NCT01306669), Nepal (clinicaltrials.gov/ct2/show/NCT01034254), and Mali (clinicaltrials.gov/ct2/show/NCT01430689), that will add to the knowledge on infant outcomes of maternal influenza vaccination, other studies, in other parts of the world are needed to ensure a robust evidence base, as influenza viruses are variable.
References
- World Health Organization. Influenza. 2003.
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44 - 52; http://dx.doi.org/10.1016/S1473-3099(07)70311-0; PMID: 18156088
- Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998; 148:1094 - 102; PMID: 9850132
- Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Hospitalizations with respiratory illness among pregnant women during influenza season. Obstet Gynecol 2006; 107:1315 - 22; http://dx.doi.org/10.1097/01.AOG.0000218702.92005.bb; PMID: 16738158
- Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ 2007; 176:463 - 8; http://dx.doi.org/10.1503/cmaj.061435; PMID: 17296958
- Hartert TV, Neuzil KM, Shintani AK, Mitchel EF Jr., Snowden MS, Wood LB, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol 2003; 189:1705 - 12; http://dx.doi.org/10.1016/S0002-9378(03)00857-3; PMID: 14710102
- Schanzer DL, Langley JM, Tam TW. Influenza-attributed hospitalization rates among pregnant women in Canada 1994-2000. J Obstet Gynaecol Can 2007; 29:622 - 9; PMID: 17714614
- Harris JW. Influenza occurring in pregnant women. JAMA 1919; 72:972 - 80; http://dx.doi.org/10.1001/jama.1919.02610140008002
- Bloom-Feshbach K, Simonsen L, Viboud C, Molbak K, Miller MA, Gottfredsson M, et al. Natality decline and miscarriages associated with the 1918 influenza pandemic: the scandinavian and United States experiences. J Infect Dis 2011; 204:1157 - 64; http://dx.doi.org/10.1093/infdis/jir510; PMID: 21917887
- Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol 1959; 78:1172 - 5; PMID: 13824729
- Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med 2005; 33:S390 - 7; http://dx.doi.org/10.1097/01.CCM.0000182483.24836.66; PMID: 16215363
- Maternal and Infant Outcomes Among Severely Ill Pregnant and Postpartum Women with 2009 Pandemic Influenza A (H1N1)—United States, April 2009–August 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1193 - 6; PMID: 21900872
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451 - 8; http://dx.doi.org/10.1016/S0140-6736(09)61304-0; PMID: 19643469
- Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303:1517 - 25; http://dx.doi.org/10.1001/jama.2010.479; PMID: 20407061
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis 2006; 12:1638 - 43; http://dx.doi.org/10.3201/eid1211.060152; PMID: 17283611
- Gaunt G, Ramin K. Immunological tolerance of the human fetus. Am J Perinatol 2001; 18:299 - 312; http://dx.doi.org/10.1055/s-2001-17861; PMID: 11607848
- Saleeby E, Chapman J, Morse J, Bryant A. H1N1 influenza in pregnancy: cause for concern. Obstet Gynecol 2009; 114:885 - 91; http://dx.doi.org/10.1097/AOG.0b013e3181bb44bb; PMID: 19888049
- Gehrz RC, Christianson WR, Linner KM, Conroy MM, McCue SA, Balfour HH Jr. A longitudinal analysis of lymphocyte proliferative responses to mitogens and antigens during human pregnancy. Am J Obstet Gynecol 1981; 140:665 - 70; PMID: 6973274
- Lederman MM. Cell-mediated immunity and pregnancy. Chest 1984; 86:6S - 9S; PMID: 6088180
- Sridama V, Pacini F, Yang SL, Moawad A, Reilly M, DeGroot LJ. Decreased levels of helper T cells: a possible cause of immunodeficiency in pregnancy. N Engl J Med 1982; 307:352 - 6; http://dx.doi.org/10.1056/NEJM198208053070606; PMID: 6979710
- Davies M, Browne CM. Pregnancy-associated nonspecific immunosuppression: kinetics of the generation and identification of the active factors. Am J Reprod Immunol Microbiol 1985; 9:77 - 83; PMID: 4073339
- Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J 2004; 23:S188 - 92; http://dx.doi.org/10.1097/01.inf.0000144660.53024.64; PMID: 15577572
- Healy CM, Baker CJ. Prospects for prevention of childhood infections by maternal immunization. Curr Opin Infect Dis 2006; 19:271 - 6; http://dx.doi.org/10.1097/01.qco.0000224822.65599.5b; PMID: 16645489
- Heikkinen T. Influenza in children. Acta Paediatr 2006; 95:778 - 84; http://dx.doi.org/10.1080/08035250600612272; PMID: 16801171
- Iskander M, Booy R, Lambert S. The burden of influenza in children. Curr Opin Infect Dis 2007; 20:259 - 63; http://dx.doi.org/10.1097/QCO.0b013e3280ad4687; PMID: 17471035
- Iwane MK, Edwards KM, Szilagyi PG, Walker FJ, Griffin MR, Weinberg GA, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 2004; 113:1758 - 64; http://dx.doi.org/10.1542/peds.113.6.1758; PMID: 15173503
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31 - 40; http://dx.doi.org/10.1056/NEJMoa054869; PMID: 16822994
- Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1 - 8; PMID: 19713882
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232 - 9; http://dx.doi.org/10.1056/NEJM200001273420402; PMID: 10648764
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333 - 40; http://dx.doi.org/10.1001/jama.292.11.1333; PMID: 15367555
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 86; http://dx.doi.org/10.1001/jama.289.2.179; PMID: 12517228
- Update: influenza activity - United States, August 30-October 31, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:1236 - 41; PMID: 19910911
- Steinhoff MC. Epidemiology and prevention of influenza. In: Nelson KE, Williams CM, eds. Infectious disease epidemiology; theory and practice. Sudbury, Mass: Jones and Bartlett Publishers, 2007:577-600.
- Glezen WP, Taber LH, Frank AL, Gruber WC, Piedra PA. Influenza virus infections in infants. Pediatr Infect Dis J 1997; 16:1065 - 8; http://dx.doi.org/10.1097/00006454-199711000-00012; PMID: 9384341
- Schrag SJ, Shay DK, Gershman K, Thomas A, Craig AS, Schaffner W, et al. Multistate surveillance for laboratory-confirmed, influenza-associated hospitalizations in children: 2003-2004. Pediatr Infect Dis J 2006; 25:395 - 400; http://dx.doi.org/10.1097/01.inf.0000214988.81379.71; PMID: 16645501
- 2009 pandemic influenza A (H1N1) virus infections - Chicago, Illinois, April-July 2009. MMWR Morb Mortal Wkly Rep 2009; 58:913 - 8; PMID: 19713879
- 2010-2011 Influenza Season Week 34 ending August 27, 2011. 2011.
- Hardy JM, Azarowicz EN, Mannini A, Medearis DN Jr., Cooke RE. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957-1958. Am J Public Health Nations Health 1961; 51:1182 - 8; http://dx.doi.org/10.2105/AJPH.51.8.1182; PMID: 13711529
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371:75 - 84; http://dx.doi.org/10.1016/S0140-6736(08)60074-4; PMID: 18177778
- McNeil SA, Dodds LA, Fell DB, Allen VM, Halperin BA, Steinhoff MC, et al. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol 2011; 204:S54 - 7; http://dx.doi.org/10.1016/j.ajog.2011.04.031; PMID: 21640231
- Nuzum JW. Pandemic influenza and pneumonia in a large civilian hospital. JAMA 1918; 71:1562 - 5; http://dx.doi.org/10.1001/jama.1918.26020450009011a
- Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ 2011; 342:d3214; http://dx.doi.org/10.1136/bmj.d3214; PMID: 21672992
- Li Z, Ren A, Liu J, Pei L, Zhang L, Guo Z. Maternal flu or fever, medication use, and neural tube defects: a population-based case-control study in Northern China. Birth Defects Res A Clin Mol Teratol 2007; 79:295 - 300; http://dx.doi.org/10.1002/bdra.20342; PMID: 17216625
- Ács N, Banhidy F, Puho E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989 - 96; http://dx.doi.org/10.1002/bdra.20195; PMID: 16323157
- Ács N, Banhidy F, Puho E, Czeizel AE. Pregnancy complications and delivery outcomes of pregnant women with influenza. J Matern Fetal Neonatal Med 2006; 19:135 - 40; http://dx.doi.org/10.1080/14767050500381180; PMID: 16690505
- France EK, Smith-Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med 2006; 160:1277 - 83; http://dx.doi.org/10.1001/archpedi.160.12.1277; PMID: 17146026
- Irving WL, James DK, Stephenson T, Laing P, Jameson C, Oxford JS, et al. Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG 2000; 107:1282 - 9; http://dx.doi.org/10.1111/j.1471-0528.2000.tb11621.x; PMID: 11028582
- Rasmussen SA, Jamieson DJ, Macfarlane K, Cragan JD, Williams J, Henderson Z. Pandemic Influenza and Pregnant Women: Summary of a Meeting of Experts. Am J Public Health 2009; 99:Suppl 2 S248 - 54; http://dx.doi.org/10.2105/AJPH.2008.152900; PMID: 19461110
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007; 25:021 - 39; http://dx.doi.org/10.1055/s-2006-956773; PMID: 17205421
- Uchide N, Ohyama K, Bessho T, Toyoda H. Induction of pro-inflammatory cytokine gene expression and apoptosis in human chorion cells of fetal membranes by influenza virus infection: possible implications for maintenance and interruption of pregnancy during infection. Med Sci Monit 2005; 11:RA7 - 16; PMID: 15614205
- Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 1989; 37:13 - 22; http://dx.doi.org/10.1016/0090-6980(89)90028-2; PMID: 2785698
- Challis JR, Sloboda DM, Alfaidy N, Lye SJ, Gibb W, Patel FA, et al. Prostaglandins and mechanisms of preterm birth. Reproduction 2002; 124:1 - 17; http://dx.doi.org/10.1530/rep.0.1240001; PMID: 12090913
- Fidel PL Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994; 170:1467 - 75; PMID: 8178889
- Deinard AS, Ogburn P Jr. A/NJ/8/76 influenza vaccination program: effects on maternal health and pregnancy outcome. Am J Obstet Gynecol 1981; 140:240 - 5; PMID: 7246624
- Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis 1993; 168:647 - 56; http://dx.doi.org/10.1093/infdis/168.3.647; PMID: 8354906
- Hulka JF. Effectiveness of polyvalent influenza vaccine in pregnancy. REPORT OF A CONTROLLED STUDY DURING AN OUTBREAK OF ASIAN INFLUENZA. Obstet Gynecol 1964; 23:830 - 7; PMID: 14168242
- Jackson LA, Patel SM, Swamy GK, Frey SE, Creech CB, Munoz FM, et al. Immunogenicity of an Inactivated Monovalent 2009 H1N1 Influenza Vaccine in Pregnant Women. J Infect Dis 2011; 204:854 - 63; http://dx.doi.org/10.1093/infdis/jir440; PMID: 21849282
- Murray DL, Imagawa DT, Okada DM, St Geme JWJ. Antibody response to monovalent A/New Jersey/8/76 influenza vaccine in pregnant women. J Clin Microbiol 1979; 10:184 - 7; PMID: 583151
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, et al. Influenza immunization in pregnancy–antibody responses in mothers and infants. N Engl J Med 2010; 362:1644 - 6; http://dx.doi.org/10.1056/NEJMc0912599; PMID: 20427817
- Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. J Infect Dis 1979; 140:141 - 6; http://dx.doi.org/10.1093/infdis/140.2.141; PMID: 479636
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555 - 64; http://dx.doi.org/10.1056/NEJMoa0708630; PMID: 18799552
- Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2005; 192:1098 - 106; http://dx.doi.org/10.1016/j.ajog.2004.12.019; PMID: 15846187
- Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol 2004; 21:333 - 9; http://dx.doi.org/10.1055/s-2004-831888; PMID: 15311370
- Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med 2011; 8:e1000441; http://dx.doi.org/10.1371/journal.pmed.1000441; PMID: 21655318
- Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt H, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406 - 16; http://dx.doi.org/10.1056/NEJMoa1010331; PMID: 21995388
- Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 2010; 51:1355 - 61; http://dx.doi.org/10.1086/657309; PMID: 21058908
- Poehling KA, Szilagyi PG, Staat MA, Snively BM, Payne DC, Bridges CB, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204:S141 - 8; http://dx.doi.org/10.1016/j.ajog.2011.02.042; PMID: 21492825
- Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, Santosham M, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 2011; 165:104 - 11; http://dx.doi.org/10.1001/archpediatrics.2010.192; PMID: 20921345
- Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004; 10:811 - 3; http://dx.doi.org/10.1038/nm1077; PMID: 15247911
- Klugman KP, Chien YW, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combinationVaccine200927:C9-17.
- Long PH. RECOMMENDATIONS FOR INFLUENZA IMMUNIZATION AND CONTROL: 1964-1965. Med Times 1964; 92:1203 - 5; PMID: 14197263
- Centers for Disease Control and Prevention. Influenza immunization and control. MMWR Morb Mortal Wkly Rep 1965; 14:203 - 8
- Centers for Disease Control and Prevention. Influenza vaccine-civilian use-1966-67. MMWR Morb Mortal Wkly Rep 1966; 15:238 - 9
- Prevention and control of influenza recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. MMWR Recomm Rep 1995; 44:1 - 22; PMID: 7746261
- ACOG committee opinion number 305, November 2004. Influenza vaccination and treatment during pregnancy. Obstet Gynecol 2004; 104:1125 - 6; http://dx.doi.org/10.1097/00006250-200411000-00058; PMID: 15516422
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004; 53:1 - 40; PMID: 15163927
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1 - 62; PMID: 20689501
- Influenza vaccines. Wkly Epidemiol Rec 2005; 80:279 - 87; PMID: 16171031
- Statement on influenza vaccination for the 2007-2008 season. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2007; 33:1 - 38; PMID: 17663034
- National Health and Medical Research Council. Vaccination of women planning pregnancy, pregnant or breastfeeding women, and preterm infants. The Australian immunization handbook, 2008.
- Influenza. In: Salisbury D, Ramsay M, Noakes K, eds. Immunisation against infectious disease- “the Green Book”: Department of Health, 2006.
- Mereckiene J, Cotter S, Nicoll A, Levy-Bruhl D, Ferro A, Tridente G, et al. National seasonal influenza vaccination survey in Europe, 2008. Euro Surveill 2008; 13:pii: 19017; PMID: 18947524
- Ropero-Alvarez AM, Kurtis HJ, Danovaro-Holliday MC, Ruiz-Matus C, Andrus JK. Expansion of seasonal influenza vaccination in the Americas. BMC Public Health 2009; 9:361; http://dx.doi.org/10.1186/1471-2458-9-361; PMID: 19778430
- Vilca Yengle LM, Campins MM, Cabero RL, Rodrigo Pendas JA, Martinez GX, Hermosilla PE, et al. [Influenza vaccination in pregnant women. Coverage, practices and knowledge among obstetricians.]. Med Clin (Barc) 2010; 134:146 - 51; http://dx.doi.org/10.1016/j.medcli.2009.10.004; PMID: 19942237
- Yudin MH, Salaripour M, Sgro MD. Pregnant women's knowledge of influenza and the use and safety of the influenza vaccine during pregnancy. J Obstet Gynaecol Can 2009; 31:120 - 5; PMID: 19327210
- Seasonal influenza and 2009 H1N1 influenza vaccination coverage among pregnant women–10 states, 2009-10 influenza season. MMWR Morb Mortal Wkly Rep 2010; 59:1541 - 5; PMID: 21124293
- Centers for Disease Control and Prevention. Self-reported influenza vaccination coverage trends 1989-2008 among adults by age group, risk group, race/ethnicity, health-care worker status and pregnancy status, United States, National Health Interview Survey (NHIS). Atlanta, GA: Centers for Disease Control and Prevention, 2009.
- Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev 2006; 28:47 - 53; http://dx.doi.org/10.1093/epirev/mxj002; PMID: 16731574
- Silverman NS, Greif A. Influenza vaccination during pregnancy. Patients' and physicians' attitudes. J Reprod Med 2001; 46:989 - 94; PMID: 11762156