Abstract
Interleukin-2 and its downstream target STAT5 have effects on many aspects of immune function. This has been perhaps best documented in regulatory T cells. In this review we summarize the initial findings supporting a role for IL2 and STAT5 in regulatory T cell development and outline more recent studies describing how this critical signaling pathway entrains regulatory T cell differentiation and affects regulatory T cell function.
Introduction
It has now been 20 years since the first reports appeared demonstrating that interleukin-2 is required to prevent the development of systemic autoimmune disease.Citation1 Subsequent studies by Sakaguchi and colleagues identified CD25, the interleukin-2 receptor α chain (IL2Rα), as one of the first useful markers for the identification of regulatory T cells (Tregs).Citation2 This led to the initial hypothesis that IL2 is required for the development or function of Tregs and more recently the implementation in the clinic of agonist IL2:anti-IL2 complexes for the treatment of autoimmune and inflammatory conditions.Citation3,Citation4
Supporting the initial hypothesis that IL2 is involved in Treg development, work by Malek and colleagues demonstrated that the autoimmune disease that developed in Il2rb−/− mice could be prevented by the transfer of CD25+ Tregs from WT mice into Il2rb−/− host mice. These studies demonstrated that Il2rb−/− mice lacked a functional population of Tregs.Citation5 Additional work by this same group demonstrated that expression of an Il2rb transgene that was expressed solely in the thymus was sufficient to rescue the defect in Treg development suggesting that the defect in Il2rb−/− mice is due to a failure of Treg development in the absence of IL2.Citation6 In contrast, Lafaille and colleagues found that transfer of CD4+ T cells from Il2−/− mice into a mouse model of experimental autoimmune encephalomyelitis prevented disease, while CD4+ T cells from Il2ra−/− mice did not. These results suggested that CD4+ T cells in Il2−/− mice are capable of developing into and functioning as Tregs.Citation7 Supporting this observation, two other groups used either Foxp3-GFP reporter mice, or the ability to stain for intracellular FOXP3, to demonstrate that young Il2−/− mice have FOXP3+ Tregs and that the defect in these mice had to do with reduced function or “fitness” of these cells.Citation8,Citation9 Finally, work from our group and Steve Ziegler’s was able to reconcile these findings by demonstrating that while young Il2−/− mice do not lack FOXP3+ Tregs, comparable Il2rb−/− mice have a substantial defect in Treg development.Citation10,Citation11 This latter result reflects redundancy between IL2 and IL15 as Il2−/− x Il15−/− mice mimic the defect in Treg development observed in Il2rb−/− mice.Citation10 It is important to point out that under physiological circumstances IL15 does not play a role in Treg development or function as IL2 signaling in Tregs leads to downregulation of the IL15Rα chain, thereby rendering these cells much less responsive to IL15.Citation12 Thus, subsequent studies have demonstrated that the original experiments by Malek and Lafaille and colleagues were both correct as IL2 plays an important role in both Treg development and function.
STAT5 Activation Drives Thymic Treg Lineage Commitment
CD4+CD25+FOXP3+ Tregs that develop in the thymus (also known as “natural Tregs”) constitute 2–4% of CD4 single positive (CD4SP) thymocytes, yet this relatively small population plays a critical role in maintaining peripheral tolerance and preventing autoimmunity. The T cell receptor (TCR) repertoire of these natural Tregs overlaps with that of non-regulatory T cell populations but is skewed to favor TCRs that interact with higher affinity to self-antigens in the thymus.Citation13-Citation18 The molecular mechanisms that drive Treg development have been tied to three primary signaling modules. First, TCR signaling plays a key role as TCRs with higher affinity for self-antigen are preferentially selected into the Treg lineage.Citation15,Citation19 Second, the costimulatory receptor CD28 also plays an important role as Cd28−/− and B7-1/B7-2−/− mice both show clear defects in Treg development.Citation20-Citation23 Third, signals emanating from the interleukin-2 receptor are also required for Treg differentiation in the thymus.Citation10,Citation11 These observations culminated in the development of a two-step model of thymic Treg development, in which a TCR- and CD28-dependent, but cytokine-independent first step generates an IL2-responsive intermediate “Treg progenitor” that lacks FOXP3 expression. Subsequently, a TCR-independent, IL2/STAT5-dependent second step results in the rapid conversion of Treg progenitors into mature FOXP3+ TregsCitation24,Citation25 (). We examine this model in further detail below.
Figure 1. Two-step model of thymic Treg development. (A) CD4SP thymocytes perceiving high affinity/avidity signals emanating from TCR/CD28 are first programmed via the NFκB pathway to express IL2Rα and IL2Rβ, rendering them highly responsive to IL2. A second step, which is TCR-independent, but cytokine-dependent, is completed when Treg progenitors receive IL2 signals transmitted via STAT5 to subsequently drive expression of Foxp3. This second step yields mature, fully functional FOXP3+ Tregs. (B) CD4SP thymocytes plotted on the basis of CD25 and FOXP3 expression can be categorized into (1) conventional or non-Treg cells which are CD4+CD25−FOXP3− (gated in red), (2) CD4+CD25+FOXP3− Treg progenitors, which are also CD122hi and GITRhi (gated in green) and (3) CD4+CD25+FOXP3+ mature Tregs (gated in blue). The representative TCR signal strength of each of these populations, reported via NUR77-GFP expression, is shown in the histogram on the right.
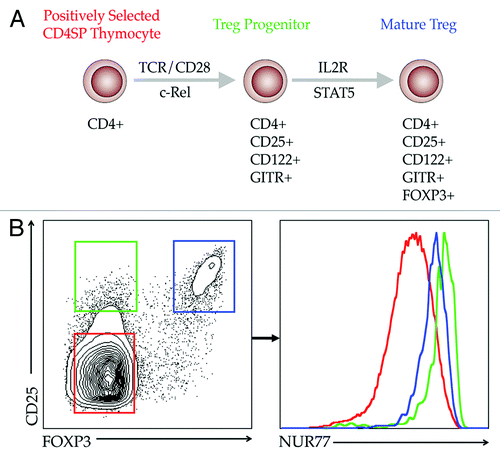
Upon interacting with medullary antigen presenting cells (APC) presenting self-peptide:MHC II complexes, strong TCR signals in a fraction of CD4SP thymocytes cause them to differentiate into Treg progenitors, marked by elevated expression of the high-affinity IL2Rα chain (CD25), the IL2Rβ chain (CD122), and the costimulatory TNF receptor superfamily member, glucocorticoid-induced TNF-related protein (GITR).Citation24,Citation25 The emergence of this CD4+CD25+CD122hiGITRhiFOXP3− Treg progenitor population requires canonical activation of the NFκB pathway downstream of TCR and CD28 ligation. Paired activation of LCK from these receptors signals through the canonical NFκB pathway to ultimately promote nuclear translocation of c-REL and REL-A.Citation22,Citation26-Citation29 The requirement for NFκB activation in Treg differentiation is demonstrated by the absence of thymic Tregs—and importantly, Treg progenitors—in animals deficient in Cd28, Prkcq, Carma1, Bcl10 and Rel.Citation21,Citation22,Citation30-Citation32 Further studies revealed that c-REL binds the conserved non-coding sequence 3 (CNS3) located in the Foxp3 gene to promote epigenetic modification of Foxp3 rendering it permissive for subsequent transcription initiation.Citation33
The conversion of FOXP3− Treg progenitors into mature FOXP3+ Tregs in the thymus occurs via a TCR-independent but IL2/STAT5-dependent process.Citation24,Citation25 Ligand binding by the high affinity IL2R complex leads to phosphorylation of three key tyrosine residues located in the cytoplasmic domain of IL2Rβ by the kinases JAK1 and JAK3. Phosphorylation of Tyr-338 recruits the SH2-containing adaptor molecule, SHC, facilitating activation of the RAS/MAPK/ERK and PI3K/AKT pathways via GRB2 and GAB2, respectively. Phosphorylation of IL2Rβ at Tyr-510 (and to a lesser degree Tyr-392) is critical for recruiting and activating STAT5.Citation34 The importance of IL2R signaling in thymic Treg differentiation is clearly demonstrated by the fact that the lethal autoimmunity in mice lacking Il2rb is due to a failure to generate thymic Tregs, and this phenotype is completely restored by adoptive transfer of small numbers of wild type Tregs.Citation5 Moreover, retroviral transduction of Il2rb−/− bone marrow with wild type Il2rb, or a mutant construct capable of activating only STAT5 via Tyr-510, restored thymic Treg generation in bone marrow chimeric mice. In contrast, restoration of Treg development did not occur when mutant constructs capable of activating RAS/PI3K, but not STAT5, were transduced into Il2rb−/− bone marrow cells and engrafted into recipient mice.Citation10 Likewise, crossing Il2rb−/− mice to transgenic mice expressing a constitutively active form of STAT5b (Stat5b-CA mice) restored Treg development in the thymus.Citation10 Additional support for the role of STAT5 in Treg development came from two studies that demonstrated that conditional deletion of STAT5 in DP thymocytes (i.e., Cd4-Cre × Stat5a/bFL/FL mice) had minimal effects on CD4SP thymocytes with the exception of CD4+FOXP3+ thymic Tregs.Citation10,Citation35 Together, these findings indicate that STAT5 activation downstream of IL2R is required for thymic Treg development.
Two groups have demonstrated that CD4+CD25+FOXP3− thymocytes are direct precursors of FOXP3+ Tregs, which require only an additional IL2R/STAT5-dependent signal to express FOXP3 (). First, Hsieh and colleagues showed that adoptive transfer of CD4+CD25+FOXP3− thymocytes, but not CD4+CD25−FOXP3− thymocytes, into the thymii of wild-type hosts resulted in the development of CD4+FOXP3+ Tregs. Similar results were observed upon adoptive transfer into MHCII-deficient mice demonstrating that the conversion process did not require additional signals via the TCR.Citation24 In addition, stimulation of sorted Treg progenitors with IL2 in vitro led to induction of Foxp3 mRNA within a few hours followed by the development of CD4+FOXP3+ Tregs 24 h later. These findings were subsequently confirmed by Burchill and colleagues.Citation25
Interestingly, IL2R/STAT5 signaling also influences selection of the thymic Treg TCR repertoire. Several studies indicate that the Treg TCR repertoire is biased toward self-reactivity, although there is some overlap with the conventional CD4+FOXP3− TCR repertoire.Citation16-Citation18 Initial studies by Burchill et al. found that augmented STAT5 signaling clearly altered the Treg TCR repertoire. Specifically, this study evaluated the effect of forced STAT5 activation on the Treg TCR repertoire by tracking the frequency of CD4+FOXP3+ cells specific for a peptide called 2W1S bound to I-Ab MHC class II molecules using peptide:MHCII tetramers (in this study the 2W1S:I-Ab tetramer) in littermate control and Stat5b-CA mice.Citation25 A simple comparison of the ratio of CD4+FOXP3− to CD4+FOXP3+ cells among total and 2W1S-specific T cells revealed that this TCR is dramatically underrepresented in the Treg pool of wild type mice. The frequency of CD4+FOXP3+ T cells specific for 2W1S:I-Ab in WT mice (~3–6%) was much lower than that observed for the average of all other Treg TCR specificities (~15%); in contrast, in Stat5b-CA mice the frequency of 2W1S:I-Ab specific Tregs was identical to the average of all other Treg TCR specificities. This finding demonstrated that for at least one TCR specificity regulation of STAT5 signaling had a profound effect on its distribution within the Treg repertoire. Thus, ectopic STAT5 activation removed the TCR selection bias that typically results in underrepresentation of 2W1S:I-Ab specific T cells in the Treg TCR repertoire.Citation25
To extend the observations on the role of STAT5 and the Treg TCR repertoire beyond a single TCR, a fixed TCRβ transgenic system was employed to partially restrict the repertoire, and sequencing was performed on over 1,000 productive Vα2 rearrangements from thymocytes isolated from Stat5b-CA mice or their WT littermate controls. Tonic STAT5 signaling in Stat5b-CA mice led to a dramatic expansion in the diversity of productive rearrangements among CD4+FOXP3+ Tregs including substantial numbers of TCRs that were typically not found in the Treg TCR repertoire.Citation25 These initial studies have been confirmed more recently by Moran and Hogquist using an independent approach.Citation15 This latter study used BAC transgenic mice in which a GFP reporter had been knocked into the Nur77 gene locus. These mice accurately measure TCR signal strength as assessed by overall GFP expression. Importantly, this study confirmed that signal strength for TCRs expressed by Tregs is higher than that for conventional CD4+ T cells (). When the Nur77-GFP mice were crossed to Stat5b-CA mice, it was observed that the Treg TCR repertoire was much broader and included substantial numbers of T cells with TCRs that signaled with significantly lower signal strength as assessed using the NUR77-GFP reporter.Citation15 Together, these observations indicate that limiting IL2R/STAT5 signaling helps to focus the thymic Treg TCR repertoire on TCRs with higher intrinsic signal strength.Citation25 This suggests that IL2R/STAT5 signaling plays an important role in ensuring the preferential development of Tregs with higher reactivity to self-antigens, which may be important in preventing autoimmunity.
Several questions remain unanswered pertaining to the role of IL2 and STAT5 in promoting thymic Treg differentiation. First, what cell subsets in the thymus actually synthesize the IL2 required for completing the second step of Treg development? IL2 was originally described as a cytokine made by activated T cells to drive proliferation and survival of T cells, thus amplifying effector responses.Citation36 In this regard, a reasonable assumption might be that developing thymocytes produce the IL2 needed for thymic Treg development. However, thymocytes make exceedingly low levels of IL2 (if any) relative to splenocytes upon stimulation (S.A.M. and M.A.F., unpublished observation). The observation that dendritic cells and B cells can also make IL2 suggest that these cell subsets might play an important role in producing the IL2 needed for Treg development in the thymus.Citation37,Citation38 Further work in which IL2 can be conditionally deleted in T cells, dendritic cells and B cells will be needed to definitively address this question. A second question has to do with what signals actually induce or regulate IL2 production in the thymus; such signals remain undefined. Finally, a defining feature of Treg progenitors is their high expression of GITR. However, the functional significance of this high level upregulation of GITR remains unclear.
The molecular mechanism by which STAT5 affects Foxp3 transcription is also unclear. STAT5 binding sites have been found in the Foxp3 promoter region as well as within the CNS2 region of intron 1 in the Foxp3 gene and several studies have shown STAT5 binding to those sites.Citation10,Citation35 The effect of STAT5 binding to these sites is not yet clear. Deletion of the entire CNS2 region including the STAT5 binding sites did not prevent Treg development although it did have an effect on stability of FOXP3 expression.Citation33 However, the CNS2 region is highly methylated in non-Tregs and typically completely demethylated in natural FOXP3+ Tregs. If methylation of this region normally represses Foxp3 transcription, then deletion of the entire region would remove the need for any factors that typically reverse this methylation state. Thus, whether STAT5 binding to CNS2 plays a role in Treg development is difficult to determine based on studies deleting the entire CNS2 region.
The critical co-factors that interact with STAT5 to promote Treg development are also poorly characterized. STAT5 is known to interact with a variety of both co-activators, such as CBP and p300, and co-repressors such as NCOR2.Citation39,Citation40 How these function in Treg differentiation remains untested. Intriguingly, treatment of Treg progenitors with two distinct histone deactylase (HDAC) inhibitors prevented the IL2/STAT5-dependent conversion of Treg progenitors into Tregs.Citation25 Although this result at first appears counterintuitive, it is consistent with several reports demonstrating that STAT-dependent gene transcription frequently requires HDAC activity.Citation41,Citation42 Whether NCOR2 and associated HDACs are recruited to the Foxp3 locus by STAT5 during thymic Treg differentiation, and if so, how this complex regulates Foxp3 transcription, remains to be elucidated. Thus, the molecular mechanisms by which STAT5 alters transcription of genes involved in Treg differentiation remains to be established.
IL2, STAT5 and Induced Tregs
An important feature of peripheral tolerance is the conversion of naïve CD4+ T cells into induced regulatory T cells (iTregs) in peripheral lymphoid organs. iTregs have important roles in protecting against chronic inflammatory conditions, and likely play a key role in regulating immune responses to commensal microorganisms.Citation43,Citation44 The differentiation of iTregs, like nTregs in the thymus, requires both TCR- and IL2-dependent signals. However, unlike nTregs, iTregs require transforming growth factor-β (TGFβ) for their differentiation.Citation45 Moreover, while the CARMA1/NFκB pathway is required for the development of nTregs it actually antagonizes iTreg differentiation.Citation46 Finally, the stability of iTregs is lower than that of nTregs,Citation47 a feature which correlates with the greater degree of DNA methylation of the CNS2 region of the Foxp3 gene in iTregs vs. nTregs.Citation48,Citation49 Thus, iTregs differ in several ways from nTregs.
A role for IL2 in iTreg development was established many years ago in studies documenting the role of both TGFβ and IL2 in iTreg differentiation.Citation50,Citation51 Likewise, STAT5 also plays an important role in iTreg differentiation in vitro.Citation35 More recent studies have demonstrated that IL2 and STAT5 also play critical roles in maintaining stability of the iTreg lineage.Citation47 Specifically, these studies demonstrated that transfer of iTregs into congenic hosts resulted in loss of FOXP3 expression in the transferred iTregs. This result could be blocked by co-administration of agonist IL2: anti-IL2 complexes indicating that IL2 was required to maintain FOXP3 expression in iTregs in vivo. These studies further documented that the loss of Foxp3 expression correlated with re-methylation of the CNS2 region (also referred to as regulatory T cell specific demethylated region or TSDR) of the Foxp3 gene, and that IL2 stimulation prevented this re-methylation process. The mechanism by which this occurs remains unclear. However, STAT5 binding sites are found in the CNS2 region, which may be important for maintaining Foxp3 expression. It is also possible that STAT5 directly initiates demethylation of this region in naïve CD4+FOXP3− T cells as they are being converted into CD4+FOXP3+ iTregs. Arguing against this possibility is evidence that STAT5 binds poorly to its cognate DNA binding site when it is methylated.Citation52,Citation53 However, only one of the three potential STAT5 binding sites found in the CNS2 region contains a CpG motif that could be methylated.Citation10 Thus, whether IL2 and STAT5 promote demethylation of CNS2 requires additional study.
STAT5 also governs Treg function. For example, Blazar and colleagues demonstrated that Tregs expressing a constitutively active form of STAT5b (Stat5b-CA mice) are superior to WT Tregs in protecting mice from graft-versus-host disease.Citation54 This was due to a number of factors including (1) improved homeostasis of transferred Tregs, (2) augmented Treg suppressor function and (3) reduced ability of Stat5b-CA effector T cells to differentiate into TH1 and TH17 cells. Supporting these observations, Malek and colleagues demonstrated that IL2 and STAT5 signaling are required for development of a population of KLRG1+ Treg cells that appear to express elevated levels of many factors required for Treg function, such as IL10.Citation55 Interestingly, this report documented that quite modest levels of IL2/STAT5 signals are required for thymic Treg development and peripheral survival. In contrast, the development of KLRG1+ Tregs, which represent a terminally differentiated form of Treg with augmented suppressor function, require much stronger IL2/STAT5 dependent signaling. In many ways this latter population resembles KLRG1+ effector CD8+ T cells suggesting that these two populations may develop via similar mechanisms.
STAT Family Influences on Treg Differentiation
As mentioned above, STAT5 has been shown to inhibit the production of IL17 both in vivo and in vitro. The mechanism underlying this has been attributed to the ability of STAT5 to directly compete with IL6-dependent STAT3 binding to enhancers within the Il17 gene locus.Citation56 STAT5 binding within the Il17 gene locus correlated with recruitment of the co-repressor NCOR2, which is known to interact with STAT5. Furthermore, IL2 downregulates IL6 receptor expression on iTregs which further acts to prevent iTregs from differentiating into TH17 cells.Citation57 Thus the balance between STAT5 and STAT3 signaling plays an important role in directing iTreg development.
Other cytokines including IL4 and IL12 have also been shown to antagonize Treg development.Citation58,Citation59 These cytokines activate STAT6 and STAT4 respectively, which are required for inhibiting Treg differentiation.Citation58 While the mechanism by which STAT6 and STAT4 inhibit Treg development remains to be precisely defined, both of these factors have been shown to reduce STAT5 binding to the promoter and/or CNS2 region of the Foxp3 gene.Citation58 Thus, cytokine crosstalk plays an important role in directing the differentiation of iTregs.
As mentioned above with regard to thymic Treg development, STAT5 interacts with a number of potential binding partners. The role of these binding partners in iTreg development remains to be defined. Interestingly, recent work from the O’Shea lab has shown that micro RNAs activated by TGFβ and retinoic acid receptor α (RARα) suppress the expression of one of these binding partners, the co-repressor NCOR2.Citation60 Specifically, this study demonstrated that conversion of iTregs into T follicular helper cells (TFH) is limited when mir10a is expressed at high levels, such as is the case following TGFβ and RARα signaling in the periphery. It appears that mir10a may have a role in fixing the iTreg cell lineage by suppressing conversion of iTregs into either TH17 or TFH cells. Additional studies are needed to more precisely define the role of NCOR2 and other STAT5 interacting partners on the development and maintenance of regulatory T cells.
Conclusions
Work over the past 20 years has clearly documented an important role for IL2 and STAT5 in shaping the development of both natural and induced Tregs. More recent studies point to a critical role for IL2/STAT5 signals in modifying the functional activity of regulatory T cells. However, the molecular mechanisms by which STAT5 and its many potential binding partners influence Treg biology are just beginning to be explored. A better understanding of how the IL2/STAT5 pathway governs Treg biology may allow for more targeted approaches for augmenting or inhibiting Treg function. Such information could be particularly useful in developing strategies to inhibit Treg responses to tumors or to augment Treg responses in autoimmunity.
| Abbreviations: | ||
| Tregs | = | regulatory T cells |
| nTregs | = | natural Tregs |
| iTregs | = | induced Tregs |
| SP | = | single positive |
| IL2 | = | interleukin-2 |
| IL2R | = | interleukin-2 receptor |
| STAT5 | = | signal transducer and activator of transcription 5 |
| FOXP3 | = | Forkhead box P3 |
| IL15 | = | interleukin-15 |
| TCR | = | T cell receptor |
| CNS | = | conserved non-coding DNA sequence |
| TGF-β | = | transforming growth factor-β |
| IL17 | = | interleukin-17 |
| TH1 | = | T helper type 1 cells |
| TH17 | = | T helper type 17 cells |
Acknowledgments
S.A.M. was supported by an immunology training grant (2T32AI007313), the University of Minnesota Medical Scientist Training Program (5T32GM008244) and by an individual predoctoral F30 fellowship from the NIH (1F30DK096844). M.A.F. was supported by grants CA154998, CA151845 and AI061165 from the NIH and by a Leukemia and Lymphoma Society Scholar Award.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993; 75:253 - 61; http://dx.doi.org/10.1016/0092-8674(93)80067-O; PMID: 8402910
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151 - 64; PMID: 7636184
- Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011; 365:2067 - 77; http://dx.doi.org/10.1056/NEJMoa1105143; PMID: 22129253
- Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011; 365:2055 - 66; http://dx.doi.org/10.1056/NEJMoa1108188; PMID: 22129252
- Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity 2002; 17:167 - 78; http://dx.doi.org/10.1016/S1074-7613(02)00367-9; PMID: 12196288
- Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol 2000; 164:2905 - 14; PMID: 10706676
- Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med 2002; 196:851 - 7; http://dx.doi.org/10.1084/jem.20020190; PMID: 12235217
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005; 6:1142 - 51; http://dx.doi.org/10.1038/ni1263; PMID: 16227984
- D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 2005; 6:1152 - 9; http://dx.doi.org/10.1038/ni1264; PMID: 16227983
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007; 178:280 - 90; PMID: 17182565
- Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur J Immunol 2007; 37:1817 - 26; http://dx.doi.org/10.1002/eji.200737101; PMID: 17559173
- Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol 2008; 181:3285 - 90; PMID: 18714000
- Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol 2012; 12:157 - 67; PMID: 22322317
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001; 2:301 - 6; http://dx.doi.org/10.1038/86302; PMID: 11276200
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011; 208:1279 - 89; http://dx.doi.org/10.1084/jem.20110308; PMID: 21606508
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 2004; 21:267 - 77; http://dx.doi.org/10.1016/j.immuni.2004.07.009; PMID: 15308106
- Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity 2006; 25:249 - 59; http://dx.doi.org/10.1016/j.immuni.2006.05.016; PMID: 16879995
- Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol 2007; 178:7032 - 41; PMID: 17513752
- Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity 2012; 37:475 - 86; http://dx.doi.org/10.1016/j.immuni.2012.07.009; PMID: 22921379
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000; 12:431 - 40; http://dx.doi.org/10.1016/S1074-7613(00)80195-8; PMID: 10795741
- Lio CW, Dodson LF, Deppong CM, Hsieh CS, Green JM. CD28 facilitates the generation of Foxp3(-) cytokine responsive regulatory T cell precursors. J Immunol 2010; 184:6007 - 13; http://dx.doi.org/10.4049/jimmunol.1000019; PMID: 20421644
- Vang KB, Yang J, Pagán AJ, Li LX, Wang J, Green JM, et al. Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J Immunol 2010; 184:4074 - 7; http://dx.doi.org/10.4049/jimmunol.0903933; PMID: 20228198
- Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 2005; 6:152 - 62; http://dx.doi.org/10.1038/ni1160; PMID: 15640801
- Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity 2008; 28:100 - 11; http://dx.doi.org/10.1016/j.immuni.2007.11.021; PMID: 18199417
- Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 2008; 28:112 - 21; http://dx.doi.org/10.1016/j.immuni.2007.11.022; PMID: 18199418
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 2009; 31:921 - 31; http://dx.doi.org/10.1016/j.immuni.2009.09.022; PMID: 20064449
- Deenick EK, Elford AR, Pellegrini M, Hall H, Mak TW, Ohashi PS. c-Rel but not NF-kappaB1 is important for T regulatory cell development. Eur J Immunol 2010; 40:677 - 81; http://dx.doi.org/10.1002/eji.201040298; PMID: 20082358
- Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity 2009; 31:932 - 40; http://dx.doi.org/10.1016/j.immuni.2009.10.006; PMID: 20064450
- Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med 2009; 206:3001 - 14; http://dx.doi.org/10.1084/jem.20091411; PMID: 19995950
- Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol 2008; 46:213 - 24; http://dx.doi.org/10.1016/j.molimm.2008.08.275; PMID: 18842300
- Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J Immunol 2009; 182:6736 - 43; http://dx.doi.org/10.4049/jimmunol.0900498; PMID: 19454668
- Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol 2009; 10:689 - 95; http://dx.doi.org/10.1038/ni.1760; PMID: 19536194
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 2010; 463:808 - 12; http://dx.doi.org/10.1038/nature08750; PMID: 20072126
- Fujii H, Ogasawara K, Otsuka H, Suzuki M, Yamamura K, Yokochi T, et al. Functional dissection of the cytoplasmic subregions of the IL-2 receptor betac chain in primary lymphocyte populations. EMBO J 1998; 17:6551 - 7; http://dx.doi.org/10.1093/emboj/17.22.6551; PMID: 9822600
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 2007; 109:4368 - 75; http://dx.doi.org/10.1182/blood-2006-11-055756; PMID: 17227828
- Smith KA, Gillis S, Baker PE, McKenzie D, Ruscetti FW. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci 1979; 332:423 - 32; http://dx.doi.org/10.1111/j.1749-6632.1979.tb47136.x; PMID: 316981
- Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol 2001; 2:882 - 8; http://dx.doi.org/10.1038/ni0901-882; PMID: 11526406
- Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity 2009; 30:421 - 33; http://dx.doi.org/10.1016/j.immuni.2009.01.006; PMID: 19249230
- Pfitzner E, Jähne R, Wissler M, Stoecklin E, Groner B. p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the transactivation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol Endocrinol 1998; 12:1582 - 93; http://dx.doi.org/10.1210/me.12.10.1582; PMID: 9773981
- Nakajima H, Brindle PK, Handa M, Ihle JN. Functional interaction of STAT5 and nuclear receptor co-repressor SMRT: implications in negative regulation of STAT5-dependent transcription. EMBO J 2001; 20:6836 - 44; http://dx.doi.org/10.1093/emboj/20.23.6836; PMID: 11726519
- Rascle A, Johnston JA, Amati B. Deacetylase activity is required for recruitment of the basal transcription machinery and transactivation by STAT5. Mol Cell Biol 2003; 23:4162 - 73; http://dx.doi.org/10.1128/MCB.23.12.4162-4173.2003; PMID: 12773560
- Nusinzon I, Horvath CM. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc Natl Acad Sci U S A 2003; 100:14742 - 7; http://dx.doi.org/10.1073/pnas.2433987100; PMID: 14645718
- Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 2011; 35:109 - 22; http://dx.doi.org/10.1016/j.immuni.2011.03.029; PMID: 21723159
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011; 478:250 - 4; http://dx.doi.org/10.1038/nature10434; PMID: 21937990
- Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol 2004; 173:6526 - 31; PMID: 15557141
- Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner. J Immunol 2011; 186:4609 - 17; http://dx.doi.org/10.4049/jimmunol.1002361; PMID: 21411734
- Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol 2011; 186:6329 - 37; http://dx.doi.org/10.4049/jimmunol.1100061; PMID: 21525380
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5:e38; http://dx.doi.org/10.1371/journal.pbio.0050038; PMID: 17298177
- Beres A, Komorowski R, Mihara M, Drobyski WR. Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clin Cancer Res 2011; 17:3969 - 83; http://dx.doi.org/10.1158/1078-0432.CCR-10-3347; PMID: 21558402
- Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur J Immunol 2008; 38:912 - 5; http://dx.doi.org/10.1002/eji.200738109; PMID: 18395858
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007; 178:2018 - 27; PMID: 17277105
- Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol 2009; 182:259 - 73; PMID: 19109157
- Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med 2007; 204:1543 - 51; PMID: 17591856
- Vogtenhuber C, Bucher C, Highfill SL, Koch LK, Goren E, Panoskaltsis-Mortari A, et al. Constitutively active Stat5b in CD4+ T cells inhibits graft-versus-host disease lethality associated with increased regulatory T-cell potency and decreased T effector cell responses. Blood 2010; 116:466 - 74; http://dx.doi.org/10.1182/blood-2009-11-252825; PMID: 20442366
- Cheng G, Yuan X, Tsai MS, Podack ER, Yu A, Malek TR. IL-2 receptor signaling is essential for the development of Klrg1+ terminally differentiated T regulatory cells. J Immunol 2012; 189:1780 - 91; http://dx.doi.org/10.4049/jimmunol.1103768; PMID: 22786769
- Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol 2011; 12:247 - 54; http://dx.doi.org/10.1038/ni.1995; PMID: 21278738
- Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol 2008; 180:7112 - 6; PMID: 18490709
- O’Malley JT, Sehra S, Thieu VT, Yu Q, Chang HC, Stritesky GL, et al. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology 2009; 127:587 - 95; http://dx.doi.org/10.1111/j.1365-2567.2008.03037.x; PMID: 19604309
- Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 2007; 104:18169 - 74; http://dx.doi.org/10.1073/pnas.0703642104; PMID: 17978190
- Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciumè G, Muljo SA, et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol 2012; 13:587 - 95; http://dx.doi.org/10.1038/ni.2286; PMID: 22544395