Abstract
The transcription factor STAT5A is essential for two processes during mammary gland development. First, it controls the development of luminal progenitor cells from stem cellsCitation1 and second, it has a role during pregnancy where it is required for alveologenesisCitation2,Citation3 the production of clusters of luminal cells that synthesize and secrete milk during lactation. Thus, deletion of STAT5A in late pregnancy results in lactation failure. Alveologenesis requires the proliferation of a different lineage of luminal epithelial cells in response to the pregnancy hormones progesterone and prolactin, the latter of which activates STAT5. Prolactin is required additionally during lactation to ensure adequate milk production and the transcription of several milk protein genes has been shown to be regulated by STAT5.Citation4,Citation5 On the other hand, the PI3K/Akt pathway is essential for the synthesis of other milk components such as lipids and lactose.Citation6 In recent elegant work from Lewis Chodosh’s laboratory, published in Genes and Development, these two pathways are now shown to be directly linked.Citation7 More specifically, it is shown that the PI3K/Akt pathway induces autocrine prolactin production and that this is required for the initiation of lactation.
The mammary gland is comprised of a branched network of ducts that are embedded within a fatty stroma. The ducts are composed of a bi-layered epithelium with a network of basal myoepithelial cells surrounding an inner layer of luminal epithelial cells.Citation8 Stem cells are predominantly found dispersed throughout the basal layer although unipotent stem cells are found in both luminal and basal locations.Citation9 The mammary gland undergoes a cycle of further development with every pregnancy. In response to the major hormones of pregnancy, progesterone and prolactin, tertiary branching and the formation of lobuloalveolar structures is initiated. The progenitors for this lineage have not been clearly defined but it is thought that the alveolar lineage arises from progenitors that are localized in the basal layer of epithelium. Several studies using genetically altered mice have demonstrated the importance of the prolactin/JAK2/STAT5 pathway for mammary gland development and mice deficient in pathway components have similar phenotypes characterized by the absence of alveolar structures and failed lactation.Citation2,Citation10-Citation12
While the prolactin/JAK2/STAT5 pathway is essential for alveologenesis and milk protein gene expression, the PI3K/Akt pathway is important for the synthesis of lactose and lipids in addition to glucose transport. The three principal components of the PI3K/Akt pathway are phosphatidylinositol 3-kinase (PI3K), its antagonist phosphatase and tensin homolog deleted on chromosome ten (Pten) and the serine/threonine kinase Akt/protein kinase B. There are three different Akt isoforms, Akt1, Akt2 and Akt3, which are encoded by different genes but share structural similarities.Citation13 The specific functions of these isoforms was revealed by the use of knockout mice which demonstrated that Akt1 and Akt2 have opposing functions in mammary gland since ablation of Akt1 results in delayed development and differentiation during pregnancy and lactation whereas loss of Akt2 promotes precocious differentiation. The consequences of Akt1 loss include a failure to upregulate a number of processes including glucose uptake, Glut1 surface localization, and lipid synthesis concomitantly with a failure to downregulate lipid catabolic enzymes.Citation14 Furthermore, Akt1-deficient glands involute more rapidly following lactation while this process is delayed in Akt2-deficient mammary glands. Akt3 seems to have a minor role.Citation15 Interestingly, only the Akt1 knockout phenotype is cell autonomous and furthermore, affects phosphorylation of STAT5a during late pregnancy and lactation. Thus Akt appears to regulate STAT5 signaling during pregnancy.
Although prolactin is secreted by the pituitary gland, it is also known to be produced elsewhere in the body where it may have autocrine effects although its regulation is not clearly defined. During early pregnancy in rodents, the levels of prolactin are low but rise toward the end of pregnancy. One of the genes regulated by prolactin is Elf5, a master regulator of the alveolar lineage and forced expression of Elf5 in virgin mice induced alveologenesis and milk production.Citation16
An exciting new insight into crosstalk between STAT5, prolactin and PI3K/Akt signaling was recently revealed in the study by Chen and coworkers.Citation7 Using a number of mouse models, they show that Akt signaling induces the production of prolactin by the mammary epithelium and that this induces, in an autocrine manner, STAT5 activation and terminal differentiation of mammary epithelium. First, the authors conditionally activated the PI3K pathway in virgin mice in two ways: (1) by expressing a doxycycline-inducible activated (myristoylated) allele of Akt in mammary epithelium under the control of the MMTV promoter and (2) by conditional deletion of the negative regulator of this pathway, Pten. In both situations, ducts became distended with lipid and protein and resembled alveoli in lactating tissue. Remarkably, milk proteins were expressed almost at lactation levels. Thus, activation of the PI3K pathway induces secretory differentiation in vivo in the absence of other signals.
It was then shown that activation of Akt, or deletion of Pten, results in phosphorylation of STAT5. This was confirmed to be due to Akt expression in the mammary epithelium by fat pad transplantation experiments of Akt1-expressing cells into wild-type fat pads. Thus, STAT5 is activated in a cell intrinsic manner.
The raises the obvious question—is Akt-induced differentiation dependent on STAT5? To address this, mice harboring hypomorphic alleles of STAT5A/B were crossed to MTB/tAkt1 transgenic animals. Although ducts were still somewhat distended in MTB/tAkt1;Stat5a/b−/− mice compared with MTB; Stat5a/b−/−, expression of milk proteins was almost completely abrogated. This was associated with much reduced levels of pSTAT5 (a truncated protein is expressed in these hypomorphic mutants). Similar results were obtained with deletion of the prolactin receptor and in in vitro studies. The authors then hypothesized that Akt could induce the expression of a locally-acting factor and demonstrated first that conditioned medium from explanted MTB-tAkt1 mammary tissue could induce STAT5 phosphorylation and then showed that prolactin expression is induced in virgin glands of Akt1-expressing or Pten deleted mice at the protein level but not at the mRNA level. Thus Akt can regulate autocrine prolactin levels post-transcriptionally. The failure to induce pSTAT5 in explanted mammary glands from MTB/tAkt1;Prl−/− mice, and the absence of autocrine prolactin in Akt1−/−;Akt2+/− mammary glands, provides convincing evidence that Akt1 induces autocrine prolactin. Interestingly, Elf5 is induced by Akt1 in virgin MTB/tAkt1;Stat5a/b−/− and MTB/tAkt1;Prl−/− mice even though pSTAT5 is not, suggesting that Akt1 is a transcriptional regulator of Elf5 independently of STAT5. The authors conclude that activation of the PI3K-Akt pathway in mammary gland is necessary and sufficient for the induction of autocrine prolactin, activation of STAT5 and terminal differentiation (i.e., milk production) even in the absence of pregnancy hormones.
One interesting outcome of this work is that the activation of Akt1 in virgin animals causes only the ductal cells to differentiate into milk-producing cells and alveologenesis is not induced. According to previous knockout studies, both STAT5 and PrlR are required for alveolar development, which leaves us with a conundrum: why has the activation of the Prl/JAK2/STAT5 pathway not resulted in lobuloalveolar development as well as differentiation?
Is it possible the mammary gland can distinguish between autocrine and endocrine sources of prolactin? This seems unlikely as the hormone is presumably identical regardless of point of origin. The authors propose that in late pregnancy STAT5 is activated by this autocrine source of prolactin as endocrine levels are very low at this stage. This could be tested by using implanted prolactin pellets in Akt1−/−/Akt2+/− mice during pregnancy. If milk production is rescued in these mice then any source of prolactin can have the same effect and autocrine prolactin is needed simply because there is insufficient circulating endocrine prolactin. However, regardless of whether the source of prolactin that stimulates differentiation and milk production is autocrine or endocrine, this does not explain why it sometimes induces alveologenesis and sometimes differentiation and milk production.
Could it be that the autocrine prolactin must impact on alveolar progenitors? The MTB/tAkt1 model used by Chen and colleagues appears to limit expression of the transgene to the luminal cell layer, as shown by co-expression with luminal marker cytokeratin (CK)8. A co-stain for a basal marker such as CK14 would confirm that this is indeed the case. Since the stem and progenitor cell populations are thought to reside predominantly in the basal layer then the effect of Akt1 activation on these cell types will not have been addressed by this investigation. Furthermore, not all cell populations are present in the virgin gland since alveolar cells arise during pregnancy and work from our laboratory has shown that the balance of alveolar cell populations during pregnancy is necessary for normal alveolar development.Citation17 As such, the development of a model with inducible Akt1 expression in the basal layer could prove illuminating as one could postulate that the activation of STAT5 in say a multipotent progenitor may stimulate alveolar development whereas activation in a ductal/alveolar luminal cell results in differentiation. It would be interesting to explore how Akt1 and STAT5 fit into the control of the balance of alveolar cell populations.
Alternatively, the absence of alveologenesis could be due to the absence of other signaling pathways or transcription factors normally present in the pregnant gland. The most notable absence is that of progesterone which has been shown to be required for alveologenesis and is present at high levels throughout pregnancy. Ideally one could add progesterone to the MTB/tAkt1 virgin animals and observe if alveologenesis would now take place. However, since the addition of progesterone alone does stimulate some alveologenesisCitation18 an enhanced effect may not be observed with the additional activation of Akt1.
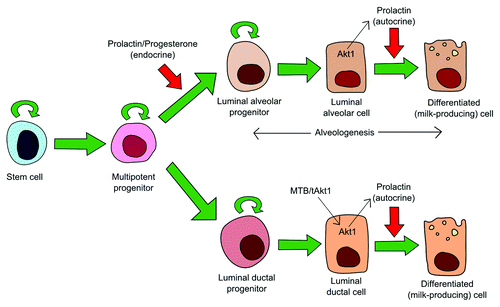
The data present here and in previous studies suggests that prolactin-induced STAT5 activation is necessary but not sufficient for alveolar formation but is necessary and sufficient for milk production. One potential model of prolactin/STAT5 action on the mammary gland, based on the work from Chen and coworkers, is outlined in . In summary, at the onset of pregnancy multipotent progenitors arise from the stem cell pool and prolactin and progesterone drive the formation of the alveolar progenitors that have activated STAT5 and thereby drive alveologenesis. Following this early pregnancy period, levels of endocrine prolactin diminish until shortly before birth. The presence of autocrine prolactin, as stimulated by activated Akt1, acts on alveolar and ductal luminal cells in later pregnancy to promote differentiation and milk protein production.
Figure 1. Model of dual role of prolactin/STAT5 in the mammary gland. Multipotent progenitors arise from the stem cell pool. These can generate a number of cell populations including ductal and alveolar progenitors. Alveolar progenitors appear during pregnancy and initiate alveologenesis. Evidence would suggest that progesterone and prolactin are required for the development of this population. Later in pregnancy, Akt1 stimulates the production of autocrine prolactin which activates STAT5 to promote the production of milk components and the terminal differentiation of luminal cells. The MTB/tAkt1 mice used in the study by Chen et al. 2012 drives the activation of Akt1 in luminal ductal cells resulting in their terminal differentiation but cannot give rise to alveologenesis as this would require prolactin to act instead on the multipotent progenitors.
Not only has this work elegantly and conclusively shown that the PI3K/Akt pathway induces autocrine prolactin production which is required for the initiation of lactation, but it has the additional merit of the use of genetically altered mouse models to study the activation of STAT5 by prolactin in vivo in the correct cellular environment so we can be confident that the data are biologically relevant. It also highlights the difficulties in interpreting and comparing data from models that use specific promoters to drive the tetracycline-dependent transactivator or Cre recombinase. How do we explain that inducible overexpression of Elf5 using MMTV-driven rtTA (MTB) mice results in precocious alveologenesisCitation16 while MTB driven overexpression of activated Akt1 (which induces expression of Elf5) results in differentiation of ducts but does not induce alveologenesis?
References
- Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, et al. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev 2009; 23:2382 - 7; http://dx.doi.org/10.1101/gad.1840109; PMID: 19833766
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 1997; 11:179 - 86; http://dx.doi.org/10.1101/gad.11.2.179; PMID: 9009201
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, et al. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 2004; 24:8037 - 47; http://dx.doi.org/10.1128/MCB.24.18.8037-8047.2004; PMID: 15340066
- Burdon TG, Maitland KA, Clark AJ, Wallace R, Watson CJ. Regulation of the sheep beta-lactoglobulin gene by lactogenic hormones is mediated by a transcription factor that binds an interferon-gamma activation site-related element. Mol Endocrinol 1994; 8:1528 - 36; http://dx.doi.org/10.1210/me.8.11.1528; PMID: 7877621
- Li S, Rosen JM. Nuclear factor I and mammary gland factor (STAT5) play a critical role in regulating rat whey acidic protein gene expression in transgenic mice. Mol Cell Biol 1995; 15:2063 - 70; PMID: 7891701
- Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: it’s not just about milk protein synthesis!. Breast Cancer Res 2007; 9:204; http://dx.doi.org/10.1186/bcr1653; PMID: 17338830
- Chen CC, Stairs DB, Boxer RB, Belka GK, Horseman ND, Alvarez JV, et al. Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways. Genes Dev 2012; 26:2154 - 68; http://dx.doi.org/10.1101/gad.197343.112; PMID: 23028142
- Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development 2008; 135:995 - 1003; http://dx.doi.org/10.1242/dev.005439; PMID: 18296651
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 2011; 479:189 - 93; http://dx.doi.org/10.1038/nature10573; PMID: 21983963
- Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol 2004; 24:5510 - 20; http://dx.doi.org/10.1128/MCB.24.12.5510-5520.2004; PMID: 15169911
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 1997; 16:6926 - 35; http://dx.doi.org/10.1093/emboj/16.23.6926; PMID: 9384572
- Ormandy CJ, Binart N, Kelly PA. Mammary gland development in prolactin receptor knockout mice. J Mammary Gland Biol Neoplasia 1997; 2:355 - 64; http://dx.doi.org/10.1023/A:1026395229025; PMID: 10935023
- Wickenden JA, Watson CJ. Key signalling nodes in mammary gland development and cancer. Signalling downstream of PI3 kinase in mammary epithelium: a play in 3 Akts. Breast Cancer Res 2010; 12:202; http://dx.doi.org/10.1186/bcr2558; PMID: 20398329
- Boxer RB, Stairs DB, Dugan KD, Notarfrancesco KL, Portocarrero CP, Keister BA, et al. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab 2006; 4:475 - 90; http://dx.doi.org/10.1016/j.cmet.2006.10.011; PMID: 17141631
- Maroulakou IG, Oemler W, Naber SP, Klebba I, Kuperwasser C, Tsichlis PN. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J Cell Physiol 2008; 217:468 - 77; http://dx.doi.org/10.1002/jcp.21518; PMID: 18561256
- Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev 2008; 22:581 - 6; http://dx.doi.org/10.1101/gad.1614608; PMID: 18316476
- Oliver CH, Khaled WT, Frend H, Nichols J, Watson CJ. The Stat6-regulated KRAB domain zinc finger protein Zfp157 regulates the balance of lineages in mammary glands and compensates for loss of Gata-3. Genes Dev 2012; 26:1086 - 97; http://dx.doi.org/10.1101/gad.184051.111; PMID: 22588720
- Fernandez-Valdivia R, Mukherjee A, Creighton CJ, Buser AC, DeMayo FJ, Edwards DP, et al. Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 2008; 149:6236 - 50; http://dx.doi.org/10.1210/en.2008-0768; PMID: 18687774