Abstract
The regulation of energy balance requires a complex system to homeostatically maintain the adult body at a precise set point. The central nervous system, particularly the hypothalamus, plays a key role in integrating a variety of signals that can relay information about the body’s energy stores. As part of this system, numerous cytokines and hormones contribute to the regulation of food intake and energy homeostasis. Cytokines, and some hormones, are known to act through JAK-STAT intracellular signaling pathways. The hormone leptin, which plays a vital role in appetite regulation, signals through the JAK-STAT pathway, and it is through this involvement that the JAK-STAT pathway has become an established component in the mechanisms regulating food intake within the body. Emerging research, however, is now showing that this involvement of JAK-STAT is not limited to its activation by leptin. Furthermore, while the JAK-STAT pathway may simply act to transmit the anorectic signal of circulating factors, this intracellular signaling pathway may also become impaired when normal regulation of energy balance is disrupted. Thus, altered JAK-STAT signaling may contribute to the breakdown of the normal homeostatic mechanisms maintaining body weight in obesity.
Introduction
The Janus kinase (JAK)-signal transducers and activators of transcription (STAT) intracellular signaling pathway is activated in response to a large number of cytokines, growth factors and hormones, and is involved in numerous functions within the body. This signaling pathway is stimulated by the interaction of ligands with their receptors, causing receptor activation and transphosphorylation of receptor-associated JAK molecules. This leads to the phosphorylation of tyrosine residues on the receptors and phosphorylation of downstream signaling molecules called STATs. STAT molecules are cytoplasmic proteins and currently seven different mammalian STAT genes have been identified, STAT1–4, 5A, 5B and 6. Once phosphorylated, STAT molecules form dimers and translocate to the nucleus where they modify gene transcription.
One of the many functions that the JAK-STAT pathway is involved in is energy homeostasis. This role for the JAK-STAT signaling pathway was discovered due to the classification of the receptor for the adipose derived hormone leptin as a member of the class I cytokine receptor superfamily.Citation1 Following this association, the ability of leptin to activate JAK-STAT signaling was investigated and in vitro studies indicated that leptin could stimulate the phosphorylation of STAT1, STAT3, STAT5 and STAT6.Citation2,Citation3 In vivo studies within the hypothalamus, an important target area for the regulation of energy balance, show that STAT3, and possibly STAT5, are the key STAT molecules involved in leptin-mediated satiety.Citation4-Citation7 For the purposes of this review, the discussion of the role of JAK-STAT signaling will mostly be focused on leptin-mediated regulation of energy balance. However, as the role of the JAK-STAT signaling pathway in the appetite suppressing effects of other hormones and cytokines is also emerging, this will also be included where possible. This review will begin with a brief overview of the key components in the regulation of energy balance then will focus on the specific involvement of the various STAT molecules.
The Regulation of Food Intake
Over a lifespan, an animal’s energy intake and expenditure remains relatively balanced and this equality is primarily due to the regulation of food intake. Through the use of parabiosis and lesion studies, it was proposed as early as the 1950s that body weight is maintained at a reasonably constant level by a signaling mechanism between the body’s fat stores and the brain.Citation8 This feedback loop was hypothesized to involve a peripherally produced factor that would relay information regarding fat stores to the hypothalamus, leading to adjustments in food intake to maintain body fat levels around a set point. The involvement of the hypothalamus in this process has long been known due to the changes in food intake and body weight following hypothalamic lesions.Citation9-Citation11 The control of appetite regulation in the hypothalamus involves a complex neuronal network including both orexigenic and anorectic neuropeptides. These neuropeptides are constantly being modulated through stimulation or inhibition by many stimuli to maintain appetite at appropriate levels for the current energy expenditure of the body and the hormone leptin is one of these key stimuli.
Leptin is an adipose-derived hormone that is secreted in proportion to size and number of adipocytes present in the body, and acts in the brain to decrease food intake and also increase metabolic rate. This creates a negative feedback loop in which body fat levels are maintained around a set point by regulating food intake and energy expenditure. Leptin is not the only factor involved in regulating energy balance but it is vital for normal energy homeostasis, as demonstrated by the extreme obesity and hyperphagia of the ob/ob mouse, which lacks functional leptin.Citation12
A number of leptin receptor (LEPR) isoforms exist due to alternative splicing of the LERP gene and LEPRb is the only isoform with full signal transduction capabilities due to the large intracellular domain.Citation13,Citation14 Leptin receptors have been detected in a wide range of tissues in the body, including liver, heart, kidneys, lungs, small intestine, testes, ovaries, spleen, pancreas, brain and adipose tissue.Citation14,Citation15 While other isoforms are the most predominant in peripheral tissues, LEPRb is most highly expressed in the central nervous system (CNS), specifically the hypothalamus.Citation14-Citation17 The db/db mouse has a mutation that results in abnormally spliced LEPRb mRNA leading to an absence of this receptor isoform.Citation3,Citation13,Citation14 The db/db mouse is obese and hyperphagic, similar to the ob/ob mouse indicating that LEPRb is the key isoform involved in energy balance.Citation12-Citation14 The conditional deletion of leptin receptors from the central nervous system (CNS) results in an obese phenotype with the same metabolic abnormalities of the ob/ob or db/db mouse, indicating the key site of leptin action in regulating energy balance is the CNS.Citation18 Also, db/db mice that have a transgenic rescue of the leptin receptor specifically in the brain are not obese, further demonstrating the central role of leptin in regulating energy balance.Citation19 As well as the hypothalamus, leptin acts in other brain areas, such as the brainstemCitation20 and the ventral tegmental area (VTA)Citation21,Citation22 to regulate food intake and metabolic rate.
STAT3 and the Regulation of Food Intake
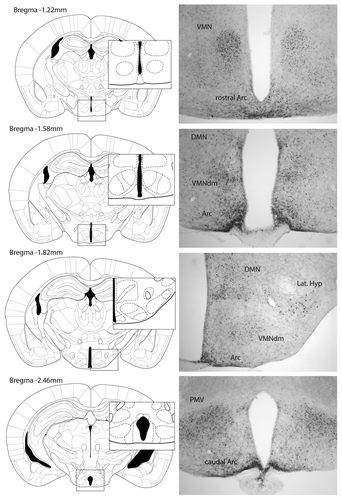
The involvement of STAT3 in the regulation of energy balance was identified due to the activation of this signaling molecule by leptin. Within the hypothalamus, leptin leads to the phosphorylation of STAT3 in areas involved in appetite regulation ().Citation6,Citation7,Citation20,Citation23 Leptin-induced phosphorylation of STAT3 is observed in other areas of the central nervous system that also contribute to the regulation of food intake, such as the VTACitation21,Citation22 and the brainstem.Citation20
Figure 1. Images show leptin-induced phospho-STAT3 immunohistochemistry in coronal sections of the hypothalamus of fasted female mice treated with an intraperitoneal injection of leptin (1 mg/kg BW).Citation67 Hypothalamic regions displaying leptin-induced phospho-STAT3 include the ventromedial nucleus (VMN), the dorsal medial area of the VMN (VMNdm), arcuate nucleus (Arc), dorsomedial nucleus (DMN), lateral hypothalamus (Lat. Hyp) and ventral premammillary nucleus (PMV).
The contribution of STAT3 in regulating food intake is clearly demonstrated in a number of transgenic mouse lines. STAT3 is widely expressed in the body, and STAT3 knockout mice are embryonic lethal thus preventing any physiological studies in these animals.Citation24 Therefore, tissue-specific conditional gene targeting approaches have been used to investigate the role of STAT3 in energy balance. Mice with a specific deletion of STAT3 from the CNS are obese and hyperphagic, demonstrating the necessity of neuronal STAT3 signaling in maintaining normal energy homeostasis.Citation25 Despite the hyperleptinemia in these transgenic mice, other leptin-induced signaling pathways do not compensate for the lack of leptin-induced STAT3 signaling,Citation25 further emphasizing the vital role of STAT3 in regulating food intake. Since this mouse is a conditional deletion of STAT3, it is possible the lack of STAT3 signaling induced by other cytokines or hormones contribute to the phenotype. The similarities in phenotype of this mouse and ob/ob or db/db mice, however, suggest that many, if not all, of the effects on food intake and body weight can be attributed to a lack of leptin-induced STAT3 signaling.Citation25
To investigate the role of STAT3 specifically in leptin signaling two transgenic mouse lines have been generated. Bates et al. (2003) developed the s/s mouse, in which the leptin receptor does not contain the Y1138 phosphorylation site that is required for leptin-induced STAT3 phosphorylation.Citation26 Due to the disrupted leptin receptor-STAT3 signaling, this mouse is hyperphagic and obese.Citation4 Another mouse generated to investigate leptin-STAT3 signaling has a specific deletion of STAT3 in leptin receptor containing neurons, and this mouse is also obese and hyperphagic.Citation27 In both this transgenic mouse and the s/s mouse, other functions of leptin such as linear growth and reproduction are not greatly affected, indicating that the key role of STAT3 in the function of leptin is the regulation of food intake and not these other functions of leptin.Citation4,Citation27 Furthermore, mice with a deletion of STAT3 from either neuropeptide Y(NPY)/agouti-related protein (Agrp) or pro-opiomelanocortin (POMC) neurons, two of the most well studied hypothalamic neuron populations involved in appetite regulation, are only slightly hyperphagic and mildly obese.Citation28,Citation29 Given the hyperphagia and obesity observed in mice with STAT3 deleted from leptin receptor containing neurons, the mild phenotype of STAT3 deletion from POMC or AgRP neurons suggests that the satiety response to leptin involves coordinated STAT3 signaling in a number of leptin-responsive neuronal populations.Citation30
Transgenic mouse models have provided a large amount of evidence indicating STAT3 is vital to the regulation of energy balance, particularly in the functioning of leptin. To approach this question in a different way, a cell-permeable phosphopeptide has been used to block leptin-mediated STAT3 activity in vivo and the acute satiety effects of leptin were shown to require functional STAT3 signaling,Citation31 confirming the conclusions reached using transgenic mouse models.
STAT3 is activated by numerous cytokines and many cytokines can influence food intake. Hence STAT3 signaling may be involved in the suppression of food intake during an immune response.Citation32 The mechanisms by which various cytokines influence food intake and the involvement of the JAK-STAT signaling pathway in this function are not well characterized. Two examples of cytokines that activate hypothalamic STAT3 are tumor necrosis factor α (TNF-α)Citation33 and ciliary neurotrophic factor (CNTF),Citation34 both of which can suppress food intake.Citation32,Citation35,Citation36 TNF-α can act synergistically with leptin to increase hypothalamic levels of STAT3 phosphorylation and has been suggested as a possible modulator of the anorectic effects of leptin.Citation33 The satiety effects of CNTF appear to be mediated through mechanisms that do not involve interactions with first-order leptin responsive neurons, as transgenic mice with a conditional deletion of a subunit of the CNTF-receptor in leptin receptor containing neurons, there is no change in the anorectic response to CNTF-receptor activation.Citation37 Interestingly, in these mice with deletions of CNTF receptor in leptin-receptor containing neurons, pSTAT3 was greatly reduced despite a normal satiety to a CNTF agonist,Citation37 indicating that the effect of CNTF on food intake may not require STAT3 activation. Further work is required to determine what role STAT3, or any of the other STAT molecules may play in the regulation of food intake by inflammatory cytokines.
Estrogen, the gonadal steroid involved in reproduction, can also influence appetite and fat mass. Infusion of estrogen into hypothalamic areas involved in appetite regulation suppresses food intake.Citation38,Citation39 It has been proposed that estrogen mediates its satiety effects by acting through STAT3.Citation25,Citation40 Estrogen administration can increase the levels of pSTAT3 in the hypothalamus of mice independent of leptin and in mice with a conditional deletion of STAT3 from the CNS, chronic estrogen treatment does not suppress food intake.Citation40 Estrogen can modulate the sensitivity to leptinCitation41 and it has been speculated that this involves the interaction of estrogen and leptin signaling at the level of STAT3 phosphorylation in the hypothalamus,Citation42 but as yet there is no direct evidence for this.
Transcriptional Control of Target Genes by STAT3
The JAK-STAT pathway provides an intracellular pathway that can relay an extracellular signal into a transcriptional response. The key function of STAT molecules upon phosphorylation is to dimerize and translocate to the cell nucleus where they bind to specific regulatory sequences to either activate or repress transcription of target genes. The transcriptional targets involved in appetite regulation of STAT3 include suppressor of cytokine signaling 3 (SOCS3), POMC and thyrotropin-releasing hormone (TRH).
SOCS molecules are one of the known families of immediate-early genes that are transcriptionally regulated by STATs.Citation43 SOCS molecules are induced by cytokines and act to decrease cytokine signaling via an intracellular negative feedback loop. Within the hypothalamus, leptin-induced STAT3 phosphorylation and the induction of SOCS3 mRNA expression overlap,Citation20,Citation44 giving anatomical support for the regulation of SOCS3 by leptin-mediated STAT3 signaling. The SOCS3 promoter has been shown to contain two STAT binding sitesCitation45 and the phosphorylation of Y1138 on the leptin receptor is required for the leptin-induced SOCS3 mRNA expression, implicating STAT3 as the mediator of leptin-induced SOCS3 gene transcription.Citation26 Furthermore, leptin-induced activation of hypothalamic STAT3 leads to the interaction of STAT3 with the proximal SOCS3 promoter, and this interaction corresponds to the duration of time that STAT3 remains phosphorylated after leptin treatment.Citation46 Increases in SOCS3 expression inhibit further leptin-STAT3 signaling.Citation47 Therefore, one mechanism by which STAT3 regulates food intake is by switching off its own signaling in first-order leptin target neurons by stimulating SOCS3 expression. Indeed, in mice with a SOCS3 haploinsufficiencyCitation48 or neuron-specific deletion of SOCS3Citation49 there is an increase in leptin sensitivity and leptin-induced phosphorylation of STAT3 is more persistent than in controls indicating that the normal regulation of SOCS3 by STAT3 is required for maintaining energy homeostasis.
STAT3 is likely to regulate expression of anorectic and orexigenic peptides, which promote changes in satiety. Within the hypothalamus, the POMC neurons are a key population that regulates appetite and energy homeostasis. The POMC gene encodes the POMC precursor polypeptide and the anorectic effects of POMC neurons are mediated through the cleavage product α-melanocyte stimulating hormone. This melanocortin system is a vital downstream pathway that mediated the anorectic responses to leptin.Citation50 In vitro, STAT3 is able to stimulate POMC transcriptionCitation20,Citation51-Citation53 and leptin stimulation of POMC mRNA is dependent on STAT3 availability.Citation20 The region of the proximal POMC gene promoter that is required for STAT3-depend activation by leptin does not bind STAT, suggesting that STAT3 may act indirectly via interaction with other transcription factors to regulate the POMC gene.Citation20 In vitro studies have showed that STAT3 regulation of POMC requires an SP1-binding site in the POMC promoter and that leptin treatment can lead to the interaction and binding of SP1 and phosho-STAT3.Citation53 Yang et al. (2009) have proposed that STAT3 stimulates POMC expression by a mechanism involving STAT3 interaction with a SP1-POMC promoter complex instead of a direct STAT3-POMC promoter interaction.Citation53
In vivo STAT3 phosphorylation and accumulation in the nucleus is seen in POMC neurons after leptin treatment.Citation20,Citation23 In the obese s/s mouse, in which leptin-STAT3 signaling is disrupted, hypothalamic POMC mRNA expression is reduced, demonstrating that STAT3 activation by leptin is required for leptin regulation of POMC.Citation4 Using β-endorphin as a marker of POMC positive cells, POMC levels were greatly reduced in mice with a deletion of STAT3 in the CNS supporting the involvement of STAT3 in regulating POMC levels.Citation25 In conclusion, the POMC gene is one of the target transcripts of STAT3 activation in the hypothalamus, and this stimulation of POMC mRNA is likely to be one of the key functions of STAT3 in the regulation of energy balance.
A second neuronal population, the NPY/Agrp neurons located in the arcuate nucleus, plays a vital role in the regulation of energy balance. Both NPY and Agrp are orexigenic and are negatively regulated by leptin.Citation54-Citation58 Given the important role of these first-order leptin target neurons in regulating energy homeostasis the question of whether STAT3 mediates their regulation by leptin arises. In the s/s mouse, NPY levels are no different to controls indicating that leptin-STAT3 signaling is unlikely to be involved in regulating NPY mRNA.Citation4 Agrp mRNA levels are significantly increased in s/s mice compared with controls but significantly less than db/db mice, suggesting that leptin-STAT3 may contribute, at least partially, to the regulation of Agrp mRNA by leptin.Citation4 Deletion of STAT3 from Agrp neurons, however, does not significantly alter Agrp mRNA levels, suggesting that STAT3 does not contribute greatly in the regulation of Agrp mRNA.Citation28,Citation59 In mice with a deletion of STAT3 in leptin receptor containing neurons, Agrp and NPY mRNA are only increased at an age of 10 weeks, once obesity is already present while total brain STAT3 deletion leads to increases in NPY and Agrp also when obesity is already apparent.Citation25,Citation27 While, these results may suggest that STAT3 is required for leptin mediated suppression in NPY and Agrp mRNA it has been suggested that these changes in mRNA expression maybe secondary effects of the obesity observed in these transgenic animals and not directly due to the specific deletions of STAT3.Citation27 In support of the latter conclusion, blocking other leptin-induced signaling pathways with antagonists prevents the regulation of Agrp and NPY mRNA by leptin despite normal leptin-induced STAT3 signaling.Citation60 Thus leading to the conclusion that leptin-induce STAT3 is insufficient to cause leptin-dependent suppression of NPY and Agrp mRNA.
Thyrotropin-releasing (TRH) hormone is another transcriptional target of leptin-mediated STAT3 activation. During fasting in rodents, metabolic rate is reduced by mechanisms involving a decrease in thyroid hormone levels.Citation61 This is achieved by a suppression of TRH in the paraventricular nucleus of the hypothalamus (PVN). TRH is a hypothalamic peptide that is essential for the normal production of thyroid-stimulating hormone in the pituitary and thyroid hormones in the thyroid gland.Citation62 Leptin is thought to play both indirect and direct roles in the regulation of TRH in the PVN. The TRH promoter has a STAT3 binding siteCitation63 and leptin treatment leads to the interaction of STAT3 with the TRH promoter region along with an increase in TRH mRNA expression.Citation46 Thus it is likely that the TRH gene is regulated by STAT3 and while it is not directly involved in food intake, the role of TRH in modulating metabolic rate demonstrates that STAT3 contributes to other functions, alongside appetite, that maintain energy homeostasis.
STAT5 and the Regulation of Food Intake
Transgenic mice with a conditional deletion of STAT5 (both STAT5A and STAT5B) in the CNS, develop severe obesity and are hyperphagic indicating that STAT5 signaling in the brain is required for the normal regulation of energy balance in the body.Citation64 These obese mice show no alterations in the expression levels of POMC, NPY and Agrp mRNA in the hypothalamus and this lack of change in mRNA expression could be a secondary effect of the obesity or that it could indicate the contribution of STAT5 in regulating energy balance within the brain does not involve the transcriptional regulation of these known anorectic and orexigenic peptides.Citation64 Further work is required to determine if STAT5 is involved in the regulation of these neuropeptides.
STAT5 signaling is activated by numerous factors in the body and which of these factors induces STAT5 mediated regulation of energy homeostasis has yet to be confirmed. In vitro, leptin has been shown to activate STAT5Citation2,Citation3,Citation5,Citation65 but there is conflicting data from in vivo studies. Using immunohistochemistry, increased translocation of STAT5 to the nucleus has been observed in hypothalamic cells in response to leptin.Citation66 Using western blot analysis, increased levels of phospho-STAT5 in the hypothalamus following leptin treatment has been shown by some,Citation5 but not others.Citation6,Citation7,Citation33 More recently, using immunohistochemistry to detect phosho-STAT5 positive cells, leptin treatment had no effect on the number of cells positive for phospho-STAT5 within the arcuate nucleus, ventromedial nucleus or the paraventricular nucleus of the hypothalamus.Citation67 It is possible that leptin acts through STAT5 in certain conditions, or in other areas of the brain. However, this study demonstrates a lack of leptin signaling through STAT5 in key hypothalamic areas involved in appetite regulation and thus it seems unlikely that STAT5 plays a major role in mediating leptin signal transduction.
STAT5 has been suggested to mediate the anorectic effects of granulocyte macrophage colony-stimulating factor (GM-CSF).Citation64 While GM-CSF is primarily thought of as a proinflammatory cytokine, it can also regulate food intake. Central administration of GM-CSF, but not peripheral, decreases food intake and bodyweight in rats and the receptor for this cytokine is located, among other areas in the brain, in the arcuate nucleus of the hypothalamus.Citation68 Central GM-CSF administration to rats leads to an increase in nuclear translocation of STAT5 in the hypothalamusCitation64 and GM-CSF knockout mice have increased body weight and body fat.Citation68 Furthermore, while administration of GM-CSF to mice decreases food intake, this effect is absent in mice with a CNS-specific STAT5 deletion, indicating that STAT5 in the CNS is required for the anorectic effects of GM-CSF.Citation64 Given the caveat that the comparison is between two different strains of mice, Lee et al. (2005) suggested that the greater degree of weight gain and increase in adiposity of mice with a CNS specific STAT5 deletion compared with the GM-CSF knockout mouse indicates that other extracellular signals, along with GM-CSF, are likely to be involved in regulating food intake and energy balance via STAT5 activation.Citation64,Citation68
Other STAT Molecules and Regulation of Food Intake
STAT1 is another molecule that was indicated in cultured cells to potentially be activated by leptin but in vivo data would suggest that leptin does not act through this signaling molecule.Citation7,Citation33 Moreover, STAT1 knockout mice have similar body weights as control mice and do not appear to have any obvious feeding or body weight dysregulation,Citation69 suggesting that STAT1 does not play a role in the regulation of energy balance.
STATs and Alterations in Energy Homeostasis
While energy balance is normally tightly regulated in the body, there are situations, both pathological and physiological, when this system is altered resulting in increased appetite and body weight. Two examples of this are obesity and pregnancy. In both these states, disruption in STAT3 signaling has been implicated in the mechanisms underlying the change in the sensitivity to the hormone leptin.
Obesity is commonly associated with hyperphagia despite increased levels of leptin, suggestive of a leptin-resistant state. Hence, during obesity there is a failure of key hypothalamic neural circuits to respond appropriately to the satiety signal of leptin. Since the phosphorylation of STAT3 is a commonly used marker of leptin activation of target neurons, in many models of obesity leptin resistance has been demonstrated by an impaired ability of leptin to induce STAT3 phosphorylation.Citation70-Citation74 The ability of leptin to induce JAK2 phosphorylation in these models of leptin resistance has not been characterized. The disruption in JAK-STAT3 signaling is likely to be an important molecular mechanism involved in leptin resistance and contributing to the dysregulation of food intake.Citation30
It is yet to be established whether impaired leptin-STAT3 signaling is a cause or consequence of leptin resistance and obesity.Citation75 To investigate this, leptin responsiveness has been examined in mice following different durations on a high fat diet. In one study, after 4 weeks on the high fat diet, mice had altered metabolism and increased leptin concentrations, suggesting the early stages of leptin resistance, yet hypothalamic leptin-induced phospho-STAT3 was unchanged.Citation70 After 15 weeks on the diet, mice had impaired leptin-induced phospho-STAT3 indicating that perhaps altered STAT3 signaling is not an initial cause of the metabolic changes associated with obesity and leptin resistance.Citation70 However, others who examined mice after only 6 d on a high fat diet, the initial time point when differences in body weight became apparent, demonstrated that leptin-induced phospho-STAT3 was already impaired.Citation72 Currently, the role of impaired STAT3 activation in development of obesity and leptin resistance remains unresolved. Nevertheless, impaired leptin-STAT3 signaling contributes to the dysfunction in the regulation of energy balance observed in obesity and leptin resistance.
The mechanisms underlying impaired leptin-STAT3 signaling during obesity and leptin resistance are yet to be fully established. Negative regulators of the JAK-STAT pathway, SOCS3 and PTP1B, have been shown to be increased in the hypothalamus during obesity, suggesting that these inhibitors of STAT3 signaling may prevent normal STAT3 signaling leading to leptin insensitivity.Citation72,Citation76-Citation78 It is unlikely that a downregulation of hypothalamic leptin receptors is an underlying cause of impaired leptin-induced phospho-STAT3 in obesity as many studies have shown either no change or an upregulation in leptin receptor expression.Citation70,Citation72,Citation79-Citation83 Impaired transcriptional activity of STAT3 due to increased levels of FOX01,Citation84 a transcription factor that can antagonize STAT3 transcriptional activity,Citation51-Citation53 also has been suggested as a mechanism that could contribute to leptin resistance associated with obesity.Citation53
Currently there is little evidence for any genetic or epigenetic mutation in the STAT3 gene that might relate to the development of leptin resistance or obesity. However, mutations in other signaling molecules that modulate JAK-STAT signaling could result in impaired leptin-induced JAK-STAT signaling. SH2B1 is an adaptor protein that can positivity modulate leptin signaling through its interaction with JAK2.Citation85-Citation87 In humans, common variants in or near the SH2B1 gene have been associated with obesity,Citation88,Citation89 and loss of function mutations of the SH2B1 gene are associated with early onset obesity.Citation90 Mice lacking SH2B1 develop hyperphagia and obesity, and have impaired hypothalamic leptin-induced phospho-STAT3.Citation87 Therefore it is likely that impaired JAK-STAT3 signaling contributes to the genetic factors that lead to obesity and leptin resistance, however, given the current data available,Citation88,Citation89,Citation91 this contribution is more likely to be through mutations in genes for associated signaling molecules and not the STAT3 gene or JAK2 gene themselves.
Unlike obesity, during pregnancy increases in food intake and body weight are beneficial, as the maternal body needs to supply the growing conceptus with all its energy demands and prepare for the energetic demands of lactation. The maternal brain undergoes adaptations in the systems that regulate energy balance to deal with these increased energy demands. Pregnancy is associated with a state of leptin resistance, so that food intake can increase despite increasing levels of body fat and hence hyperleptinemia.Citation23,Citation67 During pregnancy the ability of leptin to activate STAT3 is impaired in the ventromedial nucleus of the hypothalamus (VMN).Citation23,Citation67,Citation92 LEPRb mRNA is decreased in the VMN during pregnancy in the rat hence this reduction may underlie the decreased ability of leptin to activate STAT at this time.Citation92 Furthermore, it has been proposed that the reduced ability of leptin to phosphorylate STAT3 is due to the high levels of prolactin and its homolog, placental lactogen, during pregnancy.Citation93,Citation94 Chronic prolactin treatment in female rats leads to a suppression of leptin-induced STAT3 phosphorylation,Citation95 and psuedopregnant rats chronically treated with prolactin and progesterone to mimic pregnancy also become leptin-resistant.Citation96 How prolactin might interact with leptin-STAT3 signaling remains unknown. Although, it is unlikely that there is a direct action of prolactin on leptin responsive neurons, either by directly modulating leptin receptor expression or inducing SOCS3 expression, as there is little evidence for colocalization of prolactin receptors and leptin receptors.Citation94
Conclusion
STAT3 and STAT5 play key roles in the regulation of food intake and energy homeostasis, and the use of transgenic mice has been instrumental in demonstrating this role. Further studies are required to determine the transcriptional targets of the STATs that influence food intake and to explore the role of STAT5 in regulating energy balance, including the extracellular signals that mediated this function of STAT5. Furthermore, impaired STAT signaling is associated with obesity in many animal models and is likely to contribute to the breakdown of the normal homeostatic mechanisms maintaining body weight in obesity. Thus, increased understanding of the role of STAT molecules in the regulation of energy balance will be beneficial to investigating the mechanisms underlying disrupted energy homeostasis in obesity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995; 83:1263 - 71; http://dx.doi.org/10.1016/0092-8674(95)90151-5; PMID: 8548812
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A 1996; 93:8374 - 8; http://dx.doi.org/10.1073/pnas.93.16.8374; PMID: 8710878
- Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A 1996; 93:6231 - 5; http://dx.doi.org/10.1073/pnas.93.13.6231; PMID: 8692797
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003; 421:856 - 9; http://dx.doi.org/10.1038/nature01388; PMID: 12594516
- Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Münzberg H, Myers MG Jr.. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem 2007; 282:31019 - 27; http://dx.doi.org/10.1074/jbc.M702838200; PMID: 17726024
- McCowen KC, Chow JC, Smith RJ. Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology 1998; 139:4442 - 7; http://dx.doi.org/10.1210/en.139.11.4442; PMID: 9794450
- Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr., Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 1996; 14:95 - 7; http://dx.doi.org/10.1038/ng0996-95; PMID: 8782827
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 1953; 140:578 - 96; http://dx.doi.org/10.1098/rspb.1953.0009; PMID: 13027283
- Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med 1951; 77:323 - 4; PMID: 14854036
- Bray GA, York DA. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev 1979; 59:719 - 809; PMID: 379887
- Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec 1940; 78:149 - 72; http://dx.doi.org/10.1002/ar.1090780203
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372:425 - 32; http://dx.doi.org/10.1038/372425a0; PMID: 7984236
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996; 84:491 - 5; http://dx.doi.org/10.1016/S0092-8674(00)81294-5; PMID: 8608603
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996; 379:632 - 5; http://dx.doi.org/10.1038/379632a0; PMID: 8628397
- Chen SC, Kochan JP, Campfield LA, Burn P, Smeyne RJ. Splice variants of the OB receptor gene are differentially expressed in brain and peripheral tissues of mice. J Recept Signal Transduct Res 1999; 19:245 - 66; http://dx.doi.org/10.3109/10799899909036649; PMID: 10071762
- Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 2010; 518:459 - 76; http://dx.doi.org/10.1002/cne.22219; PMID: 20017211
- Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 1998; 395:535 - 47; http://dx.doi.org/10.1002/(SICI)1096-9861(19980615)395:4<535::AID-CNE9>3.0.CO;2-2; PMID: 9619505
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 2001; 108:1113 - 21; PMID: 11602618
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 2005; 115:3484 - 93; http://dx.doi.org/10.1172/JCI24059; PMID: 16284652
- Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 2003; 144:2121 - 31; http://dx.doi.org/10.1210/en.2002-221037; PMID: 12697721
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006; 51:811 - 22; http://dx.doi.org/10.1016/j.neuron.2006.09.006; PMID: 16982425
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006; 51:801 - 10; http://dx.doi.org/10.1016/j.neuron.2006.08.023; PMID: 16982424
- Ladyman SR, Grattan DR. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 2004; 145:3704 - 11; http://dx.doi.org/10.1210/en.2004-0338; PMID: 15142988
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 1999; 10:39 - 49; http://dx.doi.org/10.1016/S1074-7613(00)80005-9; PMID: 10023769
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A 2004; 101:4661 - 6; http://dx.doi.org/10.1073/pnas.0303992101; PMID: 15070774
- Banks AS, Davis SM, Bates SH, Myers MG Jr.. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 2000; 275:14563 - 72; http://dx.doi.org/10.1074/jbc.275.19.14563; PMID: 10799542
- Piper ML, Unger EK, Myers MG Jr., Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol 2008; 22:751 - 9; http://dx.doi.org/10.1210/me.2007-0389; PMID: 18096691
- Kaelin CB, Gong L, Xu AW, Yao F, Hockman K, Morton GJ, et al. Signal transducer and activator of transcription (stat) binding sites but not stat3 are required for fasting-induced transcription of agouti-related protein messenger ribonucleic acid. Mol Endocrinol 2006; 20:2591 - 602; http://dx.doi.org/10.1210/me.2006-0107; PMID: 16709597
- Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 2007; 148:72 - 80; http://dx.doi.org/10.1210/en.2006-1119; PMID: 17023536
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 2009; 297:E1247 - 59; http://dx.doi.org/10.1152/ajpendo.00274.2009; PMID: 19724019
- Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr., Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 2006; 4:49 - 60; http://dx.doi.org/10.1016/j.cmet.2006.04.014; PMID: 16814732
- Buchanan JB, Johnson RW. Regulation of food intake by inflammatory cytokines in the brain. Neuroendocrinology 2007; 86:183 - 90; http://dx.doi.org/10.1159/000108280; PMID: 17823502
- Rizk NM, Stammsen D, Preibisch G, Eckel J. Leptin and tumor necrosis factor-alpha induce the tyrosine phosphorylation of signal transducer and activator of transcription proteins in the hypothalamus of normal rats in vivo. Endocrinology 2001; 142:3027 - 32; http://dx.doi.org/10.1210/en.142.7.3027; PMID: 11416024
- Lambert PD, Anderson KD, Sleeman MW, Wong V, Tan J, Hijarunguru A, et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci U S A 2001; 98:4652 - 7; http://dx.doi.org/10.1073/pnas.061034298; PMID: 11259650
- Fantino M, Wieteska L. Evidence for a direct central anorectic effect of tumor-necrosis-factor-alpha in the rat. Physiol Behav 1993; 53:477 - 83; http://dx.doi.org/10.1016/0031-9384(93)90141-2; PMID: 8451312
- Plata-Salamán CR, Vasselli JR, Sonti G. Differential responsiveness of obese (fa/fa) and lean (Fa/Fa) Zucker rats to cytokine-induced anorexia. Obes Res 1997; 5:36 - 42; http://dx.doi.org/10.1002/j.1550-8528.1997.tb00281.x; PMID: 9061714
- Stefater MA, MacLennan AJ, Lee N, Patterson CM, Haller A, Sorrell J, et al. The anorectic effect of CNTF does not require action in leptin-responsive neurons. Endocrinology 2012; 153:2647 - 54; http://dx.doi.org/10.1210/en.2012-1024; PMID: 22518062
- Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res 1984; 322:41 - 8; http://dx.doi.org/10.1016/0006-8993(84)91178-8; PMID: 6518373
- Palmer K, Gray JM. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav 1986; 37:187 - 9; http://dx.doi.org/10.1016/0031-9384(86)90404-X; PMID: 3737718
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 2007; 13:89 - 94; http://dx.doi.org/10.1038/nm1525; PMID: 17195839
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006; 55:978 - 87; http://dx.doi.org/10.2337/diabetes.55.04.06.db05-1339; PMID: 16567519
- Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 2008; 294:E817 - 26; http://dx.doi.org/10.1152/ajpendo.00733.2007; PMID: 18334610
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature 1997; 387:917 - 21; http://dx.doi.org/10.1038/43206; PMID: 9202125
- Hübschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci 2001; 21:2413 - 24; PMID: 11264315
- Auernhammer CJ, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci U S A 1999; 96:6964 - 9; http://dx.doi.org/10.1073/pnas.96.12.6964; PMID: 10359822
- Guo F, Bakal K, Minokoshi Y, Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology 2004; 145:2221 - 7; http://dx.doi.org/10.1210/en.2003-1312; PMID: 14764630
- Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 1999; 274:30059 - 65; http://dx.doi.org/10.1074/jbc.274.42.30059; PMID: 10514492
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 2004; 10:734 - 8; http://dx.doi.org/10.1038/nm1072; PMID: 15220914
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 2004; 10:739 - 43; http://dx.doi.org/10.1038/nm1071; PMID: 15208705
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 1999; 21:119 - 22; http://dx.doi.org/10.1038/5070; PMID: 9916804
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci 2006; 9:901 - 6; http://dx.doi.org/10.1038/nn1731; PMID: 16783365
- Kitamura T, Feng Y, Kitamura YI, Chua SC Jr., Xu AW, Barsh GS, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 2006; 12:534 - 40; http://dx.doi.org/10.1038/nm1392; PMID: 16604086
- Yang G, Lim CY, Li C, Xiao X, Radda GK, Li C, et al. FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. J Biol Chem 2009; 284:3719 - 27; http://dx.doi.org/10.1074/jbc.M804965200; PMID: 19049975
- Korner J, Savontaus E, Chua SC Jr., Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 2001; 13:959 - 66; http://dx.doi.org/10.1046/j.1365-2826.2001.00716.x; PMID: 11737554
- Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 1999; 140:4551 - 7; http://dx.doi.org/10.1210/en.140.10.4551; PMID: 10499510
- Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology 1998; 139:2629 - 35; http://dx.doi.org/10.1210/en.139.5.2629; PMID: 9564880
- Swart I, Jahng JW, Overton JM, Houpt TA. Hypothalamic NPY, AGRP, and POMC mRNA responses to leptin and refeeding in mice. Am J Physiol Regul Integr Comp Physiol 2002; 283:R1020 - 6; PMID: 12376393
- Wilson BD, Ollmann MM, Barsh GS. The role of agouti-related protein in regulating body weight. Mol Med Today 1999; 5:250 - 6; http://dx.doi.org/10.1016/S1357-4310(99)01471-9; PMID: 10366820
- Gong L, Yao F, Hockman K, Heng HH, Morton GJ, Takeda K, et al. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology 2008; 149:3346 - 54; http://dx.doi.org/10.1210/en.2007-0945; PMID: 18403487
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 2005; 289:E1051 - 7; http://dx.doi.org/10.1152/ajpendo.00094.2005; PMID: 16046456
- Blake NG, Eckland DJ, Foster OJ, Lightman SL. Inhibition of hypothalamic thyrotropin-releasing hormone messenger ribonucleic acid during food deprivation. Endocrinology 1991; 129:2714 - 8; http://dx.doi.org/10.1210/endo-129-5-2714; PMID: 1935800
- Yamada M, Saga Y, Shibusawa N, Hirato J, Murakami M, Iwasaki T, et al. Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc Natl Acad Sci U S A 1997; 94:10862 - 7; http://dx.doi.org/10.1073/pnas.94.20.10862; PMID: 9380725
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjøorbaek C, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 2001; 107:111 - 20; http://dx.doi.org/10.1172/JCI10741; PMID: 11134186
- Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, et al. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One 2008; 3:e1639; http://dx.doi.org/10.1371/journal.pone.0001639; PMID: 18286195
- Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, et al. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J 2005; 272:109 - 19; http://dx.doi.org/10.1111/j.1432-1033.2004.04391.x; PMID: 15634336
- Mütze J, Roth J, Gerstberger R, Hübschle T. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett 2007; 417:286 - 91; http://dx.doi.org/10.1016/j.neulet.2007.02.074; PMID: 17353091
- Ladyman SR, Fieldwick DM, Grattan DR. Suppression of leptin-induced hypothalamic JAK/STAT signalling and feeding response during pregnancy in the mouse. Reproduction 2012; 144:83 - 90; http://dx.doi.org/10.1530/REP-12-0112; PMID: 22580369
- Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, et al. GM-CSF action in the CNS decreases food intake and body weight. J Clin Invest 2005; 115:3035 - 44; http://dx.doi.org/10.1172/JCI25681; PMID: 16276414
- Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 1996; 84:431 - 42; http://dx.doi.org/10.1016/S0092-8674(00)81288-X; PMID: 8608597
- El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 2000; 105:1827 - 32; http://dx.doi.org/10.1172/JCI9842; PMID: 10862798
- Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 2004; 286:R143 - 50; http://dx.doi.org/10.1152/ajpregu.00393.2003; PMID: 12958061
- Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004; 145:4880 - 9; http://dx.doi.org/10.1210/en.2004-0726; PMID: 15271881
- Scarpace PJ, Matheny M, Tümer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 2001; 104:1111 - 7; http://dx.doi.org/10.1016/S0306-4522(01)00142-7; PMID: 11457594
- Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 2003; 285:R1011 - 20; PMID: 14557234
- Myers MG Jr., Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010; 21:643 - 51; http://dx.doi.org/10.1016/j.tem.2010.08.002; PMID: 20846876
- Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1998; 1:619 - 25; http://dx.doi.org/10.1016/S1097-2765(00)80062-3; PMID: 9660946
- Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, et al. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 2007; 148:433 - 40; http://dx.doi.org/10.1210/en.2006-0672; PMID: 17038557
- White CL, Whittington A, Barnes MJ, Wang Z, Bray GA, Morrison CD. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab 2009; 296:E291 - 9; http://dx.doi.org/10.1152/ajpendo.90513.2008; PMID: 19017730
- Gamber KM, Huo L, Ha S, Hairston JE, Greeley S, Bjørbæk C. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS One 2012; 7:e30485; http://dx.doi.org/10.1371/journal.pone.0030485; PMID: 22276206
- Huang XF, Xin X, McLennan P, Storlien L. Role of fat amount and type in ameliorating diet-induced obesity: insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes Metab 2004; 6:35 - 44; http://dx.doi.org/10.1111/j.1463-1326.2004.00312.x; PMID: 14686961
- Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res 2000; 875:89 - 95; http://dx.doi.org/10.1016/S0006-8993(00)02580-4; PMID: 10967302
- Peiser C, McGregor GP, Lang RE. Leptin receptor expression and suppressor of cytokine signaling transcript levels in high-fat-fed rats. Life Sci 2000; 67:2971 - 81; http://dx.doi.org/10.1016/S0024-3205(00)00884-5; PMID: 11133009
- Sahu A, Nguyen L, O’Doherty RM. Nutritional regulation of hypothalamic leptin receptor gene expression is defective in diet-induced obesity. J Neuroendocrinol 2002; 14:887 - 93; http://dx.doi.org/10.1046/j.1365-2826.2002.00856.x; PMID: 12421342
- Ropelle ER, Pauli JR, Prada P, Cintra DE, Rocha GZ, Moraes JC, et al. Inhibition of hypothalamic Foxo1 expression reduced food intake in diet-induced obesity rats. J Physiol 2009; 587:2341 - 51; http://dx.doi.org/10.1113/jphysiol.2009.170050; PMID: 19332486
- Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem 2004; 279:43684 - 91; http://dx.doi.org/10.1074/jbc.M408495200; PMID: 15316008
- Li Z, Zhou Y, Carter-Su C, Myers MG Jr., Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol 2007; 21:2270 - 81; http://dx.doi.org/10.1210/me.2007-0111; PMID: 17565041
- Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2005; 2:95 - 104; http://dx.doi.org/10.1016/j.cmet.2005.07.004; PMID: 16098827
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 2009; 41:18 - 24; http://dx.doi.org/10.1038/ng.274; PMID: 19079260
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al, Wellcome Trust Case Control Consortium, Genetic Investigation of ANthropometric Traits Consortium. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009; 41:25 - 34; http://dx.doi.org/10.1038/ng.287; PMID: 19079261
- Doche ME, Bochukova EG, Su HW, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest 2012; 122:4732 - 6; http://dx.doi.org/10.1172/JCI62696; PMID: 23160192
- Sandholt CH, Hansen T, Pedersen O. Beyond the fourth wave of genome-wide obesity association studies. Nutr Diabetes 2012; 2:e37; http://dx.doi.org/10.1038/nutd.2012.9; PMID: 23168490
- Ladyman SR, Grattan DR. Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology 2005; 146:3868 - 74; http://dx.doi.org/10.1210/en.2005-0194; PMID: 15905318
- Augustine RA, Ladyman SR, Grattan DR. From feeding one to feeding many: hormone-induced changes in bodyweight homeostasis during pregnancy. J Physiol 2008; 586:387 - 97; http://dx.doi.org/10.1113/jphysiol.2007.146316; PMID: 18033810
- Ladyman SR, Augustine RA, Grattan DR. Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol 2010; 22:805 - 17; PMID: 20456605
- Naef L, Woodside B. Prolactin/Leptin interactions in the control of food intake in rats. Endocrinology 2007; 148:5977 - 83; http://dx.doi.org/10.1210/en.2007-0442; PMID: 17872372
- Augustine RA, Grattan DR. Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology 2008; 149:1049 - 55; http://dx.doi.org/10.1210/en.2007-1018; PMID: 18063686