Abstract
Tumor growth and cancer development are considered clear examples of Darwinian selection, whereby random mutational events in heterogeneous cancer cell populations that best fit the selective microenvironment are preferred.Citation1 As a result, cancer cells evolve resistance to apoptosis, hide from immune surveillance and acquire the ability to invade other organs. Cancer cells, however, are not necessarily passive subjects of selection; they can actively subvert the host tissue to provide a favorable habitat for their growth. Recent findings by Calon et al. convincingly demonstrate that transforming growth factor-β-induced secretion of interleukin 11 by tumor stromal fibroblasts is a necessary prerequisite for the development of distant metastases in colorectal carcinoma. Thus, understanding the complex molecular feedback loops between cancer cells and the surrounding microenvironment (i.e., the tumor-associated stroma or invaded host tissue) should aid the identification of useful molecular targets for improving clinical management of advanced metastatic cancers.
Scientific opinions on the underpinnings cancer development have changed over time as our knowledge of the biological mechanisms underlying cancer has increased. Currently, cancer growth is perceived as an outcome of a remarkably complex network of mutual interaction between malignant cells, the immune system and invaded tissue.Citation2,Citation3 Indeed, the success of specific and individualized therapy depends on detailed mapping of signaling cascades within the tumor microenvironment. Despite the complexity of such a task, the identification of individual participating factors offers new opportunities to target molecules critical for the survival of cancer cells colonizing host tissues and forming secondary tumors.
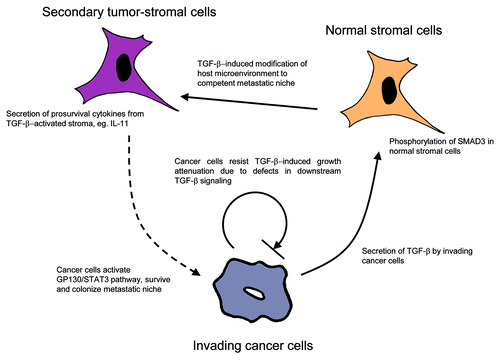
Development of distant metastases represents the most serious hindrance of clinical management of colorectal carcinoma (CRC). Although the cascade of mutational events leading to transformation of colon epithelial cells toward the malignant phenotype is relatively well understood,Citation4 a similar mechanistic description is lacking for the spread of the primary tumor. In a recent issue of Cancer Cell, Calon and colleagues identified a molecular mechanism employed by invading CRC cells and mediating transformation of surrounding tissue to competent metastatic niche. Cancer cells that fail to manipulate the neighbor microenvironment for their favorable growth are curtailed in developing secondary tumors. The molecular bearer of this regulation is transforming growth factor-β (TGF-β), a cytokine that is strongly involved in the regulation of various cellular or tissue events, ranging from cell-cycle control to immunosurveillance and tissue regeneration.Citation5 Deregulation of TGF-β expression is associated with many cancers and often correlates with poor prognosis. In the published work, Calon et al. identified TGF-β as the initiation factor launching prometastatic changes in the host tissue.
CRC is also associated with deregulated levels of TGF-β that predict poor prognosis and limited recurrence-free survival. Nonetheless, clinical outcome, even with great expression of TGF-β, depends on many factors, including the molecular history of disease development, immune system profile, and composition of microenvironment. Unfortunately, a number of studies have inherent design limitations that exhibit “promising trends” rather than true significance in short-term follow-ups.Citation6,Citation7
Calon et al. convincingly demonstrated, using a cohort of 345 cases of CRC, the predictive power of TGF-β expression. Notably, all patients whose tumors were categorized as “TGFB-low” remained without disease recurrence during 10 years of follow-up.
The epithelial CRC cells secreting active TGF-β often contain inactive downstream signaling pathways and avoid the autocrine inhibitory actions of TGF-β.Citation8 On the other hand, Calon et al. showed that nucleus-localized phosphorylated SMAD3 (p-SMAD3), a marker of TGF-β canonical signaling, was present predominantly in stroma in most CRCs. This finding indicates that various stromal cell populations, including macrophages, fibroblasts, endothelial cells or T-cells retain responsivity to TGF-β, phosphorylate downstream SMAD3 mediators and express a common set of genes termed the TGF-β response signatures (TBRS). Notably, all stromal TBRS were enriched in malignant or advanced stages of colorectal carcinoma and served as excellent predictors of disease relapse. In contrast to different stromal cell types, epithelial tumor cells minimally expressed TBRS genes. Moreover, the authors analyzed in detail stromal cell type-specific TBRS using the cells isolated from fresh CRC samples. Intriguingly, this analysis indicated that the response of cancer-associated fibroblasts and endothelial cells to TGF-β is the main contributor to poor prognosis. Concomitant loss of downstream TGF-β signaling in cancer cells together with retained sensitivity in the tumor stroma thus direct the host tissue to express prometastatic TBRS and form a competent metastatic niche.
The authors pursued a mechanistic explanation of stromal response to TGF-β secreted by cancer cells. By an elegant experiment using CRC cell lines HT29-M6 and KM12L4a, which are defective in TGF-β intracellular signaling, the authors showed it was possible to simulate the scenario of advanced disease characterized by the absence of autocrine inhibitory effects of TGF-β on epithelial cancer cells. HT29-M6TGF-β and KM12L4aTGF-β, genetically engineered to produce soluble TGF-β, showed successful initiation of metastasis in immunodeficient mice as compared with that observed by vehicle controls. Importantly, the authors also showed that blocking metastasis via pharmacological inhibition of stromal TGF-β is only effective during the initial phase of metastasis. Generally, TGF-β inhibitors seem to be a promising, specific treatment modality for advanced cancers;Citation9 however, important findings of Calon et al. contribute to a discussion on the importance of stratifying patients and choosing appropriate recipients for successful therapy.
Moreover, the authors discovered that the majority of cancer cells isolated from TGF-β-secreting tumors have a large amount of phosphorylated STAT3 molecules activated by GP130. Combined silencing of GP130 and overexpression of TGF-β in CRC HT29-M6 and KM12L4a cells significantly attenuated the capability of metastatic spreading in vivo, presumably by enhancing the apoptosis of invading CRC cells. Activation of JAK-STAT pathways, and particularly the GP130 receptor by specific molecules secreted by TGF-β-activated stroma, thus, contributes to survival of cancer cells.
The microarray experiment performed on TGF-β-stimulated fibroblasts pointed out several candidates. One of these, interleukin-11 (IL-11), a GP130 ligand, induces expression of genes (IL-11 response, IL-11RS), correlating with TBRS and also disease relapse. Metastases derived from mouse models contained increased amounts of IL-11 and, more specifically, pointed to cancer-associated fibroblasts as the major source of this cytokine, over endothelial, epithelial, or immune cells. Treatment of mice possessing CRC line-derived tumors with TGF-βR inhibitors significantly limited the expression of IL-11 by stromal fibroblasts.
Abnormal activation of STAT3 signaling by proinflammatory cytokines is well described for various gastrointestinal tumors. Changes in microenvironment architecture and cytokine context during tumor progression are also paralleled or even preceded by inflammatory states (e.g., inflammatory bowel disease). The activated GP130-STAT3 pathway not only protects the invading cancer cells from apoptosis or growth restriction but also is critical for survival and preservation of the “stemness” character of stem-like cells FACS-sorted from cultures of colon cancer cell lines.Citation10
The above-mentioned experiments increase our knowledge of TGF-β-induced alterations of the tumor microenvironment and emphasize the necessity of understanding that not only the tumor itself but also the related functional links (e.g., stromal fibroblasts) should be the target of cancer therapy to prevent successful invasion by cancer cells. Thus, TGF-β not only attenuates the immune system and enhances the tendency to epithelial-mesenchymal transition, but it also indirectly supports cancer survival by altering invaded tissue. Importantly, many biologically active molecules are part of adjuvant therapy in different treatment protocols. For example, chemotherapy-induced thrombocytopenia is treated with IL-11.Citation11,Citation12 As this cytokine supports survival of cancer cells and therefore tumor spreading, reassessment of IL-11 use in clinical praxis should be discussed.
Another important aspect raised by the authors reflects the competence of cancer cells to convert normal tissue to a metastatic site during the development of primary tumor. The expression of TBRS, which predict metastatic formation, in cells at the site of the primary tumor would be presumably recapitulated at the site of the secondary tumor, resulting in prosurvival signaling from stroma to cancer cells. Rhim et al.Citation13 discovered a similar phenomenon in the very early dissemination of pancreatic cancer, observing cells that undergo the epithelial-to-mesenchymal transition and keep stem-like characteristics at sites of inflammation. Importantly, paracrine signaling networks facilitating invasion were sensitive to immunosuppressive reagents. Thus, targeting inflammatory molecules might help abrogate the interaction between host tissue and cancer cells.
In summary, the findings of Calon et al. bring new views on intercellular communication in developing secondary tumors (). Evaluating the microenvironment response to invading cancer cells can therefore identify patients who would benefit from individualized treatment that targets particular molecules in the metastatic niche. The unraveling of such dynamic signaling networks may reveal stable and therefore druggable targets effective for therapy in advanced cancer.
Acknowledgments
Work in our laboratories is supported by grant IGA MZD NT13573-4/2012, AV ČR M200041203 and 7AMB12AT019 from the Ministry of Education, and FNUSA-ICRC (CZ.1.05/1.1.00/02.0123) from the European Regional Development Fund.
References
- Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer 2012; 12:487 - 93; http://dx.doi.org/10.1038/nrc3298; PMID: 22695393
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3:453 - 8; http://dx.doi.org/10.1038/nrc1098; PMID: 12778135
- Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta 2012; In press PMID: 23123598
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61:759 - 67; http://dx.doi.org/10.1016/0092-8674(90)90186-I; PMID: 2188735
- Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. TGF-beta signalling in colon carcinogenesis. Cancer Lett 2012; 314:1 - 7; http://dx.doi.org/10.1016/j.canlet.2011.09.041; PMID: 22018778
- Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A. TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: a retrospective study. BMC Cancer 2007; 7:156; http://dx.doi.org/10.1186/1471-2407-7-156; PMID: 17692120
- Fritzmann J, Morkel M, Besser D, Budczies J, Kosel F, Brembeck FH, et al. A colorectal cancer expression profile that includes transforming growth factor beta inhibitor BAMBI predicts metastatic potential. Gastroenterology 2009; 137:165 - 75; http://dx.doi.org/10.1053/j.gastro.2009.03.041; PMID: 19328798
- Xu WQ, Jiang XC, Zheng L, Yu YY, Tang JM. Expression of TGF-beta1, TbetaRII and Smad4 in colorectal carcinoma. Exp Mol Pathol 2007; 82:284 - 91; http://dx.doi.org/10.1016/j.yexmp.2006.10.011; PMID: 17289018
- Zhang B, Halder SK, Zhang S, Datta PK. Targeting transforming growth factor-beta signaling in liver metastasis of colon cancer. Cancer Lett 2009; 277:114 - 20; http://dx.doi.org/10.1016/j.canlet.2008.11.035; PMID: 19147275
- Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res 2011; 71:7226 - 37; http://dx.doi.org/10.1158/0008-5472.CAN-10-4660; PMID: 21900397
- Isaacs C, Robert NJ, Bailey FA, Schuster MW, Overmoyer B, Graham M, et al. Randomized placebo-controlled study of recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with breast cancer receiving dose-intensive cyclophosphamide and doxorubicin. J Clin Oncol 1997; 15:3368 - 77; PMID: 9363868
- Wilde MI, Faulds D. Oprelvekin: a review of its pharmacology and therapeutic potential in chemotherapy-induced thrombocytopenia. BioDrugs 1998; 10:159 - 71; http://dx.doi.org/10.2165/00063030-199810020-00006; PMID: 18020592
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148:349 - 61; http://dx.doi.org/10.1016/j.cell.2011.11.025; PMID: 22265420