 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Atopic dermatitis (AD), a common chronic inflammatory skin disease, is characterized by inflammatory cell skin infiltration. The JAK-STAT pathway has been shown to play an essential role in the dysregulation of immune responses in AD, including the exaggeration of Th2 cell response, the activation of eosinophils, the maturation of B cells, and the suppression of regulatory T cells (Tregs). In addition, the JAK-STAT pathway, activated by IL-4, also plays a critical role in the pathogenesis of AD by upregulating epidermal chemokines, pro-inflammatroy cytokines, and pro-angiogenic factors as well as by downregulating antimicrobial peptides (AMPs) and factors responsible for skin barrier function. In this review, we will highlight the recent advances in our understanding of the JAK-STAT pathway in the pathogenesis of AD.
Keywords: :
Introduction
Atopic dermatitis (AD) is a common skin disease manifested clinically by chronic inflammation and characterized histologically by skin infiltration of inflammatory cells including predominantly lymphocytes, eosinophils, and mast cells. It affects 10–20% of children and 1–3% of adults in developed countries,Citation1 and the prevalence is increasing. Many of these patients also suffer from asthma and allergic rhinitisCitation2 along with intense itching and skin infection. Although the pathogenesis and etiology of AD remain to be completely understood, this multifactorial disease likely results from a complex crosstalk between genetic and environmental factors. Exaggerated Th2 response, disruption of the epidermis barrier functions, high levels of serum IgE, and decreased production of antimicrobial peptides (AMPs) are the key findings in AD.Citation3,Citation4 Data from human and animal studies indicate that AD is a Th2 dominant inflammatory skin disease at the acute stage followed by Th1 involvement at the chronic stage of the disease.Citation3-Citation5 Specifically, this notion of Th2-dominance in AD is validated by a mouse model we have successfully generated through overexpressing IL-4, a key Th2 cytokine, in the basal epidermis using a epidermis-specific keratin 14 promoter.Citation6 The IL-4 transgenic (Tg) mice spontaneously develop skin lesions, serological abnormalities, and skin infection, which fulfil the clinical and histological diagnostic criteria for human AD.Citation6 Consistent with an inflammatory disease process, upregulation of chemokines,Citation7,Citation8 proinflammatory cytokines,Citation5 B cell activation molecules,Citation9 angiogenic factors,Citation10,Citation11 and several critical adhesion moleculesCitation12 has been found in these Tg mice.
The JAK-STAT pathway is a classical signal transduction pathway for numerous cytokines and growth factors. The binding of ligands to their receptors leads to JAK activation, which in turn phosphorylates and activates STATs. The activated STATs then translocate to the cell nucleus to regulate their target genes. The JAK family includes JAK1, JAK2, JAK3, and TYK2, and the STAT family includes STAT1, STAT2, STAT3, STAT5A/B, and STAT6. As a negative regulator of the JAK-STAT pathway, the SOCS family consists of cytokine-inducible SH2 domain-containing protein (CIS) and SOCS1–7. SOCSs may act on the activation loop of JAKs, or they may interact with phosphotyrosines in the cytoplasmic domain of cytokine receptors, suppressing the JAK-STAT pathway with a negative feedback mechanism.Citation13 In this review, we will discuss the roles that the JAK-STAT pathway plays in the pathophysiology of AD.
JAK-STAT in Th2 Differentiation
Since AD is a Th2-dominant disease, examination of how the JAK-STAT pathway regulates Th2 differentiation would help us to understand the possible roles that JAK-STAT play in AD. It is well established that IL-4 promotes the differentiation of Th2 cells, which in turn produce IL-4, IL-5, IL-10, and IL-13. The study of IL-4 and the IL-4 receptor α-null mice demonstrated clearly that IL-4 is required for a Th2 response and the production of Th2 cytokines.Citation14,Citation15 In addition, studies from the IL-4 Tg mice demonstrated upregulation of the Th2 cytokines.Citation5 Consistently, the IL-4 Tg mice generated on a Th2-dominant strain, BALB/c mice, develop earlier onset and worse AD-like skin lesions than the IL-4 Tg mice generated on a Th1-dominant strain, C57BL/6 mice, suggesting that the Th2 systemic immune milieu, in addition to cutaneous Th2 immune milieu, plays an essential role in the pathogenesis of AD.Citation16 Additionally, knocking out Bcl-6, a transcription factor functioning to regulate IL-4 signal transduction, leads to Th2-mediated hyperimmune response in many organs, further supporting the regulatory function of IL-4 on Th2 immunity.Citation17 Importantly, IL-4 signals through the JAKs-STAT6 pathway to regulate Th2-related target genes in lymphocytes, firmly supporting a role of JAK-STAT in Th2 regulation.Citation18
In addition to IL-4, other factors are also important for Th2 differentiation. Thymic stromal lymphopoietin (TSLP), known to promote Th2 differentiation, to activate natural killer T cells and basophils, and to affect B cell maturation, has been reported to be associated with AD.Citation19 While the murine TSLP receptor signals via STAT3, STAT5/Tec, a Src type kinase, the human TSLP receptor activates STAT1, STAT3, STAT5/JAK1, and JAK2.Citation20,Citation21
It has been reported that histamine enhances the secretion of Th2 cytokines such as IL-4, IL-5, IL-10, and IL-13 and inhibits the production of Th1 cytokines.Citation22 Histamine was shown to stimulate IL-13 production through the JAK-STAT pathway in a murine Th2 cell line.Citation23 In addition, tyrphostin, a JAK-STAT pathway inhibitor, reversed the effects of histamine on IFNγ, IL-5, and IL-10 production.Citation24 Horr et al. have shown that histamine, by acting through the H4 receptors on T cells, can suppress STAT1 activation and help to drive the Th2 response, leading to the development of AD.Citation25
It is generally accepted that TYK2, JAK2, and STAT4, which are IL-12 signaling pathway components, are essential for Th1 cell differentiation, while JAK1, JAK3, and STAT6, which are IL-4 signaling components, are critical for Th2 differentiation.Citation20 In addition, STAT5A/B, which are involved in the upregulation of GATA3 and IL-4Rα, and STAT3, which helps STAT6 bind to its target genes, also play some roles in Th2 cell differentiation.Citation20,Citation26,Citation27
STAT6 regulates genes involved in Th2 and B cell differentiation, IgE class switching, and MHC class II production.Citation15,Citation18 While STAT4 null mice fail to generate Th1 cells and conditional STAT3 knockout mice have an exaggerated Th1 response,Citation28 STAT6 null mice have impaired Th2 cell development and IgE class switching.Citation29 On the contrary, mice expressing constitutively active STAT6 (STAT6VT) develop an AD-like skin lesion.Citation30 In addition, several STAT6 polymorphisms are also associated with a predisposition to allergic diseases and high levels of IgE.Citation31,Citation32
JAK3 is expressed in natural killer cells, T cells, and B cells. A JAK3 mutation in severe combined immunodeficiency (SCID) patients leads to the absence of T cells and natural killer cells and the production of dysfunctional B cells.Citation33 JAK3-null mice also have similar defects.Citation34
In humans, a loss-of-function mutation in TYK2 leads to defects in multiple cytokine signaling pathways including type I IFN, IL-6, and IL-12.Citation35-Citation37 These patients showed impaired Th1 differentiation and accelerated Th2 differentiation with clinical manifestations including unusual susceptibility to various microorganism infections, AD-like skin lesions, and high serum IgE levels, suggesting a regulatory role of the JAK family in Th2 differentiation.
Another TYK2 null mutation and hypomorphic mutations in STAT3 are associated with the hyper-IgE syndrome (HIES), a primary immunodeficiency characterized by AD like-skin lesions, susceptibility to infections, and high serum IgE levels.Citation38 In this syndrome, IL-12 and IFN/β signal pathways are defective, thus blocking the Th1 differentiation. As a result, the Th2 response is exaggerated, leading to similar clinical findings as AD.Citation38 Because STAT3 is important for B cell proliferation and differentiation, HIES patients have decreased number of memory B cells as compared with AD patients.Citation39
As a negative regulator of the JAK-STAT pathway, suppressor of cytokine signaling 2 (SOCS2) deletion leads to increased susceptibility to AD as compared with wild-type mice. These mice show an exaggerated Th2 response along with significantly elevated serum IgE levels, eosinophilia and low IL-17 levels. SOCS2-knockout CD4+ T cells display constitutively high levels of STAT6 phosphorylation as compared with wild type T cells.Citation40 In addition, IL-2-induced STAT5 activation, also involved in the early Th2 differentiation, is exaggerated in the SOCS2-knockout CD4+ T cells.Citation40 On the other hand, IL-12-stimulated STAT4 phosphorylation is unaffected, and IL-6 mediated STAT3 phosphorylation is inhibited. Since it is generally accepted that the development of the Th1, Th2, and Th17 immune responses are mediated by STAT4/STAT1, STAT6/STAT5, and STAT3 respectively,Citation40,Citation41 the increased STAT6/STAT5 activation and suppressed STAT3 activation naturally lead to an exaggerated Th2 response at the expense of Th17.Citation40
Another example of the JAK family involvement in Th2 immunity is shown in NC/Nga mice that develop AD-like skin inflammation. Nakagawa et al. reported that Pyridone 6 (P6), a pan-JAK inhibitor, delayeded the onset and reduced the severity of AD-like skin lesions in NC/Nga mice.Citation42 P6 suppressed the IFN-γ/STAT1, IL-2/STAT5, and IL-4/STAT6 signaling pathways strongly while it suppressed IL-6/STAT3 pathway only modestly, resulting in reduction of the Th1 and Th2 responses but the enhancement of the T17 response.Citation42
JAK-STAT in Eosinophils
Eosinophil skin infiltration is frequently observed in the skin lesions of AD patients and AD mouse models.Citation43-Citation46 Although STAT6 null mice have defects in Th2 differentiation and IgE class switching,Citation47 crossing these mice with NC/Nga mice cannot prevent the development of AD-like skin lesions.Citation48 Even though these mice do not produce IgE and Th2 cytokines, the histological features of their skin lesions are similar to that of AD.Citation48 These authors suggested that a Th2 response is not absolutely necessary for the development of AD-like skin lesions; instead, IFN- γ and eosinophil skin infiltration may play an essential role.Citation48
Eosinophils express the IL-31 receptor A (IL-31RA),Citation49 and IL-31 has the ability to delay the apoptosis of eosinophils and to stimulate the secretion of pro-inflammatory cytokines and chemokines through JAK-STAT.Citation49,Citation50 In addition, IL-5, mainly involved in B cell proliferation and differentiation, was shown to induce eosinophilia when upregulated.Citation51 Conversely IL-5 or IL-5 receptor α chain (IL-5Rα) null mice show defects in B cells and eosinophils.Citation52 In eosinophils, IL-5 signals through the JAK2-STAT1/STAT5 and MAP kinases pathways to regulate genes involved in cell proliferation, survival and effector functions.Citation52-Citation54
JAK-STAT in Tregs
In the skin, regulatory T cells (Tregs) help to regulate the immune response, and in AD, Treg function is suppressed.Citation55-Citation57 Depleting Treg cells significantly increased the severity of the skin inflammation in OVA-sensitized mice.Citation58 In T cells, IL-4-activated STAT6 upregulates GATA3, the master regulator of Th2 cells. GATA3 in turn suppresses Foxp3, the master regulator in Treg cells.Citation59 This regulatory pathway could possibly explain why Treg function is suppressed in AD. In addition, STAT5A/B and STAT3 may also play important roles in the regulation of Foxp3.Citation60 On the other hand, Foxp3 can also suppress GATA3’s ability to regulate Th2 genes.Citation59 It was reported that the IFN-γ gene transfer stimulates Treg related cytokines and improves AD-like symptoms of NC/Nga mice.Citation61 This finding, however, seems to contradict the notion that IFN- γ is a promoting factor for AD.Citation48 On the clinical level, treatment with a low-dose cyclosporine A is effective in reducing skin inflammation with simultaneous increase of Treg population in AD patients, further supporting a role of Treg in AD.Citation62
JAK-STAT in Th17 Cells
STAT3, mediating the IL-23 and IL-6 pathways, is critical for the differentiation of Th17 Cell.Citation63,Citation64 In the early stage of human AD, the number of Th17 cells is increased,Citation3 and IL-17 has been reported to upregulate adhesion molecules on keratinocytes, enhancing T cell-keratinocyte adhesion and T cell-mediated cytotoxicity.Citation65 However, in chronic AD, the Th17 pathway is suppressed,Citation66 which could account for the AMP deficiency in AD.Citation3
JAK-STAT in Mast Cells
In the skin lesions of AD patients as well as AD mouse models, the number of mast cells is increased.Citation6,Citation67 IL-9 stimulates VEGF production from human mast cells through the activation of STAT3 in an in vitro experiment.Citation68 Interestingly, IL-9 serum levels in AD patients are not different from those of non-AD controls even though IL-9 and its receptors are upregulated in the lesional skin of the AD patients,Citation68 suggesting that the upregulation of VEGF in mast cells via IL-9 may be a localized cutaneous phenomenon. The prominent increase of mast cell VEGF production and angiogenesis in an AD animal model also supports the role of JAK-STAT in this immune pathwayCitation10,Citation11
JAK-STAT in Epidermal Keratinocytes
Being a major cell type of the skin and possessing the ability to participate in various immune responses, epidermal keratinocytes are likely an active player in the skin inflammation of AD.Citation69,Citation70 We and others have shown that the dysregulation of the keratinocyte genes by IL-4 play an important role in AD.Citation8,Citation71-Citation74 Two types of IL-4 receptors have been identified. The type I receptor consisting of IL-4Rα and IL-2Rγc chains is expressed in hematopoietic cells, while the type II receptor consisting of IL-4Rα and IL-13Rα1 is expressed in other cell types including keratinocytes.Citation75,Citation76
In keratinocytes, both IL-4 AND IL-13 binds to IL-4Rα and IL-13R1. IL-13Rα2, which was reported as a dominant negative inhibitor for both IL-4 and IL-13, binds to IL-13 with high affinity, but lacks a significant cytoplasmic domain.Citation77 We have previously reported that IL-13Rα2 is upregulated by IL-4 in keratinocytes.Citation71 In AD patients this gene is similarly upregulated.Citation78 This may be due to a negative feedback mechanism available to keratinocytes to maintain homeostasis.
We have also shown that IL-4 upregulates chemokines CCL8, CCL24, CCL25, CCL26, CCL3L1, CXCL6, and CXCL16,Citation8,Citation71 all known for their roles in the pathophyisology of AD. The eotaxin subfamily that consists of CCL11/eotaxin-1, CCL24/eotaxin-2, and CCL26/eotaxin-3 plays an important role in AD.Citation43,Citation79,Citation80 Eotaxins bind to the chemokine cysteine–cysteine receptor 3 (CCR3), which are expressed predominantly on eosinophils, recruiting these cells to the inflammatory site. Skin infiltrating eosinophils release a variety of cytotoxic granule proteins to cause tissue damage.Citation44 In the regulation of CCL26, though both STAT3 and STAT6 are phosphorylated by IL-4, only STAT6 is functionally activated.Citation8 Detailed analysis of the promoter of the CCL26 gene has shown that the STAT6 response element consists of two palindromic half sites TTC and GAA spaced by four nucleotides. Others also reported that STAT6, different from other STAT members, prefers the STAT sites with four spacing nucleotides.Citation81 In addition to tyrosine phosphorylation, STAT3 activation may also involve serine phosphorylationCitation82 and lysine acetylation.Citation83 Knockout mice studies indirectly support our results in that while STAT6 null mice demonstrated defects in eosinophil tissue infiltration,Citation29,Citation84 the STAT3 Cre-loxP knockout mice did not show similar defects.Citation29 Furthermore, IL-4 and IL-13 double knockout mice, when sensitized with ovalbumin, develop a skin lesion characterized by intact CD4+ dermal infiltration, severely diminished eosinophil infiltration, and undetectable OVA-specific IgE levels,Citation85 suggesting that STAT6 activation is required for eosinophil skin infiltration in AD.
In addition to chemokines, IL-4 also upregulates pro-inflammatory factors, such as IL-1α, IL-19, IL-20, IL-25, IL-27, IL-12Rβ2, IL31RA, and nitric oxide synthase 2 (NOS2).Citation71 Moreover, we demonstrated that IL-4 signals through the JAK-STAT pathway to regulate IL-19 expression in keratinocytes.Citation86
Using PCR array, we have found that that IL-4 upregulates angiogenic or lymphangiogenic-related genes, such as VEGF, ANG-1, ANGL-4, IGF1, Notch4, PGF, and MCP-1.Citation11,Citation71 Using the IL-4 Tg mice and cell culture, we showed that CD11b+ macrophages attracted to the skin lesion by MCP-1 may account for lymphangiogenesis observed in AD by secreting VEGF-C.Citation11
While IL-4 upregulates chemokines, pro-inflammatory factors, angiogenic factors in keratinocytes, it downregulates antimicrobial peptides (AMPs) or factors involved in APM production.Citation71 Other groups have also demonstrated that the dysregulation of AMPs affects the development of AD.Citation87 Human β-defensin-3 (HBD-3), which is significantly decreased in AD, is downregulated by IL-4, IL-10, and IL-13.Citation88 In addition, HBD-2 and human cathelicidin (LL-37) were also found to be reduced in AD skin lesions as compared with psoriasis.Citation89 Howell et al. suggested that low levels of LL-37 suppressed by IL-4 and IL-13 through STAT6 may be the mechanism responsible for increased vaccinia virus replication as occurred in eczema vaccinatum of AD patients.Citation90
Skin barrier function defects are critical for the development of AD.Citation91 In addition to the filaggrin mutations in some AD patients, cytokines are now known to induce the downregulation of several barrier proteins. Loricrin and involucrin are two important factors for skin barrier function. They are downregulated by IL-4 and IL-13 in keratinocytes, and in STAT6 transgenic mice, their expression is significantly decreased as well.Citation92 In addition, filaggrin, which is reduced in acute AD skin lesion, is also suppressed by IL-4 and IL-13 in keratinocytes.Citation93 Interestingly, while IL-4 markedly reduces ceramide levels in the epidermis by downregulating the expression of serine-palmitoyl transferase-2, acid sphingomyelinase, and β-glucocerebrosidase, Th1 cytokines (GM-CSF/IFN-γ/TNF-α) increase the ceramide levels.Citation94
Taken together, the role that IL-4 plays in the dysregulation of keratincoyte functions in AD involves the upregulation of chemokines, pro-inflammatory factors, and pro-angiogenic factors and the downregulation of AMPs and factors responsible for skin barrier function.
Pruritis in AD is induced by complicated crosstalks among keratinocytes, immune cells, and nerve fibers.Citation95 The data from the Nc/Nga mice studies indicate that although IL-4 is a key mediator of the inflammatory process in AD, IL-31 might be the key causative factor for pruitus.Citation50 In addition, IL-31 transgenic mice developed a Th2 immune response with severe pruritic skin lesions similar to AD.Citation96
IL-31 is upregulated in the skin of AD patients, and it induces the itching sensation either by direct stimulation of the dorsal root ganglion fibers that express IL-31RA or by indirect stimulation of pruritic factors from keratinocytes.Citation50,Citation97 IL-31 receptor activation in keratinocytes induces calcium influx and STAT3 mediated β-endorphin production, which may contribute to the peripheral itching in AD.Citation98
Conclusion
Thus far, data from our laboratory and others seem to indicate an influential role of JAK-STAT in the pathogenesis of AD, with IL-4 being a key mediator ( and ). Specifically, the JAK-STAT pathway is instrumental for the Th2 cell differentiation. In the immune milieu of AD, the enhancement of Th2 cell proliferation and their release of various cytokines via the JAK-STAT pathway is likely the critical factor for the inflammatory responses in AD. This Th2 immune upregulation could then lead to B cell maturation and plasma cell differentiation, resulting in the hyper-secretion of IgE. IgE binding to skin mast cells causes histamine release, which further exacerbates AD. Similarly, this hyper Th2 immune milieu triggers epidermal cells to release various chemokines, pro-inflammatory cytokines, and angiogenic factors that participate in AD pathophysiology. Moreover, IL-5 released from this hyper Th2 milieu could activate eosinophils that are attracted to the skin by the eotaxin subfamily, further worsening the AD condition. In addition, by way of IL-31, an inducer of pruritus, AD becomes increasingly intensified.
Figure 1. Proposed mechanism of JAK-STAT involvement in atopic dermatitis (AD) development, part I. Skin barrier function defects are critical for the development of AD. In addition to the filaggrin gene mutation defects in some AD patients, IL-4 is also able to downregulate barrier proteins filaggrin, loricrin and involucrin through the JAK-STAT pathway making the epidermis more penetrable by allergens and pathogens. Once penetrated through the epidermis, allergens/pathogens are detected by dendritic cells, which become subsequently activated to present these antigens to naïve Th0 cells. The Th0 cell can then differentiate into the Th2 cell through the JAK1,3-STAT6 pathway under the influence of IL-4. In the Th0 cells, the STAT6 pathway can also upregulate GATA3, a master regulator of Th2 cells. GATA3 in turn suppresses Foxp3, the master regulator in Treg cells, thus allowing more T cells to be activated. Black arrows indicate activation pathway. Red lines indicate inhibition pathway.
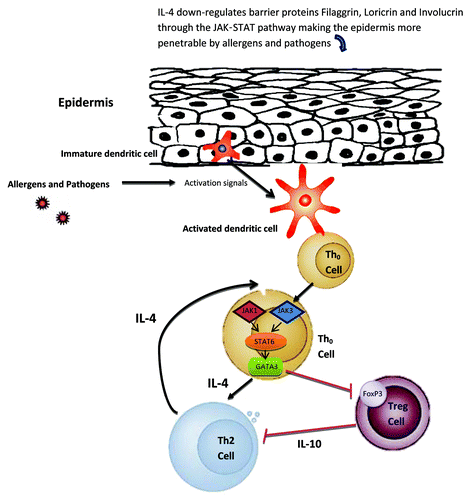
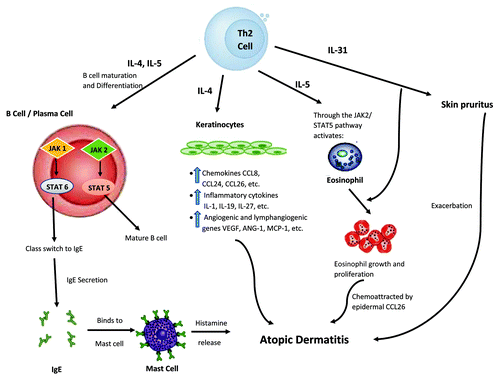
Figure 2. Proposed mechanism of JAK-STAT involvement in atopic dermatitis (AD) development, part II. Here, Th2 cells play a significant role. By their abilities to provide IL-4 and IL-5 stimulation via the JAK-STAT pathway, immature B cells could be differentiated into mature B cell and plasma cells would undergo antibody heavy chain switching to IgE class. The subsequent binding of IgE to skin mast cells could lead to release of histamine, which is known to exacerbate AD. Similarly, this hyper Th2 immune milieu, particularly IL-4, could trigger epidermal cells to produce and release various chemokines (such as CCL26), pro-inflammatory cytokines, and angiogenic factors, leading to AD pathophysiology. Moreover, IL-5 released from this hyper Th2 milieu could, through JAK-STAT pathway, activate eosinophils, and attract them to the skin via CCL26, further worsening the AD condition. In addition, by way of IL-31, an inducer of pruritus, AD symptoms could become increasingly intensified. Black arrows indicate activation pathway. Red lines indicate inhibition pathway.
Our understanding of the JAK-STAT pathway and its relationship to the dysregulated immune response and keratinocyte function is still in its infancy. We have many unanswered questions. What are JAKs’ and STATs’ tissue-specific functions? How does the JAK-STAT pathway crosstalk with other pathways in AD? What exact roles do the different JAKs and STATs play in the pathogenesis of AD? Answering these questions will enable us to target more specifically the key components of the complex pathophysiology of AD, thus providing the best treatment for this disease.
| Abbreviations: | ||
| AD | = | atopic dermatitis |
| AMP | = | antimicrobial peptide |
| Tg | = | transgenic |
| SOCS | = | suppressor of cytokine signaling |
| CIS | = | cytokine-inducible SH2 domain-containing protein |
| TSLP | = | thymic stromal lymphopoietin |
| SCID | = | severe combined immunodeficiency |
| HIES | = | hyper-IgE syndrome |
| IL-31RA | = | IL-31 receptor A |
| Tregs | = | regulatory T cells |
Acknowledgments
This work is in part supported by the Albert H. and Mary Jane Slepyn Endowed Fellowship (L.B.), and the Dr Orville J. Stone Endowed Professorship (L.S.C.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Saito H. Much atopy about the skin: genome-wide molecular analysis of atopic eczema. Int Arch Allergy Immunol 2005; 137:319 - 25; http://dx.doi.org/10.1159/000086464; PMID: 15970641
- Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol 2008; 58:68 - 73; http://dx.doi.org/10.1016/j.jaad.2007.06.041; PMID: 17692428
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol 2011; 127:1420 - 32; http://dx.doi.org/10.1016/j.jaci.2011.01.054; PMID: 21419481
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol 2011; 127:1110 - 8; http://dx.doi.org/10.1016/j.jaci.2011.01.053; PMID: 21388665
- Chen L, Martinez O, Overbergh L, Mathieu C, Prabhakar BS, Chan LS. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin Exp Immunol 2004; 138:375 - 87; http://dx.doi.org/10.1111/j.1365-2249.2004.02649.x; PMID: 15544612
- Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol 2001; 117:977 - 83; http://dx.doi.org/10.1046/j.0022-202x.2001.01484.x; PMID: 11676841
- Chen L, Lin SX, Agha-Majzoub R, Overbergh L, Mathieu C, Chan LS. CCL27 is a critical factor for the development of atopic dermatitis in the keratin-14 IL-4 transgenic mouse model. Int Immunol 2006; 18:1233 - 42; http://dx.doi.org/10.1093/intimm/dxl054; PMID: 16735375
- Bao L, Shi VY, Chan LS. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: Implication for atopic dermatitis. Mol Immunol 2012; 50:91 - 7; http://dx.doi.org/10.1016/j.molimm.2011.12.008; PMID: 22226123
- Chen L, Lin SX, Overbergh L, Mathieu C, Chan LS. The disease progression in the keratin 14 IL-4-transgenic mouse model of atopic dermatitis parallels the up-regulation of B cell activation molecules, proliferation and surface and serum IgE. Clin Exp Immunol 2005; 142:21 - 30; http://dx.doi.org/10.1111/j.1365-2249.2005.02894.x; PMID: 16178852
- Chen L, Marble DJ, Agha R, Peterson JD, Becker RP, Jin T, et al. The progression of inflammation parallels the dermal angiogenesis in a keratin 14 IL-4-transgenic model of atopic dermatitis. Microcirculation 2008; 15:49 - 64; http://dx.doi.org/10.1080/10739680701418416; PMID: 17952801
- Shi VY, Bao L, Chan LS. Inflammation-driven dermal lymphangiogenesis in atopic dermatitis is associated with CD11b+ macrophage recruitment and VEGF-C up-regulation in the IL-4-transgenic mouse model. Microcirculation 2012; 19:567 - 79; http://dx.doi.org/10.1111/j.1549-8719.2012.00189.x; PMID: 22574929
- Chen L, Lin SX, Amin S, Overbergh L, Maggiolino G, Chan LS. VCAM-1 blockade delays disease onset, reduces disease severity and inflammatory cells in an atopic dermatitis model. Immunol Cell Biol 2010; 88:334 - 42; http://dx.doi.org/10.1038/icb.2009.107; PMID: 20065994
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 2004; 22:503 - 29; http://dx.doi.org/10.1146/annurev.immunol.22.091003.090312; PMID: 15032587
- Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 1993; 362:245 - 8; http://dx.doi.org/10.1038/362245a0; PMID: 8384701
- Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest 2002; 109:1279 - 83; PMID: 12021241
- Reichle ME, Chen L, Lin SX, Chan LS. The Th2 systemic immune milieu enhances cutaneous inflammation in the K14-IL-4-transgenic atopic dermatitis model. J Invest Dermatol 2011; 131:791 - 4; http://dx.doi.org/10.1038/jid.2010.382; PMID: 21191418
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet 1997; 16:161 - 70; http://dx.doi.org/10.1038/ng0697-161; PMID: 9171827
- Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res 2011; 50:87 - 96; http://dx.doi.org/10.1007/s12026-011-8205-2; PMID: 21442426
- Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res 2012; 52:211 - 23; http://dx.doi.org/10.1007/s12026-012-8264-z; PMID: 22274860
- O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012; 36:542 - 50; http://dx.doi.org/10.1016/j.immuni.2012.03.014; PMID: 22520847
- Wohlmann A, Sebastian K, Borowski A, Krause S, Friedrich K. Signal transduction by the atopy-associated human thymic stromal lymphopoietin (TSLP) receptor depends on Janus kinase function. Biol Chem 2010; 391:181 - 6; http://dx.doi.org/10.1515/bc.2010.029; PMID: 20128689
- Packard KA, Khan MM. Effects of histamine on Th1/Th2 cytokine balance. Int Immunopharmacol 2003; 3:909 - 20; http://dx.doi.org/10.1016/S1567-5769(02)00235-7; PMID: 12810348
- Elliott KA, Osna NA, Scofield MA, Khan MM. Regulation of IL-13 production by histamine in cloned murine T helper type 2 cells. Int Immunopharmacol 2001; 1:1923 - 37; http://dx.doi.org/10.1016/S1567-5769(01)00117-5; PMID: 11606024
- Osna N, Elliott K, Chaika O, Patterson E, Lewis RE, Khan MM. Histamine utilizes JAK-STAT pathway in regulating cytokine production. Int Immunopharmacol 2001; 1:759 - 62; http://dx.doi.org/10.1016/S1567-5769(01)00009-1; PMID: 11357887
- Horr B, Borck H, Thurmond R, Grösch S, Diel F. STAT1 phosphorylation and cleavage is regulated by the histamine (H4) receptor in human atopic and non-atopic lymphocytes. Int Immunopharmacol 2006; 6:1577 - 85; http://dx.doi.org/10.1016/j.intimp.2006.06.005; PMID: 16919830
- Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, et al. The transcription factor STAT3 is required for T helper 2 cell development. Immunity 2011; 34:39 - 49; http://dx.doi.org/10.1016/j.immuni.2010.12.013; PMID: 21215659
- Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol 2008; 9:1288 - 96; http://dx.doi.org/10.1038/ni.1656; PMID: 18820682
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 1999; 10:39 - 49; http://dx.doi.org/10.1016/S1074-7613(00)80005-9; PMID: 10023769
- Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells 1999; 17:138 - 46; http://dx.doi.org/10.1002/stem.170138; PMID: 10342556
- Sehra S, Bruns HA, Ahyi AN, Nguyen ET, Schmidt NW, Michels EG, et al. IL-4 is a critical determinant in the generation of allergic inflammation initiated by a constitutively active Stat6. J Immunol 2008; 180:3551 - 9; PMID: 18292582
- Weidinger S, Klopp N, Wagenpfeil S, Rümmler L, Schedel M, Kabesch M, et al. Association of a STAT 6 haplotype with elevated serum IgE levels in a population based cohort of white adults. J Med Genet 2004; 41:658 - 63; http://dx.doi.org/10.1136/jmg.2004.020263; PMID: 15342695
- Tamura K, Arakawa H, Suzuki M, Kobayashi Y, Mochizuki H, Kato M, et al. Novel dinucleotide repeat polymorphism in the first exon of the STAT-6 gene is associated with allergic diseases. Clin Exp Allergy 2001; 31:1509 - 14; http://dx.doi.org/10.1046/j.1365-2222.2001.01191.x; PMID: 11678849
- Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 1995; 270:797 - 800; http://dx.doi.org/10.1126/science.270.5237.797; PMID: 7481768
- Ortmann RA, Cheng T, Visconti R, Frucht DM, O’Shea JJ. Janus kinases and signal transducers and activators of transcription: their roles in cytokine signaling, development and immunoregulation. Arthritis Res 2000; 2:16 - 32; http://dx.doi.org/10.1186/ar66; PMID: 11094415
- Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 2006; 25:745 - 55; http://dx.doi.org/10.1016/j.immuni.2006.09.009; PMID: 17088085
- Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med 2011; 208:235 - 49; http://dx.doi.org/10.1084/jem.20100799; PMID: 21300911
- Zhang Q, Su HC. Hyperimmunoglobulin E syndromes in pediatrics. Curr Opin Pediatr 2011; 23:653 - 8; http://dx.doi.org/10.1097/MOP.0b013e32834c7f65; PMID: 21970826
- Minegishi Y, Karasuyama H. Defects in Jak-STAT-mediated cytokine signals cause hyper-IgE syndrome: lessons from a primary immunodeficiency. Int Immunol 2009; 21:105 - 12; http://dx.doi.org/10.1093/intimm/dxn134; PMID: 19088064
- Speckmann C, Enders A, Woellner C, Thiel D, Rensing-Ehl A, Schlesier M, et al. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol 2008; 129:448 - 54; http://dx.doi.org/10.1016/j.clim.2008.08.002; PMID: 18835223
- Knosp CA, Carroll HP, Elliott J, Saunders SP, Nel HJ, Amu S, et al. SOCS2 regulates T helper type 2 differentiation and the generation of type 2 allergic responses. J Exp Med 2011; 208:1523 - 31; http://dx.doi.org/10.1084/jem.20101167; PMID: 21646394
- O’Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nat Rev Immunol 2011; 11:239 - 50; http://dx.doi.org/10.1038/nri2958; PMID: 21436836
- Nakagawa R, Yoshida H, Asakawa M, Tamiya T, Inoue N, Morita R, et al. Pyridone 6, a pan-JAK inhibitor, ameliorates allergic skin inflammation of NC/Nga mice via suppression of Th2 and enhancement of Th17. J Immunol 2011; 187:4611 - 20; http://dx.doi.org/10.4049/jimmunol.1100649; PMID: 21957150
- Owczarek W, Paplińska M, Targowski T, Jahnz-Rózyk K, Paluchowska E, Kucharczyk A, et al. Analysis of eotaxin 1/CCL11, eotaxin 2/CCL24 and eotaxin 3/CCL26 expression in lesional and non-lesional skin of patients with atopic dermatitis. Cytokine 2010; 50:181 - 5; http://dx.doi.org/10.1016/j.cyto.2010.02.016; PMID: 20236835
- Kapp A. [The role of eosinophilic granulocytes for the pathogenesis of atopic dermatitis /neurodermatitis. Eosinophilic products as markers of disease activity]. Hautarzt 1993; 44:432 - 6; PMID: 8365876
- Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med 1985; 313:282 - 5; http://dx.doi.org/10.1056/NEJM198508013130502; PMID: 3892296
- Chen L, Overbergh L, Mathieu C, Chan LS. The development of atopic dermatitis is independent of Immunoglobulin E up-regulation in the K14-IL-4 SKH1 transgenic mouse model. Clin Exp Allergy 2008; 38:1367 - 80; http://dx.doi.org/10.1111/j.1365-2222.2008.02987.x; PMID: 18489026
- Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 1996; 380:630 - 3; http://dx.doi.org/10.1038/380630a0; PMID: 8602264
- Yagi R, Nagai H, Iigo Y, Akimoto T, Arai T, Kubo M. Development of atopic dermatitis-like skin lesions in STAT6-deficient NC/Nga mice. J Immunol 2002; 168:2020 - 7; PMID: 11823539
- Cheung PF, Wong CK, Ho AW, Hu S, Chen DP, Lam CW. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol 2010; 22:453 - 67; http://dx.doi.org/10.1093/intimm/dxq027; PMID: 20410259
- Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol 2012; 91:552 - 66; http://dx.doi.org/10.1016/j.ejcb.2011.07.006; PMID: 21982586
- Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy 2012; 42:712 - 37; http://dx.doi.org/10.1111/j.1365-2222.2011.03854.x; PMID: 22092535
- Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol 2008; 20:288 - 94; http://dx.doi.org/10.1016/j.coi.2008.04.001; PMID: 18511250
- Kotsimbos AT, Hamid Q. IL-5 and IL-5 receptor in asthma. Mem Inst Oswaldo Cruz 1997; 92:Suppl 2 75 - 91; http://dx.doi.org/10.1590/S0074-02761997000800012; PMID: 9698919
- Ochiai K, Tanabe E, Ishihara C, Kagami M, Sugiyama T, Sueishi M, et al. Role of JAK2 signal transductional pathway in activation and survival of human peripheral eosinophils by interferon-gamma (IFN-gamma). Clin Exp Immunol 1999; 118:340 - 3; http://dx.doi.org/10.1046/j.1365-2249.1999.01068.x; PMID: 10594549
- Honda T, Miyachi Y, Kabashima K. Regulatory T cells in cutaneous immune responses. J Dermatol Sci 2011; 63:75 - 82; PMID: 21708454
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Curr Probl Dermatol 2011; 41:112 - 24; http://dx.doi.org/10.1159/000323305; PMID: 21576952
- Hayashida S, Uchi H, Moroi Y, Furue M. Decrease in circulating Th17 cells correlates with increased levels of CCL17, IgE and eosinophils in atopic dermatitis. J Dermatol Sci 2011; 61:180 - 6; http://dx.doi.org/10.1016/j.jdermsci.2010.10.013; PMID: 21255981
- Fyhrquist N, Lehtimäki S, Lahl K, Savinko T, Lappeteläinen AM, Sparwasser T, et al. Foxp3+ cells control Th2 responses in a murine model of atopic dermatitis. J Invest Dermatol 2012; 132:1672 - 80; http://dx.doi.org/10.1038/jid.2012.40; PMID: 22402436
- Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J Leukoc Biol 2010; 87:1011 - 8; http://dx.doi.org/10.1189/jlb.1209772; PMID: 20335310
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 2007; 109:4368 - 75; http://dx.doi.org/10.1182/blood-2006-11-055756; PMID: 17227828
- Watcharanurak K, Nishikawa M, Takahashi Y, Kabashima K, Takahashi R, Takakura Y. Regulation of immunological balance by sustained interferon-γ gene transfer for acute phase of atopic dermatitis in mice. Gene Ther 2012; 20:538 - 44; http://dx.doi.org/10.1038/gt.2012.69; PMID: 22914497
- Brandt C, Pavlovic V, Radbruch A, Worm M, Baumgrass R. Low-dose cyclosporine A therapy increases the regulatory T cell population in patients with atopic dermatitis. Allergy 2009; 64:1588 - 96; http://dx.doi.org/10.1111/j.1398-9995.2009.02054.x; PMID: 19432936
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282:9358 - 63; http://dx.doi.org/10.1074/jbc.C600321200; PMID: 17277312
- Hirahara K, Ghoreschi K, Laurence A, Yang XP, Kanno Y, O’Shea JJ. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev 2010; 21:425 - 34; http://dx.doi.org/10.1016/j.cytogfr.2010.10.006; PMID: 21084214
- Cavani A, Pennino D, Eyerich K. Th17 and Th22 in skin allergy. Chem Immunol Allergy 2012; 96:39 - 44; http://dx.doi.org/10.1159/000331870; PMID: 22433369
- Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol 2008; 181:7420 - 7; PMID: 18981165
- Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol 2009; 21:666 - 78; http://dx.doi.org/10.1016/j.coi.2009.09.006; PMID: 19828304
- Sismanopoulos N, Delivanis DA, Alysandratos KD, Angelidou A, Vasiadi M, Therianou A, et al. IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis. PLoS One 2012; 7:e33271; http://dx.doi.org/10.1371/journal.pone.0033271; PMID: 22413008
- Esche C, de Benedetto A, Beck LA. Keratinocytes in atopic dermatitis: inflammatory signals. Curr Allergy Asthma Rep 2004; 4:276 - 84; http://dx.doi.org/10.1007/s11882-004-0071-8; PMID: 15175141
- Pastore S, Mascia F, Girolomoni G. The contribution of keratinocytes to the pathogenesis of atopic dermatitis. Eur J Dermatol 2006; 16:125 - 31; PMID: 16581561
- Bao L, Shi VY, Chan LS. IL-4 up-regulates epidermal chemotactic, angiogenic, and pro-inflammatory genes and down-regulates antimicrobial genes in vivo and in vitro: Relevant in the pathogenesis of atopic dermatitis. Cytokine 2012.
- Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, et al. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol 2013; 22:30 - 5; http://dx.doi.org/10.1111/exd.12047; PMID: 23173934
- Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol 2011; 2; http://dx.doi.org/10.4172/2155-9899.1000110; PMID: 21994899
- Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol 2008; 128:2248 - 58; http://dx.doi.org/10.1038/jid.2008.74; PMID: 18385759
- Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol 2000; 105:1063 - 70; http://dx.doi.org/10.1067/mai.2000.107604; PMID: 10856136
- Wery-Zennaro S, Letourneur M, David M, Bertoglio J, Pierre J. Binding of IL-4 to the IL-13Ralpha(1)/IL-4Ralpha receptor complex leads to STAT3 phosphorylation but not to its nuclear translocation. FEBS Lett 1999; 464:91 - 6; http://dx.doi.org/10.1016/S0014-5793(99)01680-4; PMID: 10611490
- Wu AH, Low WC. Molecular cloning and identification of the human interleukin 13 alpha 2 receptor (IL-13Ra2) promoter. Neuro Oncol 2003; 5:179 - 87; http://dx.doi.org/10.1215/S1152851702000510; PMID: 12816724
- Hussein YM, Ahmad AS, Ibrahem MM, Elsherbeny HM, Shalaby SM, El-Shal AS, et al. Interleukin 13 receptors as biochemical markers in atopic patients. J Investig Allergol Clin Immunol 2011; 21:101 - 7; PMID: 21462799
- Yawalkar N, Uguccioni M, Schärer J, Braunwalder J, Karlen S, Dewald B, et al. Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J Invest Dermatol 1999; 113:43 - 8; http://dx.doi.org/10.1046/j.1523-1747.1999.00619.x; PMID: 10417617
- Kagami S, Kakinuma T, Saeki H, Tsunemi Y, Fujita H, Nakamura K, et al. Significant elevation of serum levels of eotaxin-3/CCL26, but not of eotaxin-2/CCL24, in patients with atopic dermatitis: serum eotaxin-3/CCL26 levels reflect the disease activity of atopic dermatitis. Clin Exp Immunol 2003; 134:309 - 13; http://dx.doi.org/10.1046/j.1365-2249.2003.02273.x; PMID: 14616792
- Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, et al. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem 2001; 276:6675 - 88; http://dx.doi.org/10.1074/jbc.M001748200; PMID: 11053426
- Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene 2000; 19:2628 - 37; http://dx.doi.org/10.1038/sj.onc.1203481; PMID: 10851062
- Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 2005; 307:269 - 73; http://dx.doi.org/10.1126/science.1105166; PMID: 15653507
- Miyata S, Matsuyama T, Kodama T, Nishioka Y, Kuribayashi K, Takeda K, et al. STAT6 deficiency in a mouse model of allergen-induced airways inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clin Exp Allergy 1999; 29:114 - 23; http://dx.doi.org/10.1046/j.1365-2222.1999.00405.x; PMID: 10051710
- He R, Kim HY, Yoon J, Oyoshi MK, MacGinnitie A, Goya S, et al. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. J Allergy Clin Immunol 2009; 124:761 - 70, e1; http://dx.doi.org/10.1016/j.jaci.2009.07.040; PMID: 19815118
- Bao L, Alexander J, Shi VY, Chan LS. IL-4 up-regulates epidermal IL-19 expression in atopic dermatitis. J Invest Dermatol 2012; 132:Suppl 1 S1 - S18; http://dx.doi.org/10.1038/jid.2012.79
- Schittek B. The antimicrobial skin barrier in patients with atopic dermatitis. Curr Probl Dermatol 2011; 41:54 - 67; http://dx.doi.org/10.1159/000323296; PMID: 21576947
- Howell MD, Boguniewicz M, Pastore S, Novak N, Bieber T, Girolomoni G, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol 2006; 121:332 - 8; http://dx.doi.org/10.1016/j.clim.2006.08.008; PMID: 17015038
- Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, et al. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol 2005; 125:738 - 45; http://dx.doi.org/10.1111/j.0022-202X.2005.23776.x; PMID: 16185274
- Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 2006; 24:341 - 8; http://dx.doi.org/10.1016/j.immuni.2006.02.006; PMID: 16546102
- Wolf R, Wolf D. Abnormal epidermal barrier in the pathogenesis of atopic dermatitis. Clin Dermatol 2012; 30:329 - 34; http://dx.doi.org/10.1016/j.clindermatol.2011.08.023; PMID: 22507048
- Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 2008; 126:332 - 7; http://dx.doi.org/10.1016/j.clim.2007.11.006; PMID: 18166499
- Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2007; 120:150 - 5; http://dx.doi.org/10.1016/j.jaci.2007.04.031; PMID: 17512043
- Sawada E, Yoshida N, Sugiura A, Imokawa G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: an implication for the disrupted barrier mechanism in atopic dermatitis. J Dermatol Sci 2012; 68:25 - 35; http://dx.doi.org/10.1016/j.jdermsci.2012.07.004; PMID: 22884781
- Yosipovitch G, Papoiu AD. What causes itch in atopic dermatitis?. Curr Allergy Asthma Rep 2008; 8:306 - 11; http://dx.doi.org/10.1007/s11882-008-0049-z; PMID: 18606082
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 2004; 5:752 - 60; http://dx.doi.org/10.1038/ni1084; PMID: 15184896
- Kasraie S, Niebuhr M, Baumert K, Werfel T. Functional effects of interleukin 31 in human primary keratinocytes. Allergy 2011; 66:845 - 52; http://dx.doi.org/10.1111/j.1398-9995.2011.02545.x; PMID: 21261663
- Lee CH, Hong CH, Yu WT, Chuang HY, Huang SK, Chen GS, et al. Mechanistic correlations between two itch biomarkers, cytokine interleukin-31 and neuropeptide β-endorphin, via STAT3/calcium axis in atopic dermatitis. Br J Dermatol 2012; 167:794 - 803; http://dx.doi.org/10.1111/j.1365-2133.2012.11047.x; PMID: 22578170