Abstract
Dendritic cells (DC) play a key role in immunity by recognizing and presenting antigens. Cytokines and cytokine-activated transcription factors are fundamental in the regulation of the DC differentiation and their functions. While the role of STAT3 in DC development is well established, the function of STAT5 in DC has yet to be fully elucidated. A recent study published in Nature Immunology by Bell et al., using the DC-specific deletion of Stat5, demonstrated the importance of STAT5 in the induction of a Th2 response in DC. As the activation of this transcription factor is not required for the induction of a Th1 response, the authors further investigated the role STAT5 in the signaling to thymic stromal lymphopoietin (TSLP), a cytokine known to be important for type 2 inflammatory responses. Their results demonstrate the importance of STAT5 activation during TLSP-induced Th2 responses and suggest that DC are a key TLSP target.
The dendritic cell compartment (DC) is a key cell population within the immune system. DC antigen-presenting and regulatory capacities are necessary for both an immune response and self-tolerance maintenance.Citation1 DC can be classified in two main types: plasmacytoid (pDC) and conventional (cDC) based on their phenotypes, locations, and functional properties.Citation2,Citation3 While pDC are involved in immune tolerance and T cell activation, cDC are required for adaptive immune responses.Citation4-Citation6
The role of cytokines and cytokine-responsive transcription factors in DC differentiation and maturation is well documented. For example studies in mice deficient in Fms-related tyrosine kinase 3 ligand (Flt3L) indicate that this cytokine plays nonredundant roles in DC development.Citation7 Signal transducer and activator of transcription 3 (STAT3) is known to be crucial for DC progenitor expansion and DC differentiation.Citation8 The reduction in DC numbers observed in Flt3L-deficient mice has been attributed to the absence of STAT3 activation.Citation8
In contrast to the established role of STAT3, the function of STAT5 in DC development remains unclear. Investigating the latter has been rendered difficult by the redundancy between STAT5A and STAT5B as well as the lethality of Stat5a and Stat5b double inactivation in mice.Citation9 To circumvent this obstacle, Stat5−/− chimeric mice have been used by Esashi et al. to demonstrate that GM-CSF-induced STAT5 activation inhibits pDC development.Citation10 This inhibition was shown to be due to the blockade of Irf8, an essential factor that plays a role in pDC maturation by modulating the expression of key regulatory transcription factors such as Spi-B, IRF7, and receptors like Toll-like receptor 9 and Flt3.Citation11
Like GM-CSF,Citation12 thymic stromal lymphopoietin (TSLP) induces STAT5 activation in DC. TSLP is a four-helix bundle cytokine, mostly expressed in epithelial cells.Citation13 It is an important regulator of mucosal immunity.Citation14 TLSP activates a receptor formed by the association of the TSLP and IL-7Rα receptor (R) chains.Citation15 IL-7Rα and TLSPR recruit, respectively, the Janus kinases JAK1 and JAK2.Citation16 Activation of its receptor by TLSP was shown to induce STAT5 phosphorylation and expression of the STAT5-inducible gene Cis encoding the cytokine inducible SH2-containing protein.Citation17 Activation of DC by TLSP triggers inflammatory Th2 responses. The Th2-microenvironment created by TLSP-stimulated DC is similar to that observed in allergic inflammation, suggesting that it could contribute to pathologies involving Th2 immune responses such as allergies, atopy, and asthma. In line with this hypothesis, TLSP-transgenic mice develop Th2-type inflammation at the sites of transgene expression.Citation17
To investigate the role of STAT5 in TSLP-induced Th2 responses, Bell et al. used an elegant DC-specific Stat5 deletion mouse model in which mice with a “floxed” Stat5a-Stat5b locus were crossed with CD11c-Cre transgenic mice.Citation18 Bell et al. observed that CD11c-Cre Stat5fl/fl mice (i.e., with a DC-specific deletion of Stat5) had a normal immune cellularity when compared with control littermates and no changes in specific immune cell type or DC subset (pDC, CD8+ DC, and CD4+ DC) frequencies. This led the authors to conclude that STAT5 is not required for the development of any spleen, lung, lymph node, or skin DC subtype. It is important to note that the efficiency of STAT5 depletion in the model used by Bell et al. was only 50%, thus limiting the conclusion that can be drawn regarding the roles of STAT5 in this DC subtype.
Having concluded that STAT5 deletion had no detectable effect on DC development, Bell et al. next investigated the role of STAT5 in Th2-induced responses using a mouse model of allergic contact dermatitis.Citation19 In this model, the Th2 response is induced by a combination of the hapten FITC and the phthalate ester DBP.Citation20 The mice with a CD11c-CRE induced Stat5 deletion had lower IL-4 mRNA levels, reduced ear swelling, and less DC present in the FITC/DBP exposed skin-draining lymph nodes. These results unambiguously demonstrate the role of DC STAT5 in activating antigen-specific T cells during a Th2 skin response.
The specificity of these observations for the Th2 response was established using a Th1 contact hypersensitivity model, induced by sensitization, and challenged with the hapten 2,4-dinitrofluorobenzene DNFB,Citation19 in which no difference between WT and Cre+Stat5fl/fl mice were observed.
Bell et al. further demonstrated the role of DC STAT5 in Th2 inflammation using two well established Th1 and Th2 mouse models of airway inflammation. Using ovalbumin-induced airway inflammation, they showed that DC require STAT5 for the development and promotion of Th2 responses in the lungs. Alternatively, when airway inflammation was induced by infection with influenza A virus, which induces a Th1 immune response, no difference could be observed between Cre−Stat5fl/fl and Cre+Stat5fl/fl mice, indicating that the role of STAT5 was Th2-specific and that Stat5 deletion did not simply lead to overall lung inflammation reduction. No difference between control and STAT5-deficient mice were observed in the total number of lung cells, mediastinal lymph nodes, or bronchoalveolar lavage fluids or in the immunodominant CD8 T cell response. In conclusion, STAT5 activation in DC is required for mounting an anti-ovalbumin Th2 response but unnecessary for Th1 and cytotoxic T cell response to influenza A virus.
TSLP is a cytokine known to activate STAT5; mice deficient in the TLSP receptor, like the CD11c-Cre Stat5fl/fl mice, were resistant to inflammation induced by FITC-DBC.Citation21 This led Bell et al. to hypothesize that TSLP is the cytokine that activates STAT5 in the DC of the skin and lung to promote Th2 immunity.
Previous studies have shown that Flt3L-generated bone marrow-derived DC (FL-DC) in vitro respond to TLSP.Citation22 By infecting FL-DC generated from Jak2fl/fl bone marrow infected with a retrovirus expressing the Cre recombinase and green fluorescent protein (GFP), Bell et al. generated Jak2– GFP+ and Jak2+ GFP- FL-DC. These control and Jak2– FL-DC were used to demonstrate that the TLSP sensitivity of DC also requires JAK2 as well as STAT5 activation
To complete this study, the authors analyzed the production of a direct target of TSLP, the Th2 type chemokine CCL17, as an indicator of TLSP signaling. They demonstrated, using a mouse strain with the sequence encoding GFP inserted in the Clc17 locus, that TSLP induces less CCL17 production in DC lacking STAT5 compared with control DC. As CCL17 attracts CCR4-expressing Th2 cells,Citation23 Bell et al. finally demonstrated using CD4 T cells transgenic for an OVA-specific TCR that the ability of TLSP to promote a Th2 response in vitro and in vivo was compromised in STAT5-deficient DC ().
Figure 1. Importance of STAT5 in TSLP-activated DC. The study by Bell et al. demonstrates the role of STAT5 activation by TSLP in DC. This STAT5 activation induces an upregulation of costimulation molecules like CD80, CD86, or OX40L; associated to a CCL17 production. TSLP-induced STAT5 activation mediates Th2 responses by CD4 T cells.
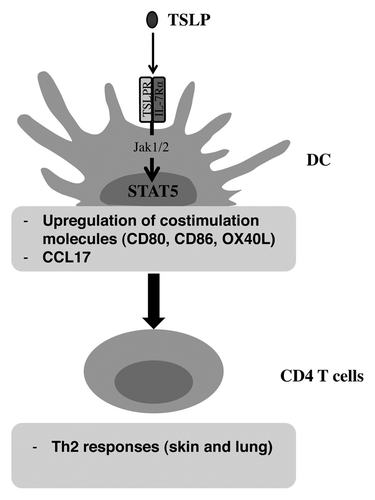
In conclusion, this study that utilized CD11c-Cre Stat5fl/fl mice demonstrate that the activation of DC STAT5 is required for the induction of Th2-type CD4 T cell response but dispensable for its Th1 counterpart. STAT5 is required for TLSP signaling in DC and for DC-mediated TLSP stimulation of Th2 responses. Altogether, the results further underline the importance of TLSP and TLSP-induction of STAT5 activation in allergic diseases and suggests that STAT5 might represent a key therapeutic target for Th2 airway and skin inflammation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245 - 52; http://dx.doi.org/10.1038/32588; PMID: 9521319
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007; 7:19 - 30; http://dx.doi.org/10.1038/nri1996; PMID: 17170756
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010; 234:45 - 54; http://dx.doi.org/10.1111/j.0105-2896.2009.00879.x; PMID: 20193011
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 2011; 29:163 - 83; http://dx.doi.org/10.1146/annurev-immunol-031210-101345; PMID: 21219184
- Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system?. Nat Rev Immunol 2011; 11:558 - 65; http://dx.doi.org/10.1038/nri3027; PMID: 21779033
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol 2007; 7:543 - 55; http://dx.doi.org/10.1038/nri2103; PMID: 17589544
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 1996; 184:1953 - 62; http://dx.doi.org/10.1084/jem.184.5.1953; PMID: 8920882
- Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity 2003; 19:903 - 12; http://dx.doi.org/10.1016/S1074-7613(03)00332-7; PMID: 14670306
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, et al. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 2004; 24:8037 - 47; http://dx.doi.org/10.1128/MCB.24.18.8037-8047.2004; PMID: 15340066
- Esashi E, Wang YH, Perng O, Qin XF, Liu YJ, Watowich SS. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity 2008; 28:509 - 20; http://dx.doi.org/10.1016/j.immuni.2008.02.013; PMID: 18342552
- Zenke M, Hieronymus T. Towards an understanding of the transcription factor network of dendritic cell development. Trends Immunol 2006; 27:140 - 5; http://dx.doi.org/10.1016/j.it.2005.12.007; PMID: 16406699
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992; 176:1693 - 702; http://dx.doi.org/10.1084/jem.176.6.1693; PMID: 1460426
- Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med 2000; 192:671 - 80; http://dx.doi.org/10.1084/jem.192.5.671; PMID: 10974033
- Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol 2007; 25:381 - 418; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141634; PMID: 17378762
- Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol 2000; 1:59 - 64; PMID: 10881176
- Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A 2010; 107:19455 - 60; http://dx.doi.org/10.1073/pnas.1008271107; PMID: 20974963
- Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt RdeW, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol 2007; 25:193 - 219; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141718; PMID: 17129180
- Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K, et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol 2013; 14:364 - 71; http://dx.doi.org/10.1038/ni.2541; PMID: 23435120
- Gorbachev AV, Fairchild RL. Induction and regulation of T-cell priming for contact hypersensitivity. Crit Rev Immunol 2001; 21:451 - 72; http://dx.doi.org/10.1615/CritRevImmunol.v21.i5.30; PMID: 11942559
- Dearman RJ, Kimber I. Role of CD4(+) T helper 2-type cells in cutaneous inflammatory responses induced by fluorescein isothiocyanate. Immunology 2000; 101:442 - 51; http://dx.doi.org/10.1046/j.1365-2567.2000.01126.x; PMID: 11122447
- Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol 2010; 184:2638 - 45; http://dx.doi.org/10.4049/jimmunol.0902960; PMID: 20124100
- D’Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med 2003; 198:293 - 303; http://dx.doi.org/10.1084/jem.20030107; PMID: 12874262
- Homey B, Zlotnik A. Chemokines in allergy. Curr Opin Immunol 1999; 11:626 - 34; http://dx.doi.org/10.1016/S0952-7915(99)00028-X; PMID: 10631546