Abstract
JAK-STAT3 signaling, while regulating many aspects of cancer development and progression, promotes invasion and metastasis through activation of key metastasis promoting genes such as WASF3. STAT3 promotes WASF3 expression and JAK2 independently activates it, which is required for invasion. JAK-STAT3 signaling is dependent on WASF3 function, since its inactivation in cells expressing JAK-STAT3 suppresses invasion. WASF3 overexpression leads to activation of NFκB and ZEB1 which also promote invasion through regulation of target genes involved in metastasis. NFκB frequently cooperates with STAT3 to upregulate metastasis promoting genes such as matrix metalloproteinases and cytokines, as well as to suppress microRNAs which can suppresses invasion. This better understanding of the complex role played by JAK-STAT3 in the regulation of cell movement, invasion, and metastasis provides opportunities to suppress this lethal aspect of cancer progression by not only targeting the JAK and STAT3 proteins directly, but also some of the downstream effectors of JAK-STAT3 signaling.
Introduction
Cancer mortality is largely due to metastatic spread of the primary tumor.Citation1 A wide variety of genes and micro-RNAs (miRNA) appear to be implicated in the metastasis process,Citation2-Citation5 in many cases independently of the dysregulation of genes leading to uncontrolled cell proliferation.Citation6 It is becoming clear, however, that despite the plethora of reports implicating single genes in metastasis, there may be a few master regulators that coordinate the process. Activation of the JAK-STAT3 pathway, in particular, has been repeatedly correlated with increased invasion and metastasis in a wide variety of cancer typesCitation7 although, until recently, there have been few mechanistic insights into how this occurs. Identification of key proteins is required for the transduction of the JAK-STAT3 signal specifically to promote invasion, however, brings many disparate observations together and begins to define critical nodes in the overall signaling process that might be exploited to suppress metastasis in a clinical setting. In this article we review the emerging evidence that specifically implicates JAK-STAT3 signaling in cell movement and invasion and investigate some of the complex regulatory relationships between other transcription factors and miRNA in this process. We now describe how activation of JAK-STAT3 signaling leads to upregulation of other key proteins that are primarily involved in orchestrating cell movement and invasion and which may prove to be targets for suppression of invasion and metastasis.
The Invasion and Metastasis Process
Not all epithelial cancer cells are capable of expressing the metastatic phenotype, and those that do must be able to escape the constraints of the primary tumor and enter the circulatory system (blood or lymphatic). This process requires production of enzymes that can break down basement membranes to allow invasion, which is a prerequisite for metastasis. Invasion also depends on reorganization of the actin cytoskeleton to facilitate migration/invasion. The ability of these cancer cells to leave the tumor mass depends on losing cell-cell contact early in the process, which has been associated with a change in cell shape referred to as the epithelial-to-mesenchyme transition (EMT). EMTCitation8 is associated with the loss of cell adhesion proteins such as E-cadherin.Citation9 Once in the circulatory system, the isolated cancer cell must survive before eventually exiting at a distant site to establish as a micro-colony. The mechanics of this process have been reviewed extensively elsewhere.Citation10 Genes that enhance all of these individual capabilities are considered metastasis promoting, and must be activated in concert for metastasis to be achieved.Citation2-Citation5 This process requires responses to external signaling cues from growth factors and cytokines, which are transmitted through extracellular receptors via adaptor protein complexes to regulate gene expression by transcription factors. Within this continuum, the same control pathways also regulate the ability of cells to move (motility) and migrate by regulating actin dynamics, and invade and metastasize by re-orchestrating gene expression. While strictly speaking, analysis of the metastatic phenotype ultimately requires an in vivo model, there are a number of in vitro phenotypes that have been commonly used to predict metastasis with remarkable accuracy and are broken down into motility, migration, and invasion. In this review we accept these phenotypes as part of the overall process in support of the role of JAK-STAT3 signaling in metastasis.
The STAT Family of Genes
Of the seven STAT family members (STAT1, 2, 3, 4, 5A, 5B, and 6),Citation11 STAT2 is largely involved with viral infectionsCitation12 and STATs 4 and 6 are primarily involved in lymphocyte function,Citation13 particularly associated with helper T cells.Citation11 While there are reports that STAT5A/B can promote invasion and metastasis,Citation14,Citation15 it now appears that STAT5, at least in prostate cancer, may be more involved in promoting growth and preventing apoptosis.Citation16 STAT1 and STAT3 have been implicated in cancer progression but with opposite roles. STAT1 appears to promote anti-proliferative and pro-apoptotic responses in cancer cellsCitation17 and, while STAT3 can clearly promote proliferation, in contrast to the other family members, it has been consistently implicated in promoting cell migration and invasionCitation17,Citation18 as well as metastasisCitation16 in many cancer cell types and is, therefore, the focus of this review.
Involvement of JAK-STAT3 Signaling in Cellular Phenotypes Associated with Metastasis
In normal cells, STAT3 signaling is under tight spatial and temporal control as a result of negative feedback mechanisms involving the suppressors of cytokine signaling (SOCS), in particular SOCS3, and tyrosine phosphatases but, in cancer cells, constitutive activation of STAT3 is ubiquitous.Citation7 STAT3 activation requires phosphorylation (typically at tyrosine 705) and this can be achieved directly by growth factor receptors that have inherent tyrosine kinase activity, such as EGFR, ERBB2, VEGFR, and PDGFR, in response to growth factor stimulation.Citation7 These receptors may also recruit non-receptor kinases such as SRC and ABL which can also activate STAT3. Constitutive activation of these receptors by either their overexpression or as a result of mutation, has been associated with increased invasion and metastasis in many cancer cell types.Citation19-Citation22 STAT3 can also be activated by members of the interleukin family of cytokines,Citation7 but since cytokine receptors do not have intrinsic tyrosine kinase activity, they must recruit kinases such as members of JAKs. JAKs phosphorylate tyrosine residues on the STAT clients facilitating STAT3 activation, which is required for its relocation to the nucleus, leading to transcription of the target genes.
The constitutive activation of STATs in cancer cells is usually facilitated through excessive stimulation by cytokines or growth factors, which may be produced by the tumors themselves (autocrine stimulation) or be derived from either stromal or inflammatory cells associated with tumor development (paracrine stimulation), which has led to the notion that the microenvironment in the metastatic niche is important for successful metastasis.Citation23 STAT3 can also stimulate its own activation by regulating genes (e.g., IL-6, IL-10, and EGF) that promote its increased activation or are themselves direct STAT3 activators (e.g., RAS, SRC, and ABL). Thus, paracrine production of IL-6 can promote autocrine STAT3 signaling.Citation24 In experimental systems, JAK-STAT3 signaling can be conveniently activated using IL-6, which has been shown to increase migration and invasion in vitroCitation25-Citation27 and metastasis in vivo.Citation24 Consistently, downregulation of cytokines and their receptors in different cell systems is associated with a less aggressive phenotype.Citation28
Phosphoactivation of STAT3 by JAKs is required for the dimerization that is essential for STAT3 to function as a transcription factor.Citation7 Intuitively, therefore, it might be expected that STAT3 promotes invasion and metastasis as a consequence of regulating genes involved in this process. Indeed, STAT3 is known to regulate expression of essential components of the metastasis continuum, such as the matrix metalloproteinases (MMP) that degrade the basement membranes and extracellular matrix.Citation29 Most aggressive cancer cells overexpress MMPs which facilitate intravasation into the vasculature and extravasation at the metastatic site. Other targets of the STAT3 transcription factor are also intimately involved in the invasion and metastasis process. It has been consistently observed, for example, that HSP70 and HSP90 promote invasion and metastasisCitation30-Citation33 and both are regulated by STAT3.Citation23 STAT3 also regulates HIF1a, which induces many other genes related to invasion and metastasis such as VEGFR.Citation23 Thus, activation of STAT3 has a broad impact on the expression of genes that promote invasion.
The idea that the pro-metastatic effects of STAT3 signaling results from upregulation of metastasis genes, however, may be too simplistic, since there are examples where, even in the presence of constitutive STAT3 signaling and IL-6 responsiveness, inactivation of other genes can still suppress invasion.Citation26 Furthermore, the fact that overexpression of these metastasis promoting genes in cells that normally do not express STAT3 can promote invasion, argues for a STAT3-independent mechanism.Citation26 We have recently demonstrated, for example, that while the expression of the WASF3 pro-metastasis gene is regulated by STAT3, its ability to regulate invasion depends on its direct phosphoactivation by JAK2, independently of STAT3 activation.Citation26
JAK-STAT3 Regulation of the WASF3 Metastasis Promoter Gene
Early evidence implicating JAK-STAT3 signaling in the invasion/metastasis process was largely correlative, with increased STAT3 expression seen in advanced stage tumors,Citation34,Citation35 at the leading edges of invasive cancersCitation24 and associated with survival.Citation36,Citation37 There were, however, few mechanistic insights into the underlying basis of this effect from these studies. For cancer cells to escape the restraints of the parental tumor, they must become able to dissociate from the surrounding cells. Epithelial cells maintain cell contact though adhesion molecules such as the claudinsCitation38 and E-cadherin.Citation9 EMT is characterized by loss of E-cadherin expressionCitation39 and aggressive subtypes of cancer have low claudin expression.Citation40 The relevance of STAT3 to this observation came with the demonstration that E-cadherin expression was downregulated by transcription factors such as ZEB, Twist, and Snail, which are activated by STAT3.Citation41,Citation42 Losing cell–cell contact by cancer cells, however, is only one component in successfully acquiring the invasive phenotype, since these cells must also be able to reorganize their actin cytoskeleton to facilitate structural changes that are essential for cell migration and invasion.Citation43 Among a variety of genes involved in actin cytoskeleton reorganization, WASF3 in particular, has been shown to be associated with invasion and metastasisCitation44 and is upregulated in high-grade tumors.Citation45
WASF3 is a member of the Wiskott–Aldrich syndrome family of proteins (WASF),Citation46 which are involved in regulating actin cytoskeleton dynamicsCitation47 through recruitment of the ARP2/3 complexCitation48,Citation49 which promotes actin polymerization. As a result, increased production of membrane structures such as lamellipodia occurs,Citation44 which is associated with cell motility, migration and invasion.Citation50 Knockdown of WASF3 in a variety of cell types in vitro, leads to loss of invasionCitation50,Citation51 and, where it has been examined, metastasis in vivo.Citation50,Citation52 This loss of invasion and metastasis is independent of the genetic background (e.g., mutant p53, mutant RAS, etc.) of the cancer cells and, whether or not they express STAT3. Overexpression of WASF3 in non-invasive cancer cells enhances invasion.Citation53 Inactivation of WASF3 in cells that constitutively express activated STAT3, however, still leads to suppression of invasion and metastasis, demonstrating that the activation of the STAT3-regulated genes described above is not sufficient to maintain the metastatic phenotype.Citation53 Several studies demonstrate that increasing WASF3 levels leads to increased invasion,Citation53,Citation54 and IL-6 treatment of cancer cells leads to increased expression levels and increased activation levels of WASF3.Citation53 STAT3 was shown to bind to elements in the WASF3 promoter, upregulating its expression in response to IL-6 treatment,Citation53 which led to increased invasion potential. To promote invasion, WASF3 must be phosphoactivated, which can be achieved by different kinases such as PI3K,Citation44 ABL,Citation55 and JAK2.Citation53 When JAK2 is deficient in these cells, however, migration is suppressed demonstrating that JAK2-dependent activation of WASF3 is required for increased migration and by inference, invasion, and metastasis, as we have shown in zebrafish models of human cancer cell metastasis.Citation56 As a consequence of loss of JAK2, WASF3 levels are further reduced because of the concomitant loss of activated STAT3. Thus, activation of WASF3 by JAK2 is the critical event promoting migration in these and other cell types, with the coincident activation of STAT3 leading to increased WASF3 protein levels (). In the study of WASF3 activation, JAK2 was shown to be the critical activating kinase, even though JAK1 was also identified in the WASF3 immunocomplex.Citation53 JAK1 is a major activator of STAT3 in many cell systemsCitation7 and, in the highly metastatic U2A fibrosarcoma cells, was required for invasion,Citation57,Citation58 with loss of JAK1 expression suppressing metastasis to a lesser extent.Citation56 It appears, therefore, that JAK1/2 may have the same role in promoting invasion but possibly in a cell context-dependent manner.
Figure 1. Summary of the IL-6/JAK-STAT interaction with the WASF3 gene. Activation of STAT3 leads to increased expression of WASF3 which is then recruited to the membrane where it is activated by JAK2 to promote invasion.
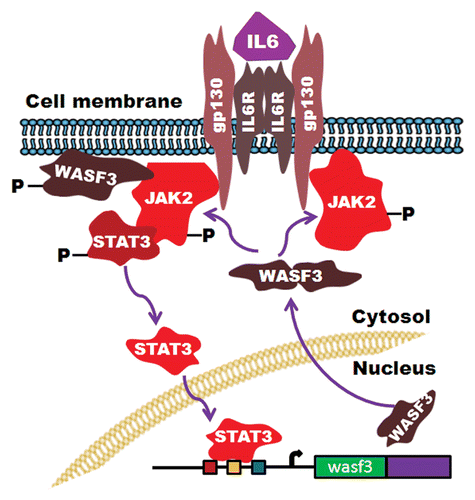
The ability of IL-6 to promote invasion and metastasis, therefore, is clearly related to its promotion of WASF3 activation, since knockdown of WASF3 in many cell types suppresses invasion despite their constitutive expression of STAT3 and JAK1/2.Citation44,Citation50,Citation51 This JAK2-dependent promotion of invasion by WASF3 appears to be due to its ability to influence expression of the KISS1 metastasis suppressor gene.Citation59 KISS1, through its receptor GPR54, regulates the function of the IκBα repressor of NFκB, which normally sequesters the p65 subunit of NFκB in the cytoplasm.Citation60 High WASF3 levels lead to downregulation of KISS1,Citation59 which in turn leads to phosphorylation-mediated degradation of IκBα, allowing NFκB to move into the nucleus and activate pro-metastasis genes such as MMPs and IL-6.Citation59,Citation60 NFκB also regulates ZEB1 expression directly,Citation61 which promotes EMT in part through downregulation of E-cadherin. ZEB1 also suppresses expression of a number of different microRNAs which have also been associated with metastasis, notably the miRNA200 familyCitation62,Citation63 which can regulate WASF3 mRNA stability.Citation64 Increased ZEB1 activity, therefore, as a result of increased NFκB activity following WASF3 overexpression, suppresses expression of miRNA200 family members, thus creating a regulatory loop to increase WASF3 levels further.Citation61 These observations demonstrate the complexity of the JAK2-STAT3 influence on invasion.
STAT3 also has indirect effects on WASF3-promotion of invasion. We recently showed that WASF3 is stabilized by HSP70,Citation54 and suppression of HSP70 function led to reduced invasion in a WASF3-dependent manner. In addition, while WASF3 is not an HSP90 client protein, it is dependent on HSP90 for its activation since ABL kinase is a client of HSP90, and ABL can activate WASF3. This loss of HSP90 prevents WASF3 activation leading to reduced invasion.Citation54 Since both HSP70 and HSP90 are regulated by STAT3,Citation23 these proteins further impact on the ability of WASF3 to control cell invasion.
MicroRNA Deregulation Promotes Cancer Progression and Motility through Regulation of JAK-STAT3
MiRNAs are non-coding RNAs that target mRNAs for decay or interfere with translation, usually through binding to the 3′ untranslated region of their targets.Citation65 Generally, miRNAs are abnormally expressed and hyper-activated in tumors, exerting oncogenic effects on multiple pathways causing increased proliferation and reduced apoptosis. There is, however, a subset of miRNAs that are directly associated with metastasis and invasion which are referred to as the “metastamirs”.Citation66 These miRNAs regulate the function of genes important in metastasis, and individual miRNAs can target many different genes simultaneously. The interaction between STAT3 and various miRNAs involves several regulatory feedback loops associated with invasionCitation67 where either STAT3 drives expression of miRNAs that promote invasionCitation68,Citation69 or where miRNAs suppress the function of STAT3 inhibitors such as SOCS and PIAS.Citation70,Citation71 MiRNAs can also bind directly to the 3′UTR of STAT3 to regulate its activity,Citation72 thereby suppressing invasion and metastasis.Citation73 As expected, downregulation of JAK2 by miRNAs has the same effect, since they lead to decreased levels of activated STAT3 which affects proliferationCitation74 and, presumably, invasion.
STAT3-NFκB Interactions
Extracellular cues transmit signals to regulatory hubs within the cancer cell that influence gene expression related to cell movement and invasion. These signals are typically not linear events and considerable cross talk between regulatory genes is involved in the complex regulation of phenotypes such as invasion. From the WASF3 studies, for example, IL-6 treatment produced a coordinated activation of NFκB through upregulation and activation of WASF3 by JAK2-STAT3. Chromatin binding studies demonstrate a significant overlap between the genes regulated by these two transcription factors, and it has been shown that they co-regulate a sub set of genes associated with invasion.Citation7 Cooperation between NFκB and STAT3 to promote invasion has also been demonstrated at a number of levels.Citation75 For example, STAT3 and NFκB both regulate the expression of proinvasive genesCitation23 including cytokines, and they are also activated by the cytokines they regulate.Citation73 Both are these transcription factors are also centrally involved with inflammation,Citation75 which also promotes tumor progression. At a mechanistic level, STAT3 can bind directly to the NFκB p65 subunit, sequestering it in the nucleus and leading to prolonged NFκB activation.Citation61,Citation76,Citation77 In our studies, we demonstrated that WASF3 can increase NFκB activity by releasing it from KISS1-mediated IκBα repression, creating a feed forward loop promoting motility and invasion through activation of IL-6-STAT3, which further increases WASF3 expression levels.
Targeting the JAK-STAT3 Pathway to Suppress Invasion and Metastasis
The clear role of JAK-STAT3 signaling in promoting invasion and metastasis opens the possibility of targeting these proteins, and possibly associated pathways, to control these phenotypes. Genetic inactivation of STAT3 and JAK2 in model cell systems leads to a reduction in motility and invasion (see above), suggesting that pharmacological targeting of these proteins might have the same effect.Citation78 Increasingly, JAK-STAT3 targeting drugs are being developed, as well as other pharmacological agents that affect genes that impact on JAK-STAT3 activation.Citation18,Citation78 For example, using antibodies that target either IL-6, or the soluble or membrane bound IL-6 receptors, STAT signaling can be downregulated, and these agents are currently in clinical trials. Direct targeting of JAK1 and JAK2 using agents such as AG490 have also been shown to suppress invasion of cancer cells in vitro,Citation78 and knockdown of these kinases suppresses metastasis in animal models.Citation24 Similarly, pharmacologically targeting STAT3 directly leads to suppression of invasion.Citation78 As the signaling pathways become more defined, it is likely that other JAK-STAT3 nodes will be identified that may be equally important targets for a more complete blockade of metastasis.
Conclusion
JAK-STAT3 signaling is clearly a central regulatory hub in the overall process leading to invasion and metastasis. It is becoming clear, however, that its role in this process is made more complex through the diversity of interactions with other signaling pathways. STAT3 also has many other functions beyond metastasis and, in considering targeting JAK-STAT3 as a treatment to suppress invasion and metastasis, a better understanding of the metastasis-specific interactions will be required to provide specificity and avoid unfortunate side effects that might be related to its other functions. In this case, defining proteins that are dependent on JAK-STAT3 function, but which are more specific for the metastasis phenotype, may present better targets. It is likely that teasing out the specific role of STAT3 in the development of metastasis, however, will depend on defining the consequences of interactions with other regulatory molecules that may complicate the analysis if they operate in a cell specific and/or temporal-specific context.
| Abbreviations: | ||
| ABL | = | Abelson leukemia virus |
| ARP | = | actin related protein |
| ERBB | = | erythroblastic leukemia viral oncogene, family member B |
| EGF | = | epidermal growth factor |
| EGFR | = | epidermal growth factor receptor |
| EMT | = | epithelial to mesenchyme transition |
| HIF | = | hypoxia inducible factor |
| HSP | = | heat shock protein |
| IκBα | = | inhibitor of NFκB |
| IL | = | interleukin |
| JAK | = | Janus kinase |
| KISS | = | kisspeptin |
| MMP | = | matrix metalloproteinase |
| NFκB | = | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PDGFR | = | platelet derived growth factor receptor |
| PIAS | = | protein inhibitor of activated STAT |
| RAS | = | Rat sarcoma gene |
| SOCS | = | suppressor of cytokine signaling |
| SRC | = | sarcoma kinase |
| STAT | = | signal transducer and activator of transcription |
| VEGFR | = | vascular endothelial growth factor receptor |
| WASF | = | Wiskott Aldrich syndrome family |
| ZEB | = | zinc finger homeodomain enhancer-binding protein |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol 2013; 15:546 - 54; http://dx.doi.org/10.1038/ncb2769; PMID: 23728460
- Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol 2013; 87:1 - 11; http://dx.doi.org/10.1016/j.critrevonc.2012.12.007; PMID: 23332547
- Trapé AP, Gonzalez-Angulo AM. Breast cancer and metastasis: on the way toward individualized therapy. Cancer Genomics Proteomics 2012; 9:297 - 310; PMID: 22990109
- Roodman GD. Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev 2012; 31:569 - 78; http://dx.doi.org/10.1007/s10555-012-9372-x; PMID: 22706844
- Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer 2003; 3:55 - 63; http://dx.doi.org/10.1038/nrc967; PMID: 12509767
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer 2004; 4:97 - 105; http://dx.doi.org/10.1038/nrc1275; PMID: 14964307
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420 - 8; http://dx.doi.org/10.1172/JCI39104; PMID: 19487818
- Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci 2013; 126:393 - 401; http://dx.doi.org/10.1242/jcs.100115; PMID: 23525005
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009; 9:274 - 84; http://dx.doi.org/10.1038/nrc2622; PMID: 19308067
- Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science 2002; 296:1653 - 5; http://dx.doi.org/10.1126/science.1071545; PMID: 12040185
- Chowdhury FZ, Farrar JD. STAT2: A shape-shifting anti-viral super STAT. JAKSTAT 2013; 2:e23633; http://dx.doi.org/10.4161/jkst.23633; PMID: 24058798
- Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene 2000; 19:2577 - 84; http://dx.doi.org/10.1038/sj.onc.1203485; PMID: 10851056
- Moser C, Ruemmele P, Gehmert S, Schenk H, Kreutz MP, Mycielska ME, Hackl C, Kroemer A, Schnitzbauer AA, Stoeltzing O, et al. STAT5b as molecular target in pancreatic cancer--inhibition of tumor growth, angiogenesis, and metastases. Neoplasia 2012; 14:915 - 25; PMID: 23097626
- Gu L, Vogiatzi P, Puhr M, Dagvadorj A, Lutz J, Ryder A, Addya S, Fortina P, Cooper C, Leiby B, et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer 2010; 17:481 - 93; http://dx.doi.org/10.1677/ERC-09-0328; PMID: 20233708
- Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Addya S, Fortina P, Dasgupta A, Hyslop T, et al. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am J Pathol 2010; 176:1959 - 72; http://dx.doi.org/10.2353/ajpath.2010.090653; PMID: 20167868
- Avalle L, Pensa S, Regis G, Novelli F, Poli V. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAKSTAT 2012; 1:65 - 72; http://dx.doi.org/10.4161/jkst.20045; PMID: 24058752
- Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med 2009; 9:626 - 33; http://dx.doi.org/10.2174/156652409788488720; PMID: 19601811
- Chuang TC, Hsu SC, Cheng YT, Shao WS, Wu K, Fang GS, Ou CC, Wang V. Magnolol down-regulates HER2 gene expression, leading to inhibition of HER2-mediated metastatic potential in ovarian cancer cells. Cancer Lett 2011; 311:11 - 9; http://dx.doi.org/10.1016/j.canlet.2011.06.007; PMID: 21757288
- Johnson E, Seachrist DD, DeLeon-Rodriguez CM, Lozada KL, Miedler J, Abdul-Karim FW, Keri RA. HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. J Biol Chem 2010; 285:29491 - 501; http://dx.doi.org/10.1074/jbc.M110.136770; PMID: 20595387
- Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H, Grünert S. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest 2006; 116:1561 - 70; http://dx.doi.org/10.1172/JCI24652; PMID: 16741576
- Yue P, Zhang X, Paladino D, Sengupta B, Ahmad S, Holloway RW, Ingersoll SB, Turkson J. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene 2012; 31:2309 - 22; http://dx.doi.org/10.1038/onc.2011.409; PMID: 21909139
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798 - 809; http://dx.doi.org/10.1038/nrc2734; PMID: 19851315
- Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al. The IL-6/JAK-STAT3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 2013; 15:848 - 62; PMID: 23814496
- Liu Q, Li G, Li R, Shen J, He Q, Deng L, Zhang C, Zhang J. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J Neurooncol 2010; 100:165 - 76; http://dx.doi.org/10.1007/s11060-010-0158-0; PMID: 20361349
- Teng Y, Ghoshal P, Ngoka L, Mei Y, Cowell JK. Critical role of the WASF3 gene in JAK2/STAT3 regulation of cancer cell motility. Carcinogenesis 2013; 34:1994 - 9; http://dx.doi.org/10.1093/carcin/bgt167; PMID: 23677069
- Huang C, Yang G, Jiang T, Huang K, Cao J, Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res 2010; 29:51; http://dx.doi.org/10.1186/1756-9966-29-51; PMID: 20482858
- Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK-STAT and FAK signalings in human hepatocellular carcinoma. Oncogene 2005; 24:6406 - 17; PMID: 16007195
- Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol 2013; 34:2041 - 51; http://dx.doi.org/10.1007/s13277-013-0842-8; PMID: 23681802
- Taiyab A, Rao ChM. HSP90 modulates actin dynamics: inhibition of HSP90 leads to decreased cell motility and impairs invasion. Biochim Biophys Acta 2011; 1813:213 - 21; http://dx.doi.org/10.1016/j.bbamcr.2010.09.012; PMID: 20883729
- Garg M, Kanojia D, Saini S, Suri S, Gupta A, Surolia A, Suri A. Germ cell-specific heat shock protein 70-2 is expressed in cervical carcinoma and is involved in the growth, migration, and invasion of cervical cells. Cancer 2010; 116:3785 - 96; http://dx.doi.org/10.1002/cncr.25218; PMID: 20564126
- Garg M, Kanojia D, Seth A, Kumar R, Gupta A, Surolia A, Suri A. Heat shock protein 70-2 (HSP70-2) expression in bladder urothelial carcinoma is associated with tumour progression and promotes migration and invasion. Eur J Cancer 2010; 46:207 - 15; http://dx.doi.org/10.1016/j.ejca.2009.10.020; PMID: 19914824
- Liu X, Yan Z, Huang L, Guo M, Zhang Z, Guo C. Cell surface heat shock protein 90 modulates prostate cancer cell adhesion and invasion through the integrin-β1/focal adhesion kinase/c-Src signaling pathway. Oncol Rep 2011; 25:1343 - 51; PMID: 21369706
- Jiang R, Jin Z, Liu Z, Sun L, Wang L, Li K. Correlation of activated STAT3 expression with clinicopathologic features in lung adenocarcinoma and squamous cell carcinoma. Mol Diagn Ther 2011; 15:347 - 52; http://dx.doi.org/10.1007/BF03256470; PMID: 22208386
- Lassmann S, Schuster I, Walch A, Göbel H, Jütting U, Makowiec F, Hopt U, Werner M. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol 2007; 60:173 - 9; http://dx.doi.org/10.1136/jcp.2005.035113; PMID: 17264243
- Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol 2010; 16:5380 - 7; http://dx.doi.org/10.3748/wjg.v16.i42.5380; PMID: 21072904
- Takemoto S, Ushijima K, Kawano K, Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M, Kakuma T, et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer 2009; 101:967 - 72; http://dx.doi.org/10.1038/sj.bjc.6605212; PMID: 19638983
- Turksen K, Troy TC. Junctions gone bad: claudins and loss of the barrier in cancer. Biochim Biophys Acta 2011; 1816:73 - 9; PMID: 21515339
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871 - 90; http://dx.doi.org/10.1016/j.cell.2009.11.007; PMID: 19945376
- Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 2010; 12:R68; http://dx.doi.org/10.1186/bcr2635; PMID: 20813035
- Xiong H, Hong J, Du W, Lin YW, Ren LL, Wang YC, Su WY, Wang JL, Cui Y, Wang ZH, et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem 2012; 287:5819 - 32; http://dx.doi.org/10.1074/jbc.M111.295964; PMID: 22205702
- Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res 2012; 22:90 - 106; http://dx.doi.org/10.1038/cr.2011.144; PMID: 21876555
- Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol 2012; 24:277 - 83; http://dx.doi.org/10.1016/j.ceb.2011.12.004; PMID: 22209238
- Sossey-Alaoui K, Li X, Ranalli TA, Cowell JK. WAVE-3 mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J Biol Chem 2005; 280:21748 - 55; http://dx.doi.org/10.1074/jbc.M500503200; PMID: 15826941
- Kulkarni S, Augoff K, Rivera L, McCue B, Khoury T, Groman A, Zhang L, Tian L, Sossey-Alaoui K. Increased expression levels of WAVE3 are associated with the progression and metastasis of triple negative breast cancer. PLoS One 2012; 7:e42895; http://dx.doi.org/10.1371/journal.pone.0042895; PMID: 22952619
- Sossey-Alaoui K, Su G, Malaj E, Roe B, Cowell JK. WAVE3, an actin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene 2002; 21:5967 - 74; http://dx.doi.org/10.1038/sj.onc.1205734; PMID: 12185600
- Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci 2001; 114:1801 - 9; PMID: 11329366
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112:453 - 65; http://dx.doi.org/10.1016/S0092-8674(03)00120-X; PMID: 12600310
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 2007; 8:37 - 48; http://dx.doi.org/10.1038/nrm2069; PMID: 17183359
- Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK. WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res 2005; 308:135 - 45; http://dx.doi.org/10.1016/j.yexcr.2005.04.011; PMID: 15907837
- Teng Y, Ren MQ, Cheney R, Sharma S, Cowell JK. Inactivation of the WASF3 gene in prostate cancer cells leads to suppression of tumorigenicity and metastases. Br J Cancer 2010; 103:1066 - 75; http://dx.doi.org/10.1038/sj.bjc.6605850; PMID: 20717117
- Sossey-Alaoui K, Safina A, Li X, Vaughan MM, Hicks DG, Bakin AV, Cowell JK. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol 2007; 170:2112 - 21; http://dx.doi.org/10.2353/ajpath.2007.060975; PMID: 17525277
- Teng Y, Ghoshal P, Ngoka L, Mei Y, Cowell JK. Critical role of the WASF3 gene in JAK2/STAT3 regulation of cancer cell motility. Carcinogenesis 2013; 34:1994 - 9; http://dx.doi.org/10.1093/carcin/bgt167; PMID: 23677069
- Ghoshal P, Teng Y, Lesoon LA, Cowell JK. HIF1A induces expression of the WASF3 metastasis-associated gene under hypoxic conditions. Int J Cancer 2012; 131:E905 - 15; http://dx.doi.org/10.1002/ijc.27631; PMID: 22581642
- Sossey-Alaoui K, Li X, Cowell JK. c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J Biol Chem 2007; 282:26257 - 65; http://dx.doi.org/10.1074/jbc.M701484200; PMID: 17623672
- Teng Y, Xie X, Walker S, White DT, Mumm JS, Cowell JK. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 2013; 13:453; http://dx.doi.org/10.1186/1471-2407-13-453; PMID: 24089705
- Burfoot MS, Rogers NC, Watling D, Smith JM, Pons S, Paonessaw G, Pellegrini S, White MF, Kerr IM. Janus kinase-dependent activation of insulin receptor substrate 1 in response to interleukin-4, oncostatin M, and the interferons. J Biol Chem 1997; 272:24183 - 90; http://dx.doi.org/10.1074/jbc.272.39.24183; PMID: 9305869
- Kohlhuber F, Rogers NC, Watling D, Feng J, Guschin D, Briscoe J, Witthuhn BA, Kotenko SV, Pestka S, Stark GR, et al. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol Cell Biol 1997; 17:695 - 706; PMID: 9001223
- Teng Y, Liu M, Cowell JK. Functional interrelationship between the WASF3 and KISS1 metastasis-associated genes in breast cancer cells. Int J Cancer 2011; 129:2825 - 35; http://dx.doi.org/10.1002/ijc.25964; PMID: 21544801
- Cho SG, Li D, Stafford LJ, Luo J, Rodriguez-Villanueva M, Wang Y, Liu M. KiSS1 suppresses TNFalpha-induced breast cancer cell invasion via an inhibition of RhoA-mediated NF-kappaB activation. J Cell Biochem 2009; 107:1139 - 49; http://dx.doi.org/10.1002/jcb.22216; PMID: 19533666
- Teng Y, Mei Y, Hawthorn L, Cowell JK. WASF3 regulates miR-200 inactivation by ZEB1 through suppression of KISS1 leading to increased invasiveness in breast cancer cells. Oncogene 2014; 33:203 - 11; http://dx.doi.org/10.1038/onc.2012.565; PMID: 23318438
- Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008; 68:7846 - 54; http://dx.doi.org/10.1158/0008-5472.CAN-08-1942; PMID: 18829540
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008; 10:593 - 601; http://dx.doi.org/10.1038/ncb1722; PMID: 18376396
- Sossey-Alaoui K, Bialkowska K, Plow EF. The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J Biol Chem 2009; 284:33019 - 29; http://dx.doi.org/10.1074/jbc.M109.034553; PMID: 19801681
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature 2005; 435:828 - 33; http://dx.doi.org/10.1038/nature03552; PMID: 15944707
- Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res 2009; 69:7495 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-09-2111; PMID: 19773429
- Cao Q, Li YY, He WF, Zhang ZZ, Zhou Q, Liu X, Shen Y, Huang TT. Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiol Genomics 2013; 45:1206 - 14; http://dx.doi.org/10.1152/physiolgenomics.00122.2013; PMID: 24192393
- Lin HY, Chiang CH, Hung WC. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br J Cancer 2013; 109:731 - 8; http://dx.doi.org/10.1038/bjc.2013.349; PMID: 23820254
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 2012; 31:3513 - 23; http://dx.doi.org/10.1038/emboj.2012.183; PMID: 22773185
- Collins AS, McCoy CE, Lloyd AT, O’Farrelly C, Stevenson NJ. miR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signalling. PLoS One 2013; 8:e69090; http://dx.doi.org/10.1371/journal.pone.0069090; PMID: 23894411
- Wu W, Takanashi M, Borjigin N, Ohno SI, Fujita K, Hoshino S, Osaka Y, Tsuchida A, Kuroda M. MicroRNA-18a modulates STAT3 activity through negative regulation of PIAS3 during gastric adenocarcinogenesis. Br J Cancer 2013; 108:653 - 61; http://dx.doi.org/10.1038/bjc.2012.587; PMID: 23322197
- Zhao G, Zhang JG, Shi Y, Qin Q, Liu Y, Wang B, Tian K, Deng SC, Li X, Zhu S, et al. MiR-130b is a prognostic marker and inhibits cell proliferation and invasion in pancreatic cancer through targeting STAT3. PLoS One 2013; 8:e73803; http://dx.doi.org/10.1371/journal.pone.0073803; PMID: 24040078
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 2012; 31:3513 - 23; http://dx.doi.org/10.1038/emboj.2012.183; PMID: 22773185
- Wu H, Huang M, Cao P, Wang T, Shu Y, Liu P. MiR-135a targets JAK2 and inhibits gastric cancer cell proliferation. Cancer Biol Ther 2012; 13:281 - 8; http://dx.doi.org/10.4161/cbt.18943; PMID: 22310976
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013; 12:86; http://dx.doi.org/10.1186/1476-4598-12-86; PMID: 23915189
- Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 2009; 15:283 - 93; http://dx.doi.org/10.1016/j.ccr.2009.02.015; PMID: 19345327
- Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 2010; 21:11 - 9; http://dx.doi.org/10.1016/j.cytogfr.2009.11.005; PMID: 20018552
- Sansone P, Bromberg J. Targeting the interleukin-6/JAK-STAT pathway in human malignancies. J Clin Oncol 2012; 30:1005 - 14; http://dx.doi.org/10.1200/JCO.2010.31.8907; PMID: 22355058
