Abstract
NKG2D is a surface receptor expressed on NK cells but also on CD8+ T cells, γδ T cells, and auto-reactive CD4+/CD28- T cells of patients with rheumatoid arthritis. Various studies suggested that NKG2D plays a critical role in autoimmune diseases, e.g., in diabetes, celiac disease and rheumatoid arthritis (RA), rendering the activating receptor a potential target for antibody-based therapies. Here, we describe the generation and characteristics of a panel of human, high-affinity anti-NKG2D IgG1 monoclonal antibodies (mAbs) derived by phage display. The lead molecule mAb E4 bound with an affinity (KD) of 2.7 ± 1.4 x 10-11 M to soluble and membrane-bound human NKG2D, and cross-reacted with NKG2D from cynomolgus macaque, indicating potential suitability for studies in a relevant primate model. MAb E4 potently antagonized the cytolytic activity of NKL cells against BaF/3-MICA cells expressing NKG2D ligand, and blocked the NKG2D ligand-induced secretion of TNFα, IFNγ and GM-CSF, as well as surface expression of CRTAM by NK cells cultured on immobilized MICA or ULBP-1 ligands. The antibody did not show a detectable loss of binding to NKG2D after 7 days in human serum at 37°C, and resisted thermal inactivation up to 70°C. Based on these results, anti-human NKG2D mAb E4 provides an ideal candidate for development of a novel therapeutic agent antagonizing a key receptor of NK and cytotoxic T cells with implications in autoimmune diseases.
Introduction
In humans, the NKG2D receptor is expressed on all NK cells, CD8+ αβ T cells and γδ T cells.Citation1–Citation3 It is a homodimeric type II transmembrane protein with an extracellular C-type lectin-like domain, to which specific ligands bind in a Ca2+-independent manner.Citation4,Citation5 Ligands of NKG2D are very diverse and differ in their structure, expression pattern and binding affinity.Citation1,Citation6–Citation8 The first human ligands described for NKG2D were MHC class I-related molecules A and B (MICA and MICB), which are closely related and highly polymorphic.Citation9,Citation10 Other ligands are UL16-binding proteins ULBP-1, -2, -3 and -4. These ligands are poorly expressed by healthy cells but frequently found upregulated in transformed cells after induction of the so-called DNA damage response pathway, as well as after infection with viruses or other pathogens.Citation3,Citation11–Citation14 The expression of NKG2D ligands is important for the recognition of transformed or infected cells by NK cells. According to the ‘missing-self’ hypotheses, as postulated by Ljunggren and Kärre, healthy cells express sufficient MHC class I molecules on their surface that protect them from NK cell-mediated cytolysis by interaction with inhibitory receptors on NK cells. On the other hand, reduced levels of MHC class I on transformed cells and the expression of alternative MHC class I-related surface molecules activate receptors on NK cells, which leads to elimination of the target cell by release of granzymes and perforin.Citation15 The activating receptor NKG2D and its interaction with ligands have an essential function in tumor surveillance and immunity against pathogens.Citation2,Citation16,Citation17
NKG2D not only mediates the activation of NK but also of CD8+ T cells. While human NK cells can directly become activated through interaction of NKG2D and its ligands, CD8+ T cells need additional signals.Citation2,Citation18 For signaling and expression on the cell surface, NKG2D associates with adaptor molecules DAP10 in humans, and with DAP10 or DAP12 in mice.Citation19,Citation20 Ligand binding to the NKG2D/DAP10 receptor complex is leading to tyrosine phosphorylation within the YxxM motif in the cytoplasmic tail of DAP10 and induces cytotoxicity and cytokine release.Citation21,Citation22
Dysfunction of NKG2D can lead to autoimmune diseases like diabetes mellitus type I, RA, celiac disease, and multiple sclerosis, and might also be involved in graft rejection.Citation23–Citation26 In a type I diabetes model in NOD mice, pancreatic tissue was found to express Rae-1, a ligand of murine NKG2D. NKG2D-expressing auto-reactive CD8+ T cells were observed to infiltrate the pancreas, leading to tissue destruction. Inhibition of the NKG2D/Rae-1 interaction by an antagonistic anti-NKG2D antibody could stop progression of the disease even if the antibody was administered to late prediabetic mice.Citation27
In celiac disease, an inflammation of the colon induced by wheat protein gluten leads to destruction of the epithelial layer of the colon due to adaptive and innate immune responses.Citation28 Several studies suggested that interaction of MICA-expressing epithelial cells with NKG2D-expressing, intraepithelial lymphocytes caused destruction of the colon tissue.Citation29,Citation30
NKG2D and its ligands also seem to be involved in the onset of rheumatoid arthritis.Citation31 Proinflammatory factors like IL-15 and TNFα induce expression of NKG2D on auto-reactive CD4+/CD28− T cells, which recognize the MICA/MICB-expressing synovial tissue and infiltrate the synovium.Citation32–Citation34 These studies indicate that antibodies blocking interactions of NKG2D with ligands might have potential for the treatment of certain autoimmune diseases.
We here describe generation and characteristics of a new human monoclonal anti-NKG2D antibody called E4, which by several criteria appears ideal for development of a human therapeutic. E4 has high target affinity for NKG2D, is stable in serum and at elevated temperature, is of lowest possible immunogenicity by bearing a fully human variable light chain and humanized variable heavy chain, and shows the necessary antagonist activity for intercepting with biological activities of NKG2D.
Results
Generation of human anti-NKG2D mAb E4.
Human single-chain antibodies (scFv) were generated by phage display and guided selection of human light chains (VL) and stepwise humanization of the heavy chains (VH) as outlined in . ScFv-based phage libraries were prepared using VL and VH sequences derived from murine hNKG2D-binding and neutralizing monoclonal antibody (mAb) 6H7 (Micromet AG). Because guided selection failed to identify a human VH sequence that would preserve the biological activity of the lead antibody, stepwise humanization of the VH framework region (FR) was performed, while retaining the murine complementarity-determining regions (CDR). During this process, each chimeric scFv variant was expressed and tested for binding and neutralizing activity. By successive replacement of murine with human framework amino acids and after conversion into the human IgG1 antibody format, mAb E1 was obtained, which showed a significant loss in binding affinity and biological function. Affinity maturation of scFv from mAb E1 was performed by sequential randomization of the CDR3 domains of VL and VH domains. This resulted in five different anti-hNKG2D scFv of increased affinity. All five human candidates were converted into human IgG1 by recombinant fusion with constant domains Cλ, CH1, CH2 and CH3 resulting in mAbs B1, E4, C3, C12 and H5.
Biological activity of human anti-NKG2D monoclonal antibodies.
MAbs B1, E4, C3, C12 and H5 were investigated in a FACS-based assay for cell surface binding to NKL cells expressing human NKG2D (). Except for mAb E1, all five affinity maturated candidates showed comparable binding to human NKG2D expressed on NKL cells, as detected by titration analysis using FACS. To determine association (ka) and dissociation rate constants (kd) for NKG2D-specific binding, all mAbs were analyzed by surface plasmon resonance spectroscopy using soluble recombinant human NKG2D/Fc bound to the surface of a sensor chip. The resulting ka and kd values were used to calculate the equilibrium dissociation constant (KD) ().
The two candidates with highest affinity for human NKG2D were mAb E4 and mAb B1 with association rate constants of ka = 3.53 ± 0.25 × 105 M−1s−1 and 4.30 ± 0.42 × 105 M−1s−1, dissociation rate constants of kd = 9.30 ± 4.53 × 10−6 s−1 and 9.95 ± 2.90 × 10−6 s−1, and KD values of 2.70 ± 1.40 × 10−11 M and 2.25 ± 0.35 × 10−11 M, respectively. These two antibodies were selected for further functional and biophysical analyses. To test their inhibitory potential, mAb E4 and mAb B1 were compared in a 51Cr-release cytotoxicity assay using NKL cells as effectors and BaF/MICA transfectants as target cells (). In this assay, NKG2D receptor-positive NKL cells recognized MICA expressed on BaF cells leading to subsequent lysis of 51Cr-loaded target cells. While the non-optimized mAb E1 had an IC50 value of 31 ± 15 nM, the two selected lead candidates showed approximately 100-fold improved IC50 values of 0.23 ± 0.12 nM (mAb B1) and 0.22 ± 0.08 nM (mAb E4), indicating that the affinity maturation process was also successful in improving the biological activity of the two antibodies.
To analyze the activation potential of the mAbs, a redirected lysis experiment was performed with NKL cells as effectors and 51Cr-labelled Fcγ receptor expressing P815 cells (). In this assay, the Fc part of anti-NKG2D antibodies binds to Fc receptors expressed on P815 cells, which induces the aggregation of NKG2D receptors leading to NKL cell-mediated target cell lysis. The EC50 values observed were 21.20 ± 13.60 pM and 19.40 ± 11.80 pM for mAb B1 and mAb E4, respectively, and 1.30 ± 1.10 nM for mAb E1, again showing a 100-fold difference in potency.
Serum and thermostability of mAb E4 and mAb B1.
The two selected antibodies E4 and B1, and parental mAb E1 were tested for their resistance to elevated temperatures as a means to determine overall stability. The antibodies were incubated for five minutes at various temperatures between 37°C and 100°C and tested after cooling for their ability to bind NKG2D expressed on NKL cells using FACS. All three antibodies preserved 100% of their binding capability up to a treatment at 70°C (data not shown). To determine stability and protease resistance in human serum, the three antibodies were incubated at 37°C for up to seven days and thereafter binding to NKG2D on NKL cells analyzed at various concentrations by FACS analysis (data not shown). None of the antibodies showed a detectable loss of binding to NKG2D after incubation in human serum for up to seven days at 37°C.
Binding of mAbs E4 and B1 to NKG2D expressing human PBMC.
To verify that mAbs E4 and B1 bind to human cells expressing NKG2D, PBMC from two healthy donors were purified and stained with mAbs E4 and B1, as well as for the antigens CD4, CD8, CD14, CD16, CD19 and CD56 marking various cell populations of PBMC (). Like positive control mouse-anti-human NKG2D antibody 1D11, both antibodies bound to sub-populations of PBMC known to express NKG2D. MAbs E4 and B1 were found to selectively recognize the majority of human CD8+ (CTL), CD16+ and CD56+ cells (NK cells). In contrast, there was no evidence for binding to human CD4+ T cells, CD19+ B cells or CD14+ monocyte cell population, confirming the specificity of mAbs E4 and B1 for human NKG2D.
Cross-reactivity of mAb E4 with NKG2D from cynomolgus monkeys (M. fascicularis).
For facilitated pre-clinical development, it can be a considerable advantage if antibodies cross-react with NKG2D from non-human species to allow for safety and efficacy studies in relevant animal models. To further assess the antibodies' specificity and to analyze if mAbs E4 or B1 can bind to cells expressing NKG2D from Macaca fascicularis, PBMC were purified from whole blood of healthy animals and cells analyzed for antibody binding by co-staining for antigen markers CD3, CD8, CD16, CD19 and CD56 in a FACS-based assay (). Both mAbs E4 and B1 were found to equally well bind to macaque and human T cells and NK cells indicating binding of the mAbs to epitopes conserved between NKG2D receptors of human and macaque origin. This finding qualifies both antibody candidates for future safety and efficacy studies in non-human primates provided they can be produced in sufficient amounts. Because mAb E4 had shown higher expression levels than mAb B1, mAb E4 was finally selected as lead candidate for further development, and studied in more detail in the following.
Immobilized mAb E4 induces cytokine release by NK cells.
In addition to cytolytic activity, it has been reported that interaction of NKG2D with its ligands induces release of pro-inflammatory cytokines by NK cells. Redirected lysis experiments had shown that mAb E4 can induce NKG2D-mediated cell lysis of P815 target cells (see ). To determine if mAb E4 could also induce secretion of cytokines via crosslinking of NKG2D receptors, human NK cells pre-activated with recombinant hIL-2 were incubated with plate-immobilized mAb E4, MICA/Fc or respective controls for 20 h, and supernatants analyzed for TNFα, IFNγ and GM-CSF (). Immobilized mAb E4 as well as stimulation with ligand MICA/Fc led to significantly increased levels of cytokines TNFα (), IFNγ () and GM-CSF () in cell supernatants when compared to human isotype control antibody or control IgG Fc.
Soluble mAb E4 inhibits both expression of NK cell activation marker CRTAM, and MICA-induced cytokine release.
The therapeutic use of an anti-NKG2D antibody is determined by its potential to neutralize activation of NKG2D by its ligands. Activation of NKG2D on NK cells leads to the release of inflammatory cytokines, which play an important role in the progression of autoimmune diseases, and upregulation of activation markers, such as the class I-restricted T cell associated molecule (CRTAM).Citation21,Citation35 To test if mAb E4 can prevent expression of CRTAM on NK cells pre-stimulated with human IL-2, cells were pre-incubated with mAb E4 at 5 µg/ml for 30 min, added to plates coated with MICA () or ULBP-1 () and incubated for 20 h. FACS analysis of harvested cells showed that mAb E4 was capable of reducing MICA- and ULBP-1 induced surface expression of CRTAM, while the isotype control IgG did not.
To determine if pre-incubation with mAb E4 could also inhibit cytokine release of NK cells stimulated with plate-bound MICA/Fc, supernatants were harvested after 20 h and analyzed for cytokine levels (). For all three cytokines measured, TNFα (), IFNγ () and GM-CSF (), a potent neutralizing effect could be observed at an antibody concentration of 5 µg/ml. Our findings indicate that soluble mAb E4 can potently interfere with the interaction of hNKG2D with a ligand. The superior affinity of mAb E4 (KD 2.7 ± 1.4 × 10−11) over ligand MICA (KD 0.6–1 × 107) to the receptor may explain the potent suppression by mAb E4 of MICA ligand binding to hNKG2D.
Rapid internalization of mAb E4 after binding to NKG2D.
Soluble NKG2D ligands as shed by tumor cells can trigger internalization of NKG2D on NK cells, which can reduce their killing activity.Citation36–Citation40 To determine if mAb E4 can also lead to internalization of NKG2D, Alexa-Fluor 488-conjugated antibody (E4-488) was incubated for different time points with NKL cells and internalization of the bound antibody studied by confocal laser scanning microscopy (). Shortly after addition to cells, the labeled antibody evenly stained the cell surface (), and a polarization of bound receptors was evident after five minutes (). After 15 min, the antibody/NKG2D complex was largely internalized by NKL cells and found in a granular staining (). This fine, dotted staining could still be detected after 4 h (), indicating that the antagonistic activity of mAb E4 on NKG2D activity may have resulted at least in part from internalization of NKG2D.
Additional internalization studies of mAb E4 in a FACS-based assay confirmed the results obtained with confocal microscopy (). NKL cells were used to assess the time-dependent internalization and intracellular accumulation of E4-488 at up to 4 h. At 0 min, all cell surface-associated E4-488 could be completely removed by washing cells with low acidic pH buffer. Internalization of mAb E4 was determined as the increase of acid-insensitive cell binding over time. Intracellular accumulation of E4-488 by NKL cells at 37°C reached a maximum after 4 h (). This suggests that not only mAb E4 but also the NKG2D receptor was internalized, but testing this would require a second antibody recognizing an epitope distinct from that of mAb E4, which is not available.
Discussion
Natural killer cells are receiving increasing attention as a therapeutic target because of their potential involvement in autoimmune and inflammatory diseases. A number of recent studies suggested a role for NKG2D receptor expressed on NK cells in rheumatoid arthritis, diabetes, celiac disease and multiple sclerosis.Citation23–Citation25,Citation27 In order to control an aberrant activation of NK cells via NKG2D, two antibody-based approaches can be chosen: (1) inhibition of the activating receptor, (2) neutralization of its activating ligands. We developed human monoclonal antibodies that bind to the NKG2D receptor and prevent its interaction with activating ligands. We preferred this approach over ligand-neutralizing antibodies because of the variety of ligands potentially binding to NKG2D. Neutralization of just one ligand will not prevent NK cell activation by other ligands of NKG2D. NKG2D was selected as the target antigen because of its key role in activating NK cells and its monomorphic nature. NKG2D is also a costimulatory receptor on cytotoxic CD8+ T cells. The ability to also control auto-reactive CD8+ T cells would broaden the effect of a therapeutic antibody.
We here selected mAb E4 as a candidate for development of an antagonistic anti-NKG2D antibody. Antibody generation employed phage display guided selection using an antagonistic antibody of murine origin. A fully human light chain was identified that in combination with a humanized heavy chain and affinity maturation created a set of mAbs suitable for selection of a candidate.
A number of prerequisites need to be fulfilled for the development of a novel antagonistic anti-NKG2D as a potential drug candidate. These include lowest possible immunogenicity, high affinity and potency, high stability, cross-reactivity with test animals, defined biological activity, and in-vivo efficacy and therapeutic window. Apart from in-vivo performance, we have demonstrated that the selected mAb E4 fulfills all other requirements. Having a fully human variable light chain and a humanized variable heavy chain, E4 is as close to human germline sequences as other antibodies used for treatment of patients with inflammatory diseases. With a dissociation rate constant in the range of 30 pM and a very slow off-rate, mAb E4 should be of sufficient affinity to interact with its target antigen on NK cells at antibody doses equivalent to those of therapeutic antibodies. MAb E4 showed exquisite stability in human serum for one week at 37°C and could resist heating up to 70°C without loss of binding activity. Future in-vivo studies with mAb E4 will be facilitated by cross-reactivity of the antibody with target antigen from a non-human primate species. Antagonistic activities of mAb E4 were evident from several biological assays. Inhibition of NKG2D-mediated cytotoxicity by mAb E4 was observed as well as blockade of TNFα, IFNγ and GM-CSF release in response to stimulation of NKG2D with its ligand MICA. Half maximal inhibition of cytotoxicity was achieved at 0.22 nM mAb E4, which is close to the KD value for NKG2D binding.
Under certain conditions, mAb E4 also exhibited agonistic activities. These can be explained by situations where NKG2D is extensively cross-linked by antibody, which is either immobilized on plastic or presented by cells bearing a high density of Fcγ receptors. Recently, Kwong et al. published a study which also describes the generation and affinity maturation of a human anti-hNKG2D antibody called KYK-2.0.Citation41 Analysis of this human IgG1 antibody produced similar results as our human IgG1 antibody mAb E4. In solution, KYK-2.0 shows an antagonistic profile by interfering with the cytolytic activity of ex vivo expanded NK cells. In contrast, immobilized KYK-2.0 was found to strongly induce activation of human NK cells. We agree that the ambivalent characteristics of human anti-hNKG2D antibodies may translate into a potent therapeutic benefit. But for therapeutic application in autoimmune diseases, it will be necessary to generate non-depleting antibody formats like Fab, F(ab)2 or antibodies with mutated Fc-parts to disrupt Fc-receptor binding. Our data suggest that only cross-linking of mAb E4 appeared to stimulate NKG2D in the absence of ligands, while bivalent binding appears neutral and showed antagonistic effects in the presence of activating ligands. The neutralization potential of mAb E4 in a relevant species remains to be clarified in animal experiments where circulating cytokine levels may be a sensitive readout for potential agonistic activities.
Materials and Methods
Cell lines and purification of cells.
The human NK cell line NKL was grown in RPMI 1640 medium supplemented with 20% heat inactivated fetal calf serum (h.i. FCS) (Biochrom), 2 mM L-glutamine, 100 µg/ml Streptomycin/Penicillin and 200 U/ml of recombinant human IL-2 (rhIL-2, Novartis). The pro-B cell line, BaF/3, mock-transduced or transduced with human MICA and the FcγR-positive murine mastocytoma cell line P815 were kindly provided by A. Cerwenka and C. Watzl (DKFZ, Heidelberg, Germany). BaF/3 and P815 cells were cultured in RPMI 1640 medium supplemented with 10% h.i. FCS, 2 mM L-glutamine and 100 µg/ml Streptomycin/Penicillin.
Primary human lymphocytes were isolated from peripheral blood from healthy donors. Peripheral blood lymphocytes (PBL) were isolated using Leucosep® devices (Greiner) and Biocoll-Separating Solution (Biochrom) according to the manufacturer's instructions. Naïve NK cells were obtained from freshly isolated PBL using the NK cell negative selection kit (Dynal Biotech, Invitrogen) according to the manufacturer's protocol. The purity of isolated populations was determined by flow cytometry. Typically, at least 97% were CD3− CD14− HLA- DR− cells. Isolated NK cells were cultured in RPMI 1640 supplemented with 200 U/ml of rhIL-2, 10% h.i. FCS, 2 mM L-glutamine and 100 µg/ml Streptomycin/Penicillin.
Primary lymphocytes from two healthy cynomolgus monkeys (M. fascicularis) were isolated from peripheral blood using Leucosep® devices (Greiner) and Biocoll-Separating Solution (Biochrom). Cells were cultured for 4 days in petri dishes coated with anti-CD28 Ab (1 µg/ml, BD 556620) and anti-CD3 Ab (1 µg/ml, clone SP34, BD 557052) in RPMI 1640 medium supplemented with 10% heat inactivated fetal calf serum (h.i. FCS) (Biochrom), 2 mM L-glutamine, 100 µg/ml Streptomycin/Penicillin, 10 mM HEPES (Biochrom), 10× non-essential amino acids (Biochrom) and 5,000 U/ml of recombinant human IL-2 (rhIL-2, Novartis).
Generation of human anti-human NKG2D antibodies.
Based on the identified mouse hybridoma cell line 6H7 (Micromet AG), which produced a monoclonal antibody (mAb) neutralizing hNKG2D, humanization of single-chain antibodies (scFv) was performed using phage display guided selection. For selection of human VL, scFv libraries were generated by cloning VLϰ and VLλ pools via SacI/SpeI in phagemid vector pComb5BHis/N2 (Micromet AG) containing the sequence of parental murine VH. The selection of antigen-binding phages by panning was carried out according to the procedure as described in Raum et al.Citation42 on recombinant soluble biotinylated NKG2D antigen immobilized on streptavidin coated microtiter plates (Nunc). For periplasmic expression of scFv, the pool of fragments obtained after four rounds of panning was subcloned into pComb5BF/HdelN2 (Micromet AG). The expression of multiple different clones using E.coli TG-1 cells and preparation of periplasmic extracts was performed as described elsewhere.Citation43 ScFv were collected for screening by FACS. Because phage display guided selection method failed to select a human VH with designated binding activity, stepwise humanization of VH framework-region (FR) was performed while retaining the murine CDR-domains. The successive replacement of murine with human framework amino acids resulted, after conversion in IgG1 antibody format, in the construct mAb E1, which showed loss in binding and biological activity. Due to the fully human VL and the mostly human VH, the constructs will be referred to as ‘human’.
Affinity maturation process of scFv E1 was performed by sequential randomization of the CDR3 domains of VL and VH, and selection of scFv with high affinity by method of in solution-panning as described in Krinner et al.Citation43 Here phage particles were incubated for 1 h with recombinant hNKG2D/Fc in a total volume of 0.5 ml in PBS/0.1% BSA. Then 30 µl of protein G beads (Sigma) were added for an additional hour. Elution of binding phages was performed according to the procedure described in Krinner et al.Citation43 During phage display panning, the antigen concentration was reduced every round from 100 nM to 0.1 nM to select for scFv with high affinity. The affinity maturation process resulted in five different anti-hNKG2D scFv lead candidates with high affinity. All five human candidates were converted into IgG1 mAbs, designated mAb B1, mAb E4, mAb C3, mAb C12, mAb H5.
Expression and purification of human anti-NKG2D antibodies.
For transient transfection of HEK293-F cells or stable expression of light and heavy chain in CHO cells, separate expression vector constructs were prepared as described before.Citation42 For initial characterization of binding affinity of human anti-hNKG2D mAbs by FACS and Biacore, mAbs were expressed in transiently transfected human HEK293-F cells. Vectors encoding the heavy and light chain of mAbs were co-transfected in HEK293-F cells using the 293 fectin reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions.
For 51Cr-release and cytokine assays human anti-hNKG2D mAbs were expressed in stably transfected CHO cells as described before.Citation42
MAbs from cellular supernatants were purified by protein G affinity chromatography with HiTrap protein G HP-column according to standard protocols for the isolation of IgGs using an ÄKTAexplorer 100 Air (GE Healthcare).
Surface plasmon resonance (SPR).
The SPR measurements were performed using a BIAcore™ 2000 instrument (Biacore AB, Switzerland). The surface of the CM5 sensor chip (Biacore) was activated with NHS/EDC. Approximately 1,600 resonance units (RU) of hNKG2D were immobilized on the CM5 sensor chip by injection of 5 µg/ml hNKG2D/Fc (1299-NK, R&D Systems) in 10 mM sodium-acetate (pH 4). After immobilization of the antigen, the activated chip surface was blocked by 1 M Ethanolamine-HCl (pH 8.5). Control flow cells were prepared by carrying out the coupling reaction in the presence of coupling buffer alone. Control sensograms were automatically subtracted from sensograms obtained with immobilized hNKG2D/Fc. Binding assays were performed at 25°C in HBS-EP running buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20). Anti-hNKG2D antibodies were diluted 1:2 in HBS-EP and injected (1 min stabilization and 3 min injection) at several concentrations (1:2 serial dilution: mAbs H5, B1, E4, C3, C12 at 5–156.3 × 10−3 µg/ml or mAb E1 at 50–1.6 µg/ml) and a flow rate of 20 µl/min, followed by 5 min of buffer flow for dissociation. Regeneration was obtained with a 45 sec pulse of 100 mM glycine in 500 mM NaCl solution, pH 3. The association and dissociation rate constants (ka [1/Ms] and kd [1/s]) and the equilibrium dissociation constants KD were determined using the BIAevaluation software (3.2 RC, Biacore) with the Langmuir binding equation and global curve fitting. The equilibrium dissociation constant KD [M] was calculated from kd/ka. The analytes were produced and purified and used to record at least three independent data sets.
FACS.
Unless specified otherwise, all incubations were performed on ice in PBS-F (phosphate buffered saline, pH 7.4, 2% FCS). Routinely, washing was performed in three consecutive steps applying 200 µl washing buffer PBS-F per well each time.
(1) The binding activity of untreated human anti-hNKG2D mAbs was tested by flow cytometry on NKG2D expressing NKL cells. Per sample 2 × 105 NKL cells were seeded in a 96-well microtest plate and incubated for 30 min with different anti-hNKG2D mAbs diluted 1:3 in PBS-F (10 −5.6 × 10−5 µg/ml) or with isotype control (human IgG1, Micromet AG) on ice. Cells were washed and incubated 30 min on ice with a goat anti-human IgG mAb conjugated to PE (Jackson/Dianova) 1:100 in PBS-F. After final washing, cell associated fluorescence was determined by FACS.
(2) The purity of enriched human naïve NK cells was determined by incubation of PBL and isolated NK cells with PE- or PE-Cy5-conjugated anti-CD3 (Becton Dickinson, BD), anti-CD14 (BD), anti-HLA-DR (BD), anti-CD16 (BD), anti-CD56 (BD), anti-NKG2D (BD) mAbs and respective isotype controls. Incubation was performed on ice for 30 min. After final washing, cell associated fluorescence was determined by FACS.
(3) After stimulation of naïve human NK cells with NKG2D-ligands in the presence or absence of anti-hNKG2D mAbs the expression of the surface protein CRTAM was analyzed. Two × 105 of stimulated human NK cells were seeded in a 96-well microtest plate and incubated for 30 min on ice with 0.25 µg of anti-CRTAM mAb (R&D Systems) or the respective IgG2b isotype control. Cells were washed and incubated 30 min on ice with a goat anti-mouse IgG mAb conjugated to PE (Jackson/Dianova) 1:100 in PBS-F. After final washing, cell associated fluorescence was determined by FACS. The mean and standard deviation were generated from four different healthy donors using the software package GraphPadPrism4.
(4) Cross-reactivity of human anti-hNKG2D mAbs was analyzed with PBMC obtained from the following species: H. sapiens and M. fascicularis. Per well 5 × 105 of human or macaque PBMC were seeded in 96-well U-bottom plates. To block Fcγ receptors cells were treated with 10% human AB serum (PAA) for 15 min at 4°C. Blocked PBMC were then incubated with 1 µg of the Alexa Fluor 488-conjugated human anti-hNKG2D mAbs B1 (B1-488) or E4 (E4-488), or a human IgG1 isotype control (human IgG1-488 isotype control) (Micromet AG) in PBS-F. Incubation was performed for 30 min on ice. PBMC subpopulations were gated by co-staining with PE-Cy5-or APC-coupled mouse anti-human CD4, CD8, CD16, CD19, CD14 and CD 56 (all BD) for human cells and PerCP-, APC-, PE- or Alexa Fluor 647 coupled mouse anti-human CD3 (BD), CD19 (Beckman Coulter), CD8 (eBioscience) and CD56 (BD) for PBMC of M. fascicularis. PE-conjugated mouse antihuman NKG2D (1D11, BD) was used as positive control. After final washing, cell associated fluorescence was determined by FACS collecting 20,000 gated events for each sample. The stainings were analyzed for two different donors per species.
(5) Cytokine concentrations were measured using the CBA Th1/Th2 Cytokine Kit II (BD).
Thermal stability.
For analysis of thermal stability of human anti-hNKG2D mAbs they were diluted to a final concentration of 1 µg/ml in 1× PBS/1% h.i. FCS/0.05% sodium azide. Aliquots (100 µl) were heated in a water bath at temperatures varying from 37°C to 100°C (37°C, 50°C, 60°C, 70°C, 80°C and 100°C). After 5 min incubation the samples were cooled on ice. The stability of mAbs at different temperatures was tested by binding to NKG2D expressing NKL cells by FACS analysis. NKL cells (2 × 105 per well) were seeded in a 96-well microtest plate and 30 µl of the incubated protein solution was added. After washing with 200 µl of 1× PBS/1% h.i. FCS/0.05% sodium azide for three times the binding of the human mAbs was detected using a PE-conjugated goat anti-human mAb (Jackson/Dianova, 1:100 in 1× PBS/1% h.i. FCS/0.05% sodium azide). Data were normalized by setting the MFI of untreated antibody (4°C) at 100% binding. This assay was performed in four replicates and repeated three times.
Serum stability.
For analysis of serum stability, human anti-hNKG2D mAbs were diluted in PBS with 50% human AB serum (PAA) at a concentration of 60 µg/ml. After incubation for different time points (d0, d1, d2, d4 and d7) at 37°C the samples were frozen in liquid nitrogen (LN) and stored at −80°C. For FACS analysis of binding activity all samples were thawed simultaneously and binding of mAbs tested in 1:3 dilutions (dilution in PBS-F: 10−4.6 × 10−3 µg/ml) on NKG2D-expressing NKL cells. NKL cells (2 × 105 per well) were seeded in a 96-well microtest plate and 100 µl of the respective sample were added. For detection a PE-conjugated goat anti-human Ab (Jackson/Dianova, 1:100 in PBS-F) was used. This assay was performed in duplicates and was repeated three times.
51Cr chromium release assays.
Inhibition of NKG2D-dependend cytolytic activity in the absence or presence of different anti-hNKG2D antibodies was tested in a 4-h 51Cr-release assay. BaF/3 target cells, which were mock-transduced or transduced with human MICA, were labeled for 1 hour with 100 µCi 51Cr (Hartmann Analytics) at 37°C. The human NKL effector cell line was added at an E:T ratio of 40:1. NKL cells was washed, resuspended in RPMI 1640 medium supplemented with 10% h.i. FCS, 2 mM L-glutamine, 100 µg/ml Streptomycin/Penicillin and 200 U/ml rhIL-2 and 1.2 × 105 cells were added to the wells. The NKL cells were then pre-incubated for ca. 30 min with 1:3 diluted samples (mAb E1 and hIgG1 isotype control: 20 µg/ml−1.1 × 10−4 µg/ml or mAb B1 and mAb E4: 2.2 µg/ml−1.2 × 10−5 µg/ml) of different anti-hNKG2D mAbs or isotype controls.
To analyze Fc-part-mediated redirected lysis, a 6-hour assay was performed. NK cells were washed, resuspended in RPMI 1640 medium supplemented with 10% h.i. FCS, 2 mM L-glutamine, 100 µg/ml Streptomycin/Penicillin and 200 U/ml rhIL-2 and 1.2 × 105 cells were added to the wells. The NKL cells were then pre-incubated for ca. 30 min with 1:3 diluted samples (mAb E1 and hIgG1 isotype control: 5 µg/ml−2.8 × 10−5 µg/ml or mAb B1 and mAb E4: 0.6 µg/ml−3.1 × 10−6 µg/ml) of different anti-hNKG2D mAbs or isotype controls. Briefly, P815 target cells were labeled with 100 µCi in medium for 1 hour at 37°C. Cells were washed three times in RPMI 1640 medium supplemented with 10% h.i. FCS, 2 mM L-glutamine, 100 µg/ml Streptomycin/Penicillin and 200 U/ml rhIL-2. Cells were spun down, resuspended in medium at 1 × 106 cells/mL and plated at 3 × 103 cells/well in triplicates at an E:T ratio of 40:1.
Cytokine assays.
Primary human NK cells were cultured for two days in RPMI 1640 supplemented with 10% FCS, 2 mM L-glutamine, 100 µg/ml Streptomycin/Penicillin and 200 U/ml rhIL-2 at a cell concentration of 5 × 105 cells/ml. After pre-culture cells were washed and 1 × 105 cells per well stimulated with 10 µg/ml plate bound NKG2D-Ligands MICA/Fc, ULBP-1/Fc, control protein CD25/Fc (R&D Systems), with 5 µg/ml of immobilized human anti-hNKG2D mAb E4 or human IgG/Fc control protein (Calbiochem). To analyze the capacity of the human anti-hNKG2D mAb E4 to block NKG2D-dependend cytokine release, NK cells were pre-incubated with 5 µg/ml of mAb E4 or isotype control for 30 min at 37°C before adding the mixture to plate bound NKG2D-ligands. After incubation for 20 hours at 37°C cells were spun down and supernatants harvested. Cytokine levels in cell-free supernatants were determined by ELISA or CBA and the stimulated NK cells analyzed for surface expression of CRTAM by FACS.
ELISA.
GM-CSF levels were determined using the OptEIA Human GM-CSF ELISA Set (BD Bioscience, Heidelberg, Germany) according to manufacturer's instructions. In brief, 100 µl of capture mAb (BD) 1:250 diluted in immobilization buffer (0.1 M sodium-carbonat, pH 9) were coated on microtiter plates (Maxisorb, Nunc, Wiesbaden, Germany). After blocking of plates with PBS-T (PBS with 0.05% Tween 20) containing 10% FCS, cell culture supernatants and human GM-CSF standard (BD) were added in 1:2 dilutions and incubated for 2 hour at room temperature. Detection of bound human GM-CSF was accomplished by 1 hour incubation at room temperature using a mixture of biotinylated detection Ab and streptavidin-HRP (BD). The ELISA was developed with OPD substrate (Sigma) and absorbance was measured at 490/650 nm. All assays were performed in duplicates. The mean and standard deviation of 8 donors were calculated using the software package GraphPadPrism4. To analyze significance, data were transformed (Y = Y + K, K = 1) and tested with a ONE Way ANOVA test by using the software package GraphPadPrism4.
Cytometric bead assay.
TNFα and IFNγ levels in cell-free supernatants were analyzed using the cytometric bead assay ‘Th1/Th2 Cytokine Kit II’ (BD) according to the manufacturer's instructions. The mean and standard deviation of eight donors were calculated using the software package GraphPadPrism4. To analyze statistical significance, data were transformed [TNFα: Y = Y + K, K = 1; IFNγ: Y = Log(Y)] and tested with a ONE Way ANOVA test by using the software package GraphPadPrism4.
Alexa fluor 488 labeling of mAb E4 and mAb B1.
Chemical coupling of Alexa Fluor-NHS 488 (Invitrogen, A20000) to human anti-hNKG2D mAb E4, mAb B1 and IgG1λ isotype control (Sigma) was carried-out by a 30-fold molar excess of Alexa Fluor-NHS 488 over mAb E4 or mAb B1. The coupling reaction was performed in borat buffer containing 0.05 M boric acid (Sigma), 0.1 M sodium chloride (pH 8.5) and 5% DMSO at room temperature for 1 h. Unconjugated Alexa Fluor 488 was removed by gel filtration with PBS using a sephadex G-25 column (GE Healthcare Bio-Science, Uppsala, Sweden).
Confocal laser scanning microscopy.
Internalization of Alexa Fluor 488-conjugated human anti-hNKG2D mAb E4 (mAb E4-488) was determined by kinetic studies using confocal laser scanning microscopy (CLSM). 6 × 105 human NKL cells were seeded in 69-well round bottom plates and the Fc-receptors blocked with PBS/10% human AB serum (PAA) for 15 min at 4°C. After washing the cells were stained with 1 µg mAb E4-488 or human IgG1-488 isotype control for 45 min on ice. Cells were washed again and incubated at 37°C for 0 min, 5 min, 15 min, 30 min, 1 h, 2 h, 3 h and 4 h. Cells were then placed on ice and stained intracellularly with ∼500 nM DAPI at room temperature for 30 min using the Cytofix/Cytoperm Fixation/Permeabilization kit (BD). Cells were plated on coverslips and mounted in fluorescent mounting medium (DakoCytomation) before analysis with a Leica TCS SP2 AOBS confocal laser scanning microscope. Optical sections (0.2 µm) were prepared using a HCX PL APO 1.4 oil 63× lense. Confocal microscopy images were acquired by use of Amira 3.1.1 (visage imaging) software.
Analysis of in vitro internalization of mAb E4 by FACS.
After blocking of NKL cells with 10% human AB serum for 15 min at 4°C mAb E4-488 or a human IgG1-488 isotype control were added to blocked cells (1 µg of antibody per 5 × 105 cells in 200 µl of PBS-F for 30 min at 4°C). Then cells were pelleted, washed once with PBS-F and resuspended in RPMI 1640 medium supplemented with 10% heat inactivated fetal calf serum (h.i. FCS) (Biochrom), 2 mM L-glutamine, 100 µg/ml Streptomycin/Penicillin. Cells were transferred to a 37°C tissue culture incubator, and aliquots of 5 × 105 cells removed at various time points (5 min, 15 min, 30 min, 60 min, 120 min, 180 min and 240 min). At specified time points, cells were immediately placed on ice and stored at 4°C. At each time point, one aliquot of cells was resuspended in 200 µl of 2% paraformaldehyde, a second aliquot was resuspended in 200 µl of low pH acid wash (ultra pure water, 150 mM NaCl, HCl, pH 2.5) for 5 min at room temperature to remove non-internalized antibody prior to fixing in 2% paraformaldehyde. Fixed cells were then analyzed by FACS.
Statistical analysis.
Differences between groups were analyzed using the One Way ANOVA test. Values of p < 0.05 were considered significant.
Abbreviations
| CDR | = | complementarity determining regions |
| CRTAM | = | class I restricted T cell associated molecule |
| DAP10/12 | = | DNAX activating protein with 10 or 12 kDa |
| FACS | = | fluorescence activated cell sorter |
| FR | = | framework region |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| h | = | human |
| IFN | = | interferon |
| Ig | = | immunglobulin |
| IL-2 | = | interleukin-2 |
| ka | = | association rate constant |
| KD | = | equilibrium dissociation constant |
| kd | = | dissociation rate constant |
| mAb | = | monoclonal antibody |
| MHC | = | major histocompatibility complex |
| MIC A/B | = | MHC class I chain-related antigen A/B |
| NKG2D | = | natural killer group 2 member D of the lectin-like receptor family |
| NK cells | = | natural killer cells |
| NOD | = | nonobese diabetic |
| PBMC | = | peripheral blood mononuclear cells |
| RA | = | rheumatoid arthritis |
| Rae-1 | = | retinoic acid early inducible-1 |
| scFv | = | single-chain fragment of variable domains |
| TNF | = | tumor necrosis factor |
| ULBP | = | UL16 binding proteins |
| VL | = | variable domain of light chain |
| VH | = | variable domain of heavy chain |
Figures and Tables
Figure 1 Guided selection strategy and stepwise humanization of mouse monoclonal antibody 6H7 to affinity maturated human IgG1 antibodies E4, B1, C3, C12 and H5. Red, murine variable regions; green, human or humanized variable regions; pink, affinity maturated variable region CDR3; m, murine; h, fully human.
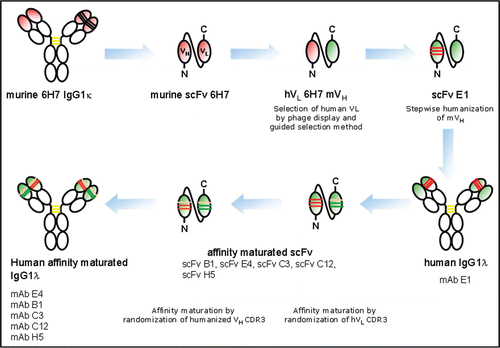
Figure 2 Characterization of affinity maturated human antibodies compared to human mAb E1. (A) Monoclonal antibodies were tested for binding to hNKG2D expressed on NKL cells. Titration curves of mAbs purified from supernatants of transiently transfected HEK293-F cells are shown: mAb C3 (▲), mAb B1 (▽), mAb E1 (□), mAb E4 (○), mAb H5 (■), mAb C12 (◆), mAb hIgG1 isotype control (*). Cell-associated mean fluorescence intensity (MFI) was monitored using FACS analysis. The Graph shows one of three representative experiments. (B) Neutralization of NKG2D-dependent cytotoxicity. mAb E4 (○), mAb E1 (□), mAb B1 (▽) and a hIgG1 isotype control mAb (▲) were purified from supernatants of stable CHO-transfectants and tested in a 51Cr-release assay for their potency to inhibit NKG2D-dependent cytolytic activity of NKL cells against BaF/3-MICA target cells. (C) NKG2D-dependend cytolytic induction activity in a redirected lysis assay. All mAbs (symbols as in B) induced lysis of FcR+ P815 target cells by NKL cells. Error bars indicate standard deviation of triplicates. Data are representative of three independent experiments. E:T = 40:1 in both experimental setups.
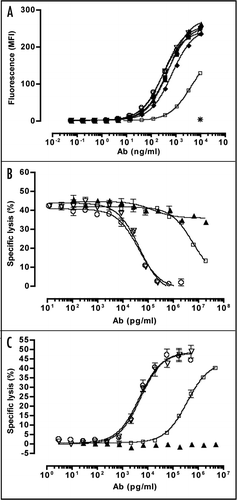
Figure 3 Analysis of binding activity of mAbs E4 and B1 to NKG2D on human PBMC subpopulations by flow cytometry. Freshly isolated human PBMC were stained with PE-Cy5-or APC-coupled mouse anti-human CD4, CD8, CD16, CD19, CD14 or CD 56 (y axes) and with anti-NKG2D mAbs B1-488 or E4-488 (x axes). Human IgG1-488 isotype was used as negative control. PE-conjugated mouse anti-human NKG2D (1D11, BD) was used as positive control. Numbers indicate percent of cells gated.
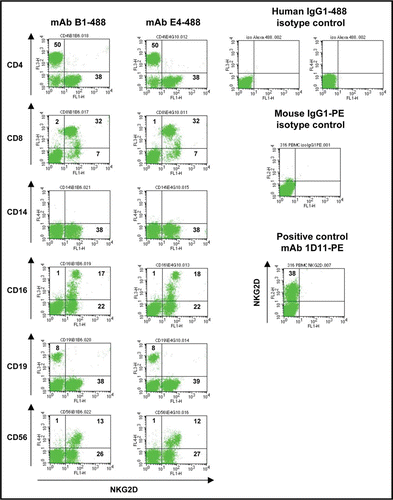
Figure 4 Binding activity of mAbs E4 and B1 to macaque PBMC subpopulations by flow cytometry. Stimulated PBMC from M. fascicularis were stained with PerCP-, APC-, PE- or Alexa 647 coupled mouse anti-human CD3 (BD), CD19 (Beckman Coulter), CD8 (eBioscience) and CD56 (BD) mAbs cross-reacting to cynomolgus monkey (y axes) and with mAb B1-488 or mAb E4-488 (x axes). Human IgG1-488 was used as negative control and PE-coupled mouse anti-human NKG2D mAb 1D11 was used as positive control. Numbers indicate percent of cells gated.
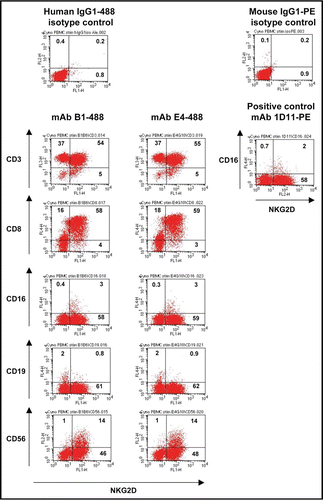
Figure 5 Cross-linking of NKG2D with immobilized mAb E4 is sufficient to activate human NK cells pre-stimulated with rhIL-2 and induce increased cytokine release. (A–C) Plate-bound mAb E4 induces IFNγ (A), TNFα (B) and GM-CSF (C) production by human NK cells. Secreted cytokines were measured in supernatants after 20 h using ELISA (GM-CSF) or CBA array (IFNγ, TNFα). Error bars show standard deviation of eight different healthy donors.(***p < 0.001)
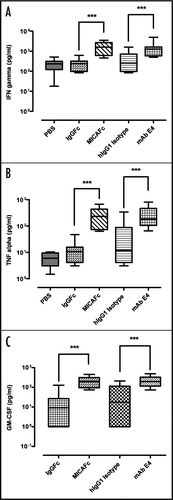
Figure 6 Inhibitory effect of soluble mAb E4 on NKG2D-dependent expression of CRTAM on NK cells and cytokine release. (A and B) mAb E4 (5 µg/ml) reduced MICA/Fc- (A) or ULBP-1/Fc (B)-induced expression of the NK cell activation marker CRTAM. Error bars show standard deviation of four healthy donors tested. No reduction was detectable with control mAb. (C–E) NK cells were pre-incubated with mAb E4 (5 µg/ml) before exposure to NKG2D-ligand MICA/Fc. The antibody reduced the release of cytokines TNFα (C), IFNγ (D) and GM-CSF (E) as measured in supernatants by CBA array or ELISA. Error bars show standard deviation of eight healthy donors. (***p < 0.001)
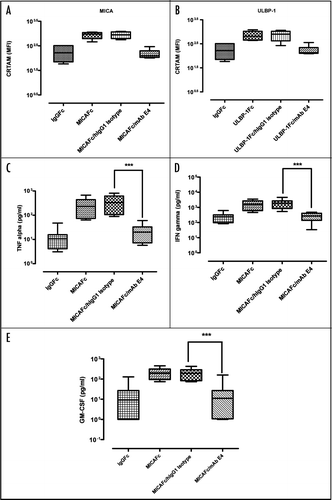
Figure 7 Internalization and subcellular localization of mAb E4. Confocal laser scanning microscopy of NKL cells incubated with mAb E4 conjugated to Alexa Fluor 488 (mAb E4-488, green). (A–D) Cells were incubated with mAb E4-488 and the nucleus labeled with DAPI (blue). The different panels show internalization of NKG2D-bound mAb E4-488 at time points 0 min (A), 5 min (B), 15 min (C) and after 4 h (D). The arrows indicate internalized anti-hNKG2D Ab vesicles. Optical sections of 0.2 µm thickness are shown. (E) Intracellular accumulation of mAb E4. The graph shows time-dependent intracellular accumulation of anti-hNKG2D antibody E4-488 (●) in NKL cells. The y-axis represents the percentage of MFI measured by flow cytometry of NKL cell population over time (x-axis) relative to total binding at 0 min. Antibody-bound cells were incubated at 37°C for 0, 5, 15, 30, 60, 120, 180 and 240 min. Each time point was replicated at least three times.
Table 1 Binding constants of affinity maturated anti-hNKG2D mAbs in comparison with the human precursor mAb E1
Table 2 Characteristics of mAb E4
Acknowledgements
We thank A. Cerwenka (DKFZ, Heidelberg) and C. Watzl (Institute for Immunology, University Heidelberg) for kindly providing the cell lines BaF/3 and P815, as well as for helpful discussions.
References
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003; 3:781 - 790
- Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8ab T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol 2001; 2:255 - 260
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA 1999; 96:6879 - 6884
- Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat Immunol 2001; 2:443 - 451
- Radaev S, Rostro B, Brooks AG, Colonna M, Sun PD. Conformational plasticity revealed by the cocrystal structure of NKG2D and its class I MHC-like ligand ULBP3. Immunity 2001; 15:1039 - 1049
- Watzl C. The NKG2D receptor and its ligands-recognition beyond the “missing self”?. Microbes Infect 2003; 5:31 - 37
- Cerwenka A, Lanier LL. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens 2003; 61:335 - 343
- Mistry AR, O'Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology 2007; 121:439 - 447
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK Cells and T Cells by NKG2D, a Receptor for Stress-Inducible MICA. Science 1999; 285:727 - 729
- Stephens HA. MICA and MICB genes: can the enigma of their polymorphism be resolved?. Trends Immunol 2001; 22:378 - 385
- Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev 2006; 214:130 - 142
- Hamerman JA, Ogasawara K, Lanier LL. NK cells in innate immunity. Curr Opin Immunol 2005; 17:29 - 35
- Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev 2001; 181:158 - 169
- Siren J, Sareneva T, Pirhonen J, Strengell M, Veckman V, Julkunen I, et al. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J Gen Virol 2004; 85:2357 - 2364
- Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med 1985; 162:1745 - 1759
- Hayakawa Y, Smyth MJ. NKG2D and cytotoxic effector function in tumor immune surveillance. Semin Immunol 2006; 18:176 - 185
- Cosman D, Müllberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, et al. ULBPs, Novel MHC Class I-Related Molecules, Bind to CMV Glycoprotein UL16 and Stimulate NK Cytotoxicity through the NKG2D Receptor. Immunity 2001; 14:123 - 133
- Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J Immunol 2005; 174:4480 - 4484
- Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cells activation and costimulation. Nat Immunol 2002; 3:1150 - 1155
- Wu J, Song Y, Bakker ABH, Bauer S, Spies T, Lanier LL, Phillips JH. An Activating Immunoreceptor Complex Formed by NKG2D and DAP10. Science 1999; 285:730 - 732
- Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res 2008; 40:18 - 34
- Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol 2003; 4:557 - 564
- Groh V, Anja B, El-Gabalawy H, Nelson JL, Thomas S. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. PNAS 2003; 100:9452 - 9457
- Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, et al. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J Neurosci 2007; 27:1220 - 1228
- Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 2004; 21:357 - 366
- Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol 2005; 6:938 - 945
- Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 2004; 20:757 - 767
- Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004; 21:367 - 377
- Martin-Pagola A, Ortiz L, Perez de Nanclares G, Vitoria JC, Castano L, Bilbao JR. Analysis of the expression of MICA in small intestinal mucosa of patients with celiac disease. J Clin Immunol 2003; 23:498 - 503
- Stepniak D, Koning F. Celiac disease—sandwiched between innate and adaptive immunity. Hum Immunol 2006; 67:460 - 468
- Caillat-Zucman S. How NKG2D ligands trigger autoimmunity?. Hum Immunol 2006; 67:204 - 207
- Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest 1996; 97:2027 - 2037
- Schmidt D, Martens PB, Weyand CM, Goronzy JJ. The repertoire of CD4+ CD28− T cells in rheumatoid arthritis. Mol Med 1996; 2:608 - 618
- Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum 1997; 40:1106 - 1114
- Boles KS, Barchet W, Diacovo T, Cella M, Colonna M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 2005; 106:779 - 786
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419:734 - 738
- Song H, Kim J, Cosman D, Choi I. Soluble ULBP suppresses natural killer cell activity via downregulating NKG2D expression. Cell Immunol 2006; 239:22 - 30
- Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, et al. Systemic NKG2D downregulation impairs NK and CD8 T cell responses in vivo. J Immunol 2005; 175:720 - 729
- Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol 2005; 6:928 - 937
- Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood 2005; 106:1711 - 1717
- Kwong KY, Baskar S, Zhang H, Mackall CL, Rader C. Generation, Affinity Maturation, and Characterization of a Human Anti-Human NKG2D Monoclonal Antibody with Dual Antagonistic and Agonistic Activity. J Mol Biol 2008; 384:1143 - 1156
- Raum T, Gruber R, Riethmuller G, Kufer P. Anti-self antibodies selected from a human IgD heavy chain repertoire: a novel approach to generate therapeutic human antibodies against tumor-associated differentiation antigens. Cancer Immunol Immunother 2001; 50:141 - 150
- Krinner EM, Hepp J, Hoffmann P, Bruckmaier S, Petersen L, Petsch S, et al. A highly stable polyethylene glycol-conjugated human single-chain antibody neutralizing granulocyte-macrophage colony stimulating factor at low nanomolar concentration. Protein Eng Des Sel 2006; 19:461 - 470