Abstract
Natural IgM antibodies play an important role in the body’s defense mechanisms against transformed cells in the human body and are currently being exploited both in prognoses of malignant lesions and in the therapy of cancer patients. However, despite growing interest and clinical promise, thus far the IgM class of antibodies has failed to gain widespread commercial interest as these are considered to be difficult to produce recombinantly. IgMs are polymeric and have a relatively large mass. In addition, IgM molecules are heavily glycosylated and, when produced in non-human cell lines, they may contain non-human glycan structures which may be potentially immunogenic. Clearly, production systems capable of expressing human recombinant IgM antibodies are needed. We have successfully used PER.C6® cells – a human cell line - to generate three separate human recombinant monoclonal IgMs in suspension cultures in protein-free medium. All three of the IgMs were constructed with joining (J) chain and were expressed in the pentameric form. One of the IgMs was also expressed as a hexamer without J chain. Clones with cell specific productivities greater than 20 pg/cell/day were generated, which led to yields of 0.5 g/L to 2g/L in fed-batch production. All the IgMs expressed were biologically active as shown in binding and cytotoxicity assays. These studies demonstrate the potential of PER.C6® cells for the production of high levels of functional recombinant IgM and other polymeric molecules, using a straightforward and rapid stable cell line generation method.
Introduction
The identification and isolation of tumor specific antibodies has been of major interest since hybridoma cell fusion technology was established by Köhler and Milstein.Citation1 While non-protein based chemotherapies have been successfully used in the clinic for treatment of a wide variety of neoplasms, many treatment options suffer from the induction of unpleasant or toxic systemic or organ-specific side effects. In contrast, monoclonal antibodies are designed to specifically target antigens associated with disease, and are therefore considered to have a better safety profile than non-protein based therapies, with fewer side effects. While the IgG class of antibodies has been widely and successfully used in both pre-clinical studies and in patients,Citation2 other immunoglobulin classes may also offer unique advantages and applications. For example, the IgM class, which naturally exists in pentameric or hexameric forms, is highly active in cytotoxic and cytolytic reactions, a result of its ability to fix complement efficiently.Citation3,Citation4 The high valency of IgMs imparts upon them a high avidity for the target antigen, and may help facilitate cell surface receptor cross-linking.
IgMs are the first immunoglobulin class to be secreted by B cells upon primary stimulation by antigen. Some cancer cell markers may be more effectively targeted by IgMs than they are by IgGs, as noted, for example, for metastatic melanomas,Citation5,Citation6 neuroblastomasCitation7 or gastric cancers.Citation8 Indeed, there is evidence that the IgM class of antibodies is involved in the natural immunosurveillance of not only foreign pathogens, but also of tumors or neoplastic cells.Citation9 Tumor-specific antibodies that have been detected so far in the humoral response are natural germ-line coded IgM antibodies, but not affinity-maturated IgGs.Citation10 These observations suggest that the IgM class of antibodies may be attractive as therapeutic agents for the treatment of tumors.
In recent years, the biopharmaceutical industry has seen a rapid increase in the volumetric yields of recombinant IgGs, with titers approaching 5 g/L in CHO cell.Citation11 Economic and competitive pressures often dictate the need for these high yields to successfully bring a therapeutic protein candidate, for example monoclonal antibodies, into the market. The same paradigm applies for IgMs, which have a similar therapeutic profile as do IgGs. However, IgMs present a much more challenging molecule for expression in heterologous host cell systems. For example, unlike IgGs, which are comprised of two heavy and two light chains (HC and LC), IgMs are significantly more complex. Five or six units, each containing an IgG-like structure of two HC and two LC, are assembled into a pentamer or hexamer. A J chain may also be present, which binds to the tailpiece at the C-terminus of the heavy chains and helps mediate multimerisation and stabilization of the pentameric form. Moreover, each IgM subunit may have five or six potential sites for N-linked glycosylation. The theoretical mass of a fully assembled and modified IgM is between 800–900 kDa, which is considerably larger than that of IgG, which is about 140–160 kDa. Nevertheless, several non-lymphoid cell lines such as C6 glioma, CHO and HeLa cells have been used to successfully produce polymeric IgMs, albeit with low reported yields (10 ng/ml for CHO cells).Citation12 Better productivity has been reported with rat hybridoma cell lines (which naturally express the J chain to produce pentameric IgM proteins), with final yields reported around 100–200 mg/L in batch cultures and 700 mg/L in a medium exchange process.Citation13 More productive cell lines (up to 30 pg/cell/day) were generated in a study producing recombinant IgM in CHO cells after gene amplification with methotrexate and 2′-deoxycoformycinCitation14 but amplification of recombinant genes has been reported to result in instability of protein expression.Citation15
Over the past two decades a large portfolio of tumor specific and cytotoxic IgM antibodies has been isolated and tested successfully.Citation10 Several IgM class antibodies have been isolated from human hybridomas generated by fusion of lymphocytes from cancer patients to the heteromyeloma HAB-1.Citation16,Citation17 One such antibody, SC-1, showed promise in an academic clinical trial.Citation8 Three additional antibodies, designated SM-6, LM-1 and CM-1, are specific for malignant tissues and mediate tumor specific apoptosis. When tested for in vitro functional activity, all three antibodies were able to inhibit tumor cell proliferation of carcinoma cells by inducing apoptosis.Citation18,Citation19
We describe here the expression of these three recombinant IgMs in the PER.C6® cell line. For all three IgMs, the recombinant heavy and light chains, as well as the J chain, were co-expressed from the same vector. In addition, for one of the IgMs, a J chain minus variant (J−) was also evaluated. These studies demonstrate the ability of PER.C6® cells to express high levels of functional IgM, using a straightforward and rapid stable cell line generation method.
Results and Discussion
Construction of IgM expressing cell lines in PER.C6®.
Three different IgMs were expressed in PER.C6® cells: SM-6, LM-1 and CM-1. SM-6 was expressed with and without J chain while LM-1 and CM-1 was only expressed with J chain (). All heterologous genes were expressed under the control of the strong constitutive promoter from human cytomegalovirus (hCMV), except for the neomycin resistance marker, which was expressed under the control of the SV40 promoter. In all cases, a single vector was constructed and used for stable transfections.
For each of the IgM versions evaluated, the expression plasmid was introduced into a serum-free and suspension adapted version of PER.C6® by electroporation, and limiting dilution cloning in 96-well plates was performed. Cells which stably incorporated the expression plasmid were selected for by survival in the presence of the drug Geneticin®. Approximately 200–300 clones were assayed for IgM production by using an anti-IgM ELISA, from which the top 20% of the clones based on the ELISA results were expanded. Further productivity assessments during cell line screening were performed, and ~20 candidate cell lines for each of the IgMs were chosen for further study. No gene amplification, retransfection or subcloning steps were introduced. A summary of the cell line generation methods is shown in .
IgM-expressing PER.C6® cells have high cell specific productivity.
PER.C6® cells have been reported to be capable of expressing the IgG class of immunoglobulin to very high levels.Citation20 Recently, volumetric yields in PER.C6® cells as high as 8 g/L in fed-batch production cultures and as high as 27 g/L in a modified perfusion/fed-batch system have been demonstrated.Citation21 We were therefore interested in determining if PER.C6® cells could also support high expression levels of IgM molecules.
The expression construct and cell line generation strategy employed for IgM production were identical to those employed for IgGs. To determine the cell specific productivities, stable cell lines expressing SM-6 (J+) and SM-6 (J−) were assessed in a 7-day batch assay. Product concentration and cell number were determined each day, and the “Qpmax”, or maximum cell specific productivity, was calculated. While the average Qpmax values for the IgM cell lines were lower than typical values observed for IgG-producing clones, the Qpmax values for the lead clones of both SM-6 (J+) and SM-6 (J−) were greater than 20 pg/cell/day (). This cell-specific productivity level is comparable with typical production-ready manufacturing clones. While the Qpmax value for the lead cell line was slightly higher for SM-6 (J−) variant, the average Qpmax value for the top four cell lines was nearly identical for the SM-6 (J−) and SM-6 (J+) variants, suggesting that co-expressing the J chain has no significant impact on the overall expression levels of IgMs in PER.C6® cells. Similar Qpmax values were also observed for the LM-1 and CM-1 variants, both co-expressed with the J chain (data not shown).
IgM-expressing PER.C6® cells have high volumetric yields.
In addition to the 7-day batch assessments, clones were also assessed in small-scale fed-batch assays. Growth profiles from the fed-batch assays of clones expressing LM-1 and CM-1 are given in and B respectively (results of SM-6 fed-batch assays not shown). The profiles point to differences in the response of individual clones to a platform fed-batch process, with maximum viable cell concentrations observed ranging from 2 to 28 × 106 and 3 to 26 × 106 viable cells/mL for the LM-1 and CM-1 clones, respectively. The volumetric productivities obtained for the LM-1 and CM-1 clones in these fed-batch assays are shown in and B, and the productivities of the lead clones for all four IgM variants tested are summarized in . Volumetric productivities obtained from the top clones expressing SM-6 (J+), SM-6 (J−) and LM-1 (J+) were similar ranging from 0.9–1.6 g/L. The CM-1 clones were more productive, reaching volumetric productivities of 2.2 to 2.3 g/L for the lead clones.
PER.C6® cells express functional and properly assembled IgMs.
The material from the fed-batch studies was further analyzed to determine if the IgM produced in PER.C6® cells adopted the proper multimeric conformations, and to determine if they were capable of binding to the target antigen. Western blotting analysis revealed that SM-6 (J−) clones produced hexameric forms of IgM, whereas only pentameric IgM species were detected in SM-6 (J+), LM-1 (J+) and CM-1 (+J) clones ().
Antibody binding to antigens on the surface of human pancreatic adenocarcinoma cells (BXPC-3) and human lung carcinoma cells (A549) was assessed by fluorescence-activated cell sorting analysis (). Recombinant SM-6 (J−), SM-6 (J+) and LM-1 antibodies isolated from the PER.C6® cell lines bound strongly to the BXPC-3 cells ( top and middle). Binding of the recombinant SM-6 (J+/−) and LM-1 antibodies to the BXPC-3 cell line was similar to the binding of the control SM-6 and LM-1 antibodies produced by the hybridoma cell lines. Similarly, PER.C6® produced CM-1 antibody bound to the A549 cells comparably to the control hybridoma produced CM-1 antibodies (, bottom).
The hybridoma produced LM-1 and SM-6 antibodies are described to induce cell death of tumor cells.Citation19,Citation22 The activity of the PER.C6® produced IgMs was also assessed in a cytotoxicity assay by measuring decrease in cell viability of HeLa tumor cells using the Calcein-AM assay (). The results for LM-1 and SM-6 PER.C6® derived antibodies show a dose-dependent decrease in viability of tumor cells which is comparable to data obtained formerly with purified hybridoma derived IgMs.
The IgM class of immunoglobulins has been implicated as a potentially powerful tool in the arsenal of anti-tumor therapeutics, given its high avidity. However, as a large and heavily glycosylated protein, the commercial production of sufficient quantities of functionally active recombinant IgMs poses potential challenges. Previous work has demonstrated that PER. C6® cells are a robust and efficient platform for the high level expression of IgGs. The results presented in this paper demonstrate that PER.C6® cells are also suitable for the high level production of IgMs, an antibody class previously believed to be more difficult to express. The full assembly and polymerization of pentameric and hexameric IgM occurs in a stepwise manner in the endoplasmic reticulum and requires an intracellular redox environment and quality control system that allows the further modification with the J chain. Notably, during B cell development, plasma cells undergo a differentiation program that enables high level secretion of fully polymerized IgMs. In contrast, B cells cannot assemble and polymerize IgM, even with the ectopic expression of J chain,Citation23 and consequently degrade free IgM heavy and light chains. The inability of B cells to efficiently express secretory IgMs is thought to be the result of the differential expression of molecules involved in ER redox control.Citation24 In this paper, we report that cell lines were generated for three different IgMs, and in one case an IgM was expressed both with and without the accessory J chain. In all cases, cell lines were isolated that exhibited productivities of at least 0.5 g/L, and in some cases the productivity exceeded 2 g/L. These results are remarkable as they suggest that PER. C6® cells are equipped for the proper assembly and secretion of complex molecules such as IgMs, in a manner that is more similar to that of professional secretory cells such as plasma cells than of undifferentiated B cells. Moreover, these cell lines were generated following a simple transfection and limiting dilution screening approach, with no amplification or cell line/cell culture optimization. On average, cell lines were assessed in a small scale fed-batch assay by about four months after transfection, reflecting the relative speed and simplicity in which cell lines can be generated in PER. C6® cells. The results presented here demonstrate the versatility and robustness of the PER.C6® cell line as a production host suitable for the high level expression of IgMs.
Materials and Methods
Generation of IgM expression constructs.
Individual IgM cDNA constructs were generated based on the published variable domain sequences of the SM-6 (GenBank accession numbers: CS105717, CS105715), LM-1 (WO/2004/081027) and CM-1 cDNAs (GenBank accession numbers: AX839424, AX839426). Total RNA was prepared from SM-6, LM-1 and CM-1 producing hybridoma cell lines using the RNeasy Mini Kit (Qiagen) according to the manufacturer instructions. The cDNAs for SM-6 and LM-1 antibodies were amplified with the Superscript III One-step RT-PCR System with platinum Taq DNA Polymerase (Invitrogen). The cDNA was directly cloned into the pJet1/blunt cloning vector (Fermentas). The cDNA sequences of several independent clones for heavy and light chains were determined. The following primers were used for amplification of the LM-1 light chain cDNA: AGG TCA CCA TCT CCT GCT CTA A and CTA TGA ACA TTC TGT AGG GGC C; the LM-1 heavy chain cDNA: CCG ACC CTG TCC CTC ACC TGC GCT and TCA GTA GCA GGT GCC AGC TGT GT; SM-6 light chain cDNA TCC TAT GTG CTG ACT CAG CCA CC and CTA TGA ACA TTC TGT AGG GGC C; SM-6 heavy chain cDNA CAG GTG CAG CTG GTG GAG TCT GG and TCA GTA GCA GGT GCC AGC TGT GT. The HAVT20 leader peptide sequence was added to the light and heavy chains by PCR.Citation25 The CM-1 heavy chain and light chain cDNAs were amplified by PCR using the PfuUltra HF DNA polymerase (Stratagene), cloned into the pCR-BluntII-TOPO vector (Invitrogen) and sequenced. Forward primers used for amplification of both light and heavy chains contained the native leader peptide sequences. The following primers were used for amplification of the heavy chain cDNA: ACC ATG GAG TTT GGG CTG AGC and TCA GTA GCA GGT GCC AGC T. The following primers were used for amplification of the light chain cDNA: ACC ATG GCC TGG ATC CCT C and CTA TGA ACA TTC TGT AGG GGC C. Restriction sites used for cloning into the expression vector were added to cDNAs by PCR and the integrity of the sequences was confirmed by DNA sequence analysis. The heavy chain cDNAs were inserted into the pcDNA3002Neo expression vectorCitation20 via AscI and HpaI restriction sites; the light chain cDNAs were inserted via BamHI and NheI sites for the SM-6 and CM-1, and NheI site for the LM-1 IgM. The J chain cassette was subcloned into pcDNA3002Neo vector via BglII site after removal of the BamHI site within the J chain cassette.
Upon sequencing of the LM-1 heavy chain, it was discovered that the N-terminal 15 amino acids of the mature heavy chain variable domain were missing since the primer used for amplification primed internal to the heavy chain variable domain. The missing 15 amino acids (QVQLQESGPGLVKPS) were introduced in two steps by PCR. In the first step, a primer encoding the first 15 amino acids (15-AS-forw: 5′-CAG GTG CAG CTG CAG GAG TCG GGC CCA GGA CTG G) and the primer TCA GTA GCA GGT GCC AGC TGT GT were used to amplify the LM-1 template with the missing 15 amino acids. This was followed by amplification by PCR with a primer (GTT GGC GCG CCG CCA CCA TGG CAT GCC CTG GCT TCC TGT GGG CAC TTG TGA TCT CCA CCT GTC TTG AAT TTT CCA TGG CTC AGG TGC AGC TGC AGG AGT CG) and TCA GTA GCA GGT GCC AGC TGT GT that reintroduces the leader sequence creating the LM-1 clone with the 15 amino acids that were earlier missing. The corrected LM-1 heavy chain fragment was recloned into the expression vector.
Transfection and clone generation.
The suspension-adapted PER.C6® cell line was transfected using the Amaxa Nucleofector according to manufacturer recommendations. Briefly, 5 µg of expression vector was mixed with 5 × 106 cells in 100 µL of solution T (Amaxa). Cells were electroporated using program A-027 and placed in 5 ml of serum-free Mab medium (SAFC) supplemented with 4 mM L-glutamine in a tissue culture flask. On average 4–5 reactions were performed and pooled together for each construct. After a two-day recovery period, cells were plated in 96-well plates in Mab medium containing 125 µg/mL of Geneticin® (Invitrogen). Plates were screened for cell growth three to four weeks after transfection and well-defined single colonies were transferred to 48-well plates. Approximately 200–400 clones were assayed using an anti-IgM ELISA, from which the top 20% of the clones based on the ELISA results were expanded. Further productivity assessments during cell line screening were performed, and ∼20 candidate cell lines for each of the IgMs were chosen for further study.
Human IgM ELISA.
Nunc MaxiSorp plates were coated overnight at 4°C with capture goat polyclonal anti-human IgM anti-body (Bethyl Labs). Blocking was done with TBS buffer (10 mM Tris, 150 mM NaCl) containing 1% BSA for 2 h at room temperature. The standard curve was generated with ChromPure Human IgM (Jackson Laboratories). Bound IgM was detected by sequential addition of horseradish peroxidase (HRP) conjugated mouse monoclonal anti-human IgM (Southern Biotech) and tetramethylbenzidine (BioFX Laboratories). Conversion of the colorimetric substrate was quantified at 450 nm using an ELISA plate reader (Molecular Devices, Sunnyvale, CA).
Batch assay.
Seven-day batch assays were performed in 250-mL shake flasks, at a 25 mL working volume. Cultures were inoculated at a target concentration of 1.0 × 106 viable cells/mL in VPRO production medium (SAFC). Cultures were incubated at 37°C and 5% CO2 on an orbital shaker platform at 125 rpm. The cultures were sampled daily (except day 5 and 6) to determine cell concentration and viability by Cedex cell culture analyzer (Innovatis, Germany) and antibody concentration by ELISA. From these results, the growth rates and the integrated viable cell concentrations (IVCC) for each of the cell lines were determined. The cell specific productivity (Qpmax) was calculated over the linear part of the growth curve during the batch assay.
Fed-batch assay.
The growth and productivity of the candidate cell lines were assessed in fed-batch assays carried out in 250-mL shake flasks, at a 50 mL initial working volume. Clones were inoculated at a target concentration of 0.5 × 106 viable cells/mL into CDM4PERMAb medium (Hyclone) containing 125 µg/mL Geneticin® (Invitrogen). The flasks were placed inside a shaker incubator under the following conditions: 36.5°C, 125 rpm, 2.5 cm shaker throw, 5% CO2 and 70–80% relative humidity. The progress of the cultures was monitored using the Vi-CELL™ cell viability analyzer (Beckman) for cell concentration and viability, BioProfile 400 analyzer (NOVA Biomedical) for metabolite concentrations and Rapidlab 248 blood gas analyzer (Bayer) for pH, pO2 and pCO2. The product titer was determined by an ELISA assay. Daily bolus feeding of the cultures was initiated when the glucose concentration decreased below 2.5 g/L (day 4–7 post inoculation). The volume of the daily feed was adjusted to match the consumption rate of the cells using glucose concentration as a marker. The feed medium was composed of a proprietary mixture of glucose, amino acids, buffer, media concentrates and Geneticin® (LM-1 and CM-1 clone assays only). When the culture viability decreased to approximately 50%, the material was harvested by centrifugation at 3,000 g for 30 minutes. The clarified harvest was used for further product analysis.
SDS-PAGE and western blotting analysis of the recombinant IgM.
IgMs were separated on a 3.7% polyacrylamide-agarose composite gel and subjected to western blotting analysis. SDS-PAGE gel was prepared by mixing 3.7 mL of 30% acrylamide (acrylamide: N,N'-methylenebisacrylamide = 29:1), 7.5 mL of 1.5 M Tris-Acetate (pH 7.0), 6.68 mL H2O, 4.5 mL of glycerol, 7.5 mL of 2 % agarose at 50°C. The mixture was left to stand at room temperature for 30 seconds, and 6 µL of TEMED and 100 µl of APS (25%) were added, followed by stirring for 15 seconds. The solution was poured into a prewarmed (50°C) gel plate (BIO-RAD DCode™ System), and acrylamide was allowed to polymerize for one hour at room temperature. A buffer for electrophoresis was prepared using 50 mM of Tris, 50 mM of Tricine and 1% of SDS. Samples were prepared by addition of 6 µL 1 M iodoacetamide to 24 µL of sample followed by incubation for 10 min at room temperature. After adding 10 µL of loading buffer (125 mM of Tris (pH 6.8), 5% of SDS, 0.25% Bromphenol blue and 25% glycerol), samples were heated to 95°C for 5 min. For each sample, 12 µL was loaded per lane (10 µg of antibody), and the gel was run for 15 h at 80 V at room temperature. Western blotting was done in wet blot running buffer (25 mM Tris, and 192 mM Glycine, pH 8.3). The gel was blotted in a wet blotting chamber (BioRad) on a PVDF-membrane (Millipore) for two hours at 250 mA. Blots were blocked in 5% dry milk in PBS containing 0.05% Tween for one hour. Peroxidase-coupled anti human IgM antibody (DAKO, 1:1,000) was applied for one hour. Blots were washed three times with PBS containing 0.05% Tween for 15 minutes and were developed with Pierce ECL Super Signal West Pico solutions.
FACS analysis of cell binding of the IgM to the tumor cell lines.
Cells (human pancreatic carcinoma cells BXPC-3 and human lung carcinoma A549) were trypsinized with cell dissociation solution (Sigma C5789), and resuspended in complete medium (RPMI 1640, PAA, E15-039; 10% Fetal bovine serum, PAA, A15-151 and 1% Glutamine, PAA, M11-004)) at a cell concentration of 2 × 105 cells/mL. After a 30 minute incubation on ice, 1 mL of cells was aliquoted and washed once with ice cold PBS, then centrifuged at 800 g at 4°C. Staining was done with 100µg/mL of IgM antibody or isotype control IgM (Chrompure IgM, Dianova 009-000-012). Antibodies were incubated 30 minutes on ice, washed with ice cold PBS and secondary anti-human IgM-FITC antibody (DAKO, F0317) was applied at a dilution of 1:50 in 200 µL per sample. After another 30 minutes of incubation in the dark, cells were washed twice with PBS, transferred to a FACS tube and the number of FITC-positive cells was determined by flow cytometry.
Purification of antibodies.
Antibody purification was performed as described previously.Citation26 Briefly, cell culture harvest was filtered through a 0.2 µm filter (Millipore) and loaded on a 15 mL Ceramic Hydroxyapatite TypII, 40 µm column (ATOLL GmbH, WEINGARTEN, GERMANY) with a flow rate of 150 cm/h. The column was washed with 10 mM sodium phosphate, 10% PEG-600, pH 7.0, followed by a wash with 100 mM sodium phosphate, 10% PEG-600, pH 7.0. The purified antibody was eluted with 325 mM phosphate, 10% PEG-600. The eluate was diluted with 2.3 volumes 10 mM sodium phosphate, 2 M urea, 2 mM EDTA, pH 7. Antibody-containing fractions were loaded to a 8 mL QA CIM column (BIA Separations, Ljubljana, Slovenia), diluting the samples by in-line dilution 1:2 with 10 mM sodium phosphate, 2 M urea, pH 7.0 (buffer A). After loading, the column was washed with 100% buffer A, followed by a wash with 15% buffer B (500 mM sodium phosphate, pH 7). Elution was performed with 50% buffer B. Antibody-containing fractions were pooled and buffer was exchanged to formulation buffer (50 mM Tris/100 mM Arginine, pH 9.5) using PD10 columns (GE Healthcare Europe, Freiburg, Germany).
Calcein-AM assay.
HeLa cells were plated at 2,000 cells per well in black 96-well plates (Nunc) and incubated with different antibody concentrations or formulation buffer alone for 48 hours. Medium was then removed and replaced with 100 µL PBS containing 1 µM Calcein-AM (Calbiochem) and cells were incubated at 37°C for 2 hours. Plates were analyzed with the fluorescence plate reader (Beckman) with excitation and emission at 485 nm and 530 nm, respectively.
Figures and Tables
Figure 1 Expression vectors expressing the SM-6 (J+) and SM-6 (J−) IgM. The heavy chain (HC), light chain (LC) and J chain are all expressed from the hCMV promoter.
Figure 2 Summary of PER.C6® cell line generation. The expression construct is introduced into serum-free and suspension adapted PER.C6® cells by electroporation. Following a short recovery period, cells are seeded into 96-well plates by limiting dilution, whereupon stable transfectants are selected by survival in the presence of the antibiotic Geneticin®. Following productivity screens in the multi-well plates, candidate cell lines are expanded and cultured in shake flasks. Batch and fed-batch assays were performed on the candidate cell lines to determine Qpmax and volumetric productivity.
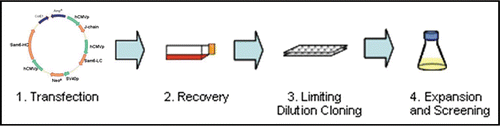
Figure 3 Productivity of IgG and IgM expressing cell lines in PER.C6® cells, in a 7-day batch process. The average Qpmax values for the top 4 cell lines, for 6 independent IgG stable cell line generation programs, are shown in the red bar (n = 24). For the SM-6 (J−) and SM-6 (J+) IgMs, the average of the top 4 cell lines for each variant is shown in the blue bars. For each variant, the Qpmax for the leading cell line is indicated by the yellow circle.
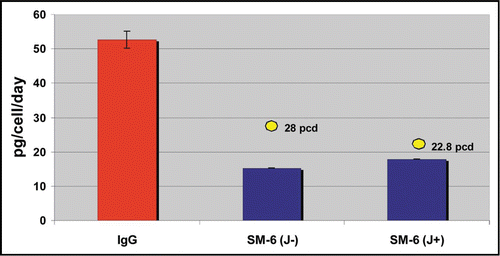
Figure 4 Cell growth profiles from a small scale fed-batch screen of 21 candidate LM-1 IgM expressing cell lines (A) and 22 candidate CM-1 IgM expressing cell lines (B). Colored traces represent results from individual clones.
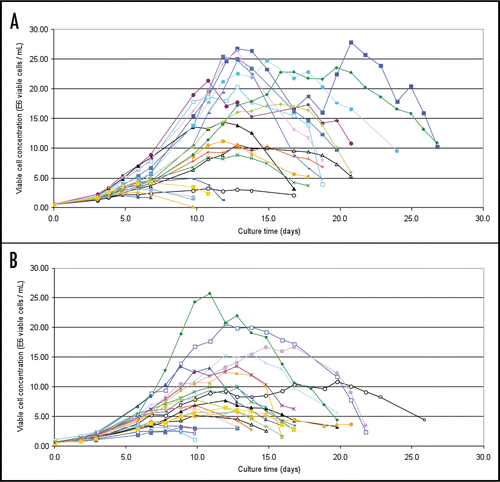
Figure 5 Production profiles from a small scale fed-batch screen of 21 candidate LM-1 IgM expressing cell lines (A) and 22 candidate CM-1 IgM expressing cell lines (B). Colored traces represent results from individual clones.
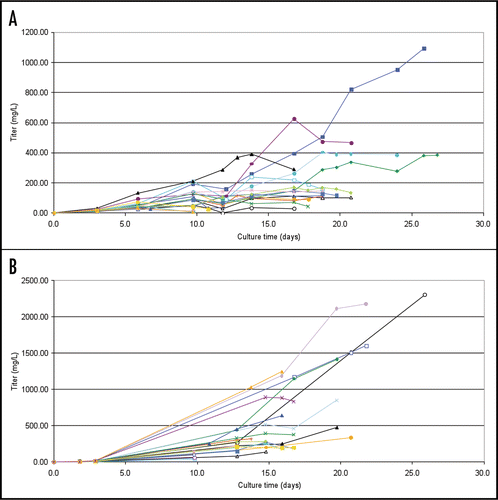
Figure 6 Evaluation of pentameric/hexameric expression of IgM in PER.C6® cells. Samples were run on 3.7% polyacrylamide-agarose composite SDS-PAGE gel and subjected to western blotting analysis. Ten micrograms of purified IgM was loaded for each recombinant IgM. Lane 1: CM-1 clone 064 (J+); Lane 2: LM-1 clone 041 (J+); Lane 3: XP control (J chain positive); Lane 4: GP control (J chain deficient); Lane 5: XP control (J chain positive); Lane 6: GP control (J chain deficient); Lane 7: SM-6 clone 089 (J−); Lane 8: SM-6 clone 130 (J−); Lane 9: SM-6 clone 450 (J+); Lane 10: SM-6 clone 528 (J+). Control IgMs were described by Collins et al.Citation3
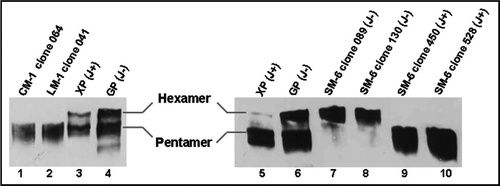
Figure 7 FACS analysis of hybridoma and PER.C6® derived IgM antibodies on tumor cells. Top: FACS analysis of SM-6 antibody binding to tumor cell line BXPC-3. Positive control SM-6 antibody (black line, 100 µg/mL) is hybridoma produced IgM. CP IgM (Chrompure; red line 100 µg/mL) was used as a negative control. PER.C6® SM-6 (J−) clones 089 and 130, and SM-6 (J+) clones 450 and 528 were tested (black line, 100 µg/mL). Middle: FACS analysis of LM-1 antibody binding to the BXPC-3 cell line. Positive control LM-1 (black line, 100 µg/mL) is hybridoma produced IgM. Negative control is CP IgM (Chrompure; red line 100 µg/mL). PER.C6® LM-1 clones 041 and 170 were tested (black line, 100 µg/mL). Bottom: FACS analysis of CM-1 antibody to the tumor cell line A549. Positive control CM-1 (black line, 100 µg/mL) is hybridoma produced IgM. Negative control is CP IgM (Chrompure; red line 100 µg/mL). PER.C6® CM-1 clones 027 and 064 were tested (100 µg/mL).
Figure 8 Calcein-AM assay with purified PER.C6® derived IgM antibodies. HeLa cells were seeded into 96-well plates and incubated with increasing amounts of antibodies or formulation buffer alone. Cell viability was measured after incubation for 2 hours with Calcein-AM. The percentage cytotoxicity was calculated using the following formula: Cytotoxicity [%] = [100/(cellsonly × cells+formulation buffer)] − [100/(cellsonly × cells+antibody)], where cellsonly are cells in RPMI medium without formulation buffer or antibody. Dose-dependent increase in cytotoxicity was observed with LM-1 IgM (A) and SM-6 IgM (B) antibodies incubated with HeLa tumor cells.
![Figure 8 Calcein-AM assay with purified PER.C6® derived IgM antibodies. HeLa cells were seeded into 96-well plates and incubated with increasing amounts of antibodies or formulation buffer alone. Cell viability was measured after incubation for 2 hours with Calcein-AM. The percentage cytotoxicity was calculated using the following formula: Cytotoxicity [%] = [100/(cellsonly × cells+formulation buffer)] − [100/(cellsonly × cells+antibody)], where cellsonly are cells in RPMI medium without formulation buffer or antibody. Dose-dependent increase in cytotoxicity was observed with LM-1 IgM (A) and SM-6 IgM (B) antibodies incubated with HeLa tumor cells.](/cms/asset/bb873658-7976-448f-a540-e40a40b6be56/kmab_a_10907945_f0008.gif)
Table 1 Productivities of lead clones for each of the four IgM variants in a fed-batch process
Acknowledgements
We thank Dr. Mark J. Shulman of Immunology Department, University of Toronto for giving us GP (J chain negative) and XP (J chain positive) hybridomas which were used as a control for western blot analysis.
References
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256:495 - 497
- Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nature Reviews 2007; 6:349 - 356
- Collins C, Tsui FWL, Shulman MJ. Differential activation of human and guinea pig complement by pentameric and hexameric IgM. Eur J Immunol 2002; 32:1802 - 1810
- Wiersma EJ, Collins C, Fazel S, Shulman MJ. Structural and functional analysis of J chain-deficient IgM. J Immunol 1998; 160:5979 - 5989
- Irie RF, Ollila DW, O'Day S, Morton DL. Phase I pilot clinical trial of human IgM monoclonal antibody to ganglioside GM3 in patients with metastatic melanoma. Cancer Immunol Immunother 2004; 53:110 - 117
- Jones PC, Sze LL, Liu PY, Morton DL, Irie RF. Prolonged survival for melanoma patients with elevated IgM antibody to oncofetal antigen. J Natl Cancer Inst 1981; 66:249 - 254
- Ollert MW, David K, Schmitt C, Hauenschild A, Bredehorst R, Erttmann R, et al. Normal human serum contains a natural IgM antibody cytotoxic for human neuroblastoma cells. Proc Natl Acad Sci USA 1996; 93:4498 - 4503
- Vollmers HP, O'Connor R, Muller J, Kirchner T, Muller-Hermelink HK. SC-1, a functional human monoclonal antibody against autologous stomach carcinoma cells. Cancer Res 1989; 49:2471 - 2476
- Brandlein S, Pohle T, Ruoff N, Wozniak E, Muller-Hermelink HK, Vollmers HP. Natural IgM antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res 2003; 63:7995 - 8005
- Vollmers HP, Brandlein S. Natural antibodies and cancer. J Autoimmune. 2007; 29:295 - 302
- Wurm F. Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology 2004; 22:1393 - 1398
- Cattaneo A, Neuberger MS. Polymeric immunoglobulin M is secreted by transfectants of non lymphoid cells in the absence of immunoglobulin J chain. EMBO J 1987; 6:2753 - 2758
- Cacciuttolo MA, Patchan M, Lamey K, Allikmets E, Tsao E. Large-scale production of a monoclonal IgM in a hybridoma suspension culture. BioPharm 1998; 20 - 29
- Wood CR, Dorner AJ, Morris GE, Alderman EM, Wilson D, O'Hara RM Jr, Kaufman RJ. High level synthesis of immunoglobulins in Chinese hamster ovary cells. J Immunol 1990; 145:3011 - 3016
- Barnes LM, Bentley CM, Dickson AJ. Stability of protein production from recombinant mammalian cells. Biotechnol Bioeng 2003; 81:631 - 639
- Faller G, Vollmers HP, Weiglein I, Marx A, Zink C, Pfaff M, Muller-Hermelink HK. HAB-1, a new heteromyeloma for continuous production of human monoclonal antibodies. Br J Cancer 1990; 62:595 - 598
- Brandlein S, Vollmers HP. Natural IgM antibodies, the ignored weapons in tumor immunity. Histol Histopathol 2004; 897 - 905
- Vollmers HP, Brandlein S. Death by stress: natural IgM-induced apoptosis. Methods Find Exp Clin Pharmacol 2005; 27:185 - 191
- Brandlein S, Lorenz J, Ruoff N, Hensel F, Beyer I, Muller J, et al. Human monoclonal IgM antibodies with apoptotic activity isolated from cancer patients. Hum Antibodies 2002; 11:107 - 119
- Jones D, Kroos N, Anema R, van Montfort B, Vooys A, van der Kraats S, et al. High-level expression of recombinant IgG in the human cell line PER.C6®. Biotechnol Prog 2003; 19:163 - 168
- 2008; DSM and Crucell press release, “DSM and Crucell announce another key achievement for PER.C6® technology: scaleup of high titer fed-batch process to 250 liters.”
- Brandlein S, Rauschert N, Rasche L, Dreykluft A, Hensel F, Conzelmann E, et al. The human IgM antibody SAM-6 induces tumor-specific apoptosis with oxidized low-density lipoprotein. Mol Cancer Ther 2007; 6:326 - 333
- Randall TD, Brewer JW, Corley RB. Direct evidence that J chain regulates the polymeric structure of IgM in antibody-secreting B cells. J Bio Chem 1992; 267:18002 - 18007
- Tagliavacca L, Anelli T, Fagioli C, Mezghrani A, Ruffato E, Sitia R. The making of a professional secretory cell: architectural and functional changes in the ER during B lymphocyte plasma cell differentiation. Biol Chem 2003; 384:1273 - 1277
- Kimura N, Toyonaga B, Yoshikai Y, Du R, Mak TW. Sequences and repertoire of the human T cell receptor α and β chain variable region genes in thymocytes. Eur J Immunol 1987; 17:375 - 383
- Gagnon P, Hensel F, Richieri R. Purification of IgM Monoclonal Antibodies: Manufacturing challenges surround the use of IgM monoclonal. BioPharm Int Sup 2008; 26 - 36