Abstract
Despite therapeutic advances, the long-term survival rates for acute myeloid leukemia (AML) are estimated to be 10% or less, pointing to the need for better treatment options. AML cells express the myeloid marker CD33, making it amenable to CD33-targeted therapy. Thus, the in vitro and in vivo anti-tumor activities of lintuzumab (SGN-33), a humanized monoclonal anti-CD33 antibody undergoing clinical evaluation, were investigated. In vitro assays were used to assess the ability of lintuzumab to mediate effector functions and to decrease the production of growth factors from AML cells. SCID mice models of disseminated AML with the multi-drug resistance (MDR)-negative HL60 and the MDR+, HEL9217 and TF1-α, cell lines were developed and applied to examine the in vivo antitumor activity. In vitro, lintuzumab significantly reduced the production of TNF-α-induced pro-inflammatory cytokines and chemokines by AML cells. Lintuzumab promoted tumor cell killing through antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) activities against MDR- and MDR+ AML cell lines and primary AML patient samples. At doses from 3 to 30 mg/kg, lintuzumab significantly enhanced survival and reduced tumor burden in vivo, regardless of MDR status. Survival of the mice was dependent upon the activity of resident macrophages and neutrophils. The results suggest that lintuzumab may exert its therapeutic effects by modulating the cytokine milieu in the tumor microenvironment and through effector mediated cell killing. Given that lintuzumab induced meaningful responses in a phase 1 clinical trial, the preclinical antitumor activities defined in this study may underlie its observed therapeutic efficacy in AML patients.
Introduction
Acute myeloid leukemia (AML) represents ∼90% of all adult acute leukemias and is the second most common pediatric leukemia.Citation1–Citation3 For 2008, the American Cancer Society projected 13,300 new cases and 8,800 deaths from AML in the US alone. It is predominantly a disease of patients older than 70 years of age, and as such, the incidence of AML will increase as the population continues to grow. AML is often preceded by a myelodysplastic phase and exhibits unfavorable cytogenetic abnormalities and older patients fare more poorly than younger patients because often, they cannot tolerate intensive chemotherapy.Citation1,Citation2,Citation4 AML in older patients also tends to be resistant to chemotherapy due to the multi-drug resistance (MDR) phenotype resulting from alterations in cellular detoxification mechanisms and enhanced expression of several genes including MDR1 and the ATP-driven toxin pump, P-glycoprotein (pgp, ABCB1).Citation5 Consequently, only one-third of older patients achieve remission and only one-fifth of patients live more than a year from diagnosis.Citation1,Citation4 MDR may also emerge following unsuccessful chemotherapy in recurrent AML.Citation6–Citation8
CD33, a myeloid lineage-specific antigen, is a sialoadhesin family member normally expressed on precursor myeloid cells and most monocytic cells,Citation9 and constitutes an important drug target on AML.Citation10,Citation11 Patients with relapsed disease can be treated with the only approved anti-CD33 drug conjugate, gemtuzumab ozogamicin (Mylotarg®), yielding an overall response rate of ∼30%. However, drawbacks to this treatment include severe neutropenia and liver toxicity.Citation12,Citation13 In addition, a subset of AML patients expressing the MDR phenotype on their AML blasts has been reported to be resistant to gemtuzumab ozogamicin.Citation14–Citation17 Therefore, there is a clear unmet medical need for therapeutics that can circumvent these obstacles.
Lintuzumab, also known as SGN-33 or HuM195,Citation18 is a humanized anti-CD33 monoclonal antibody (mAb) in clinical development. Treatment of advanced AML patients with low doses of lintuzumab has yielded multiple responses including complete remissions, stable disease, and reductions in marrow leukemic blast percentages.Citation19–Citation21 In ongoing clinical trials, the antibody is under evaluation in patients with myeloid malignancies who are not considered candidates for intensive chemotherapy. The results from a multiple dose, single arm dose escalation Phase 1 study showed that the antibody is well-tolerated and demonstrated clinical efficacy in 7 (4 complete remissions) of 17 AML patients with blast percentages ranging from 29 to 63%.Citation22,Citation23
Limited preclinical characterization of lintuzumab including efficacy studies in murine xenograft models of AML, has been done. While the activity of the murine parent (M195) has been evaluated in a MDR-negative (MDR−) xenograft model,Citation24 lintuzumab has not been previously tested in xenograft models that simulate the disseminated nature and bone marrow involvement of AML. In this study, three new disseminated models of AML have been developed and used to demonstrate that lintuzumab significantly prolongs the survival of mice in MDR− and MDR-positive (MDR+) models of AML. Additionally, the results show that lintuzumab significantly reduces the production of pro-inflammatory and tumor-promoting growth factors and interacts with the immune system to mediate antibody effector functions. These findings suggest that the antibody represents a valid targeted therapeutic for the treatment of CD33+ myeloproliferative diseases.
Results
Characterization of AML cell lines. Five human AML cell lines were evaluated with respect to their CD33 expression levels, complement regulatory protein (CRP) expression, and MDR status (). The cell surface levels of CD33 among the 5 cell lines ranged from 12,600 to 20,000 copies per cell, in agreement with those found on normal CD14+ primary human monocytes (∼8,000 copies), primary bone marrow samples from two newly diagnosed AML patients (∼11,000 copies), and those published previously.Citation10 All cell lines used in this study expressed the immune regulatory proteins, CD46 and CD55, while three of the cell lines also expressed CD59. In addition, normal CD14+ monocytes and primary AML patient samples expressed CRPs (reviewed in ref. Citation25). The cell lines bound lintuzumab to a similar extent, with an apparent Kd ∼100 pM (range of 70 to 168 pM, ).
The MDR status of the cell lines was established by assessing the expression of the Pgp pump and the efflux of rhodamine by flow cytometry. The HL60, KG-1 and U937 cell lines were MDR−, whereas HEL9217 and TF1-α cell lines were MDR+ (, , and data not shown). Consistent with these findings, gemtuzumab ozogamicin (Mylotarg®) effectively mediated cytotoxicity against the MDR− cell lines while it was refractory in the MDR+ cell lines (reviewed in refs. Citation14, Citation15 and Citation17).
Lintuzumab is a signaling mAb and reduces cytokine secretion by AML cells. While other anti-CD33 antibodies have been reported to initiate a signaling response upon ligation to CD33 on the cell surface,Citation26–Citation29 this has not been previously demonstrated for lintuzumab. The results show that in HL60 cells and primary bone marrow cells from newly diagnosed (AML #1) or relapsed (AML #2) AML patients, lintuzumab treatment induced phosphorylation of CD33 and recruitment of Shp-1 phosphatase (). Although AML #2 bone marrow sample expressed lower levels of CD33 (27% total population with 5,700 copies/cell) compared to the other AML sample (90% population with 10,000 copies/cell), increased phosphorylation of CD33 and recruitment of Shp-1 were still detectable upon ligation of CD33. A kinetic study using the HL-60 cell line revealed that the association of Shp-1 with the CD33 complex occurred rapidly, peaked between 6–15 min, and started to decline around 1 h subsequent to CD33 ligation. Unlike previous reports with other anti-CD33 antibodies,Citation27,Citation28 lintuzumab did not induce recruitment of Shp-2 to CD33 (data not shown).
Higher than normal levels of circulating cytokines and chemokines has been reported in the blood and in the bone marrow of AML patients.Citation30–Citation33 These factors may contribute to the pathogenesis of disease by promoting growth of tumor cells in the bone marrow microenvironment and/or suppressing the host immune system.Citation30–Citation33 Thus, agents that can decrease cytokine levels in AML patients may provide clinical benefits. As recruitment of Shp-1 is consistent with the hypothesis that CD33 may deliver negative signals to modulate cellular functions, the effect of lintuzumab on cytokine and chemokine secretion by AML cells was examined as part of the efforts to better define its mechanisms of action.
KG-1 cells produced modest levels of several cytokines and chemokines (). When KG-1 cells were treated with TNF-α, the production of known tumorigenic growth factors including IL-8 (CXCL8), MCP-1 (CCL2), IP-10 (CXCL10), and RANTES (CCL5) was significantly enhanced ( and data not shown). Lintuzumab, but not hIgG1κ significantly decreased the production of these factors (), with inhibition observed in a dose-dependent manner (). A F(ab')2 fragment of lintuzumab (1,000 ng/ml) was also effective in suppressing the TNF-α-induced IL-8 secretion by KG-1 cells, further confirming that ligation of CD33, but not FcγR, was required ().
Antibody effector functions of lintuzumab. A previous study using mouse AL67 fibroblasts overexpressing human CD33 demonstrated that lintuzumab displayed measurable ADCC activity.Citation18 The current study was undertaken to further characterize the effector functions of lintuzumab. The results show that lintuzumab displays multiple effector functions, including the ability to mediate ADCC and ADCP () against MDR− and MDR+ AML cell lines.
In the presence of primary human NK cells, lintuzumab mediated the tumor cell killing of MDR− KG-1 cells and the MDR+ HEL9217 and TF1-α cell lines in a dose-dependent manner (). Low, but clearly detectable, ADCC activity could also be detected against the AML blasts from an AML patient with refractory disease (). In contrast, the hIgG1κ control antibody demonstrated minimal activity. Decreased levels of specific lysis observed with primary cells as compared to the cell lines may result from reduced chromium-51 uptake by the AML patient cells and a lower cell viability contributing to a higher background lysis.
Removal of antibody-coated tumor cells by FcγR-mediated phagocytosis (ADCP) has been hypothesized to be a key mechanism for target cell depletion mediated by therapeutic mAbs in vivo.Citation34,Citation35 In the current study, the ability of lintuzumab to mediate ADCP against three different AML cell lines with macrophages generated from GM-CSF-treated normal human PBMCs as effector cells, was investigated. These macrophages expressed the relevant phenotypic markers, including CD11b, CD16, CD206, CD14 and CD33 (data not shown). Engulfment of antibody-coated tumor cells by macrophages (dual-labeled cells) was detected by flow cytometry and visualized by fluorescence microscopy. As can be seen in , lintuzumab-coated MDR− or MDR+ AML cells were effectively engulfed by macrophages compared to cells coated with the hIgG1κ control antibody.
Lintuzumab, like other anti-CD33 antibodies, mediated CDC activity against U937 cells in the presence of rabbit complement, but failed to do so with human complement ( and reviewed in refs. Citation18, Citation36 and Citation37). Although low antigen expression on the AML target cells might have prevented the antibody from exerting CDC activity with human serum,Citation18 it is also possible that CRP expression on these cells may modulate lintuzumab-mediated lysis in the presence of syngeneic complement components. As noted in , AML cell lines express CD46, CD55 and CD59 which would impact their response to CDC.Citation25,Citation38,Citation39 When AML cell lines were pre-treated with blocking antibodies to CD46 and CD55, CDC activity with human serum was detectable ( and data not shown). These findings suggest that CRP expression on AML cells may modulate their susceptibility to lintuzumab-mediated CDC.
Collectively our data demonstrate that lintuzumab can potentially mediate anti-tumor activity through cytokine modulation and effector functions (ADCC and ADCP).
In vivo efficacy of lintuzumab in disseminated models of AML. Antitumor activity in a disseminated HL-60 xenograft model has been demonstrated for the mouse parent mAb of lintuzumab, M195.Citation24 In this report, disseminated disease xenograft models of AML using the HL60, TF1-α and HEL9217 cell lines have been developed to examine the in vivo antitumor activity of lintuzumab. As exemplified by the HL-60 model, injected tumor cells stably expressed CD33 on their surface, with very little change upon further ex vivo culture (). In addition, human CD33+ tumor cells were readily detected by flow cytometry or immunohistochemistry in the blood, bone marrow, lymph nodes, as well as tumor masses in mice injected with the tumor cells ( and C).
The results of dosing tumor-bearing mice with lintuzumab 1 day or 3 days after intravenous injection of the tumor cells are shown in to D. Lintuzumab treatment had no negative side effect on the health of the mice at all of the doses tested (data not shown). In the MDR− HL60 model, lintuzumab given at 4 doses of 3, 10 or 30 mg/kg significantly improved the survival of the mice compared to untreated or hIgG1 treated mice (median survival time of >180 days for all doses of lintuzumab compared to 57 days for untreated or hIgG1-treated mice, p < 0.0001, ). In some experiments where treatment was initiated 7 days after injection of tumor cells, the survival of the mice was significantly prolonged by lintuzumab (data not shown). Mylotarg® also increased the survival of HL60 tumor-bearing mice (, p < 0.0001 compared to untreated). An apparent reduction in the levels of IL-6 and IL-8 was observed in the plasma of lintuzumab-treated mice compared to untreated or hIgG1-treated mice () three weeks after the final dose of the antibody.
Similarly, lintuzumab was efficacious in the MDR+ AML models ( and D). For the HEL9217 model, the mice were irradiated one day prior to tumor cell injection to allow tumor engraftment. In agreement with the MDR+ phenotype and expression of Pgp on TF1-α and HEL9217 cells, xenografts established from these cells were resistant to gemtuzumab ozogamicin (Mylotarg®) tested at the MTD dose. There was no significant difference in survival between untreated or hIgG1-treated groups compared to the gemtuzumab ozogamicin-treated group ( and D). In the TF1-α model, four doses of lintuzumab provided survival benefits at all doses tested (median survival time of >200 days for lintuzumab compared to 69 days for the untreated and hIgG1-treated groups, p = 0.0002, and 100 days for Mylotarg®, p = 0.015, ). Similarly for the HEL9217 model, lintuzumab at 3 or 10 mg/kg significantly prolonged the survival of the mice regardless of whether the mice were dosed 1 day or 3 days after injection of the tumor cells (median survival time of 173 days for the lintuzumab-treated groups compared to 53 days for hIgG1-treated mice, p = 0.002 and to 82 days for untreated or gemtuzumab ozogamicin-treated groups, p = 0.01, ). In these MDR+ AML models, plasma levels of cytokines including IL-6 and MIP1-α tended to be lower in lintuzumab-treated mice when compared to levels measured in untreated or hIgG1-treated mice (data not shown).
Mouse effector cells including macrophages and neutrophils but not NK cells were necessary for the survival of mice treated with lintuzumab (data not shown). The anti-leukemic effect of lintuzumab was significantly decreased in mice dosed with clodronate-encapsulated liposomes (CEL) to deplete macrophages and with an anti-GR-1 antibody to deplete neutrophils. Depletion of NK cells with anti-asialo GM-1 or TMb-1 antibody had minimal effect on lintuzumab activity (data not shown).
These studies demonstrate that lintuzumab can effectively and significantly prolong the survival of the animals, in particular in situations where gemtuzumab ozogamicin is less efficacious.
Discussion
In this study, the potential mechanisms of action for lintuzumab, a humanized anti-CD33 antibody, were investigated and characterized. The results show that targeting AML with lintuzumab may offer the opportunity for a better tolerated therapeutic that is active irrespective of MDR status of the AML cells.
Lintuzumab, like other anti-CD33 antibodies, can initiate intracellular signaling processes upon CD33 ligation. This process includes rapid recruitment of Shp-1,Citation27–Citation29 but not of Shp-2,Citation29 phosphatase. Decreased calcium mobilization or proliferation in AML cells treated with anti-CD33 antibodies has been associated with enhanced activities of various downstream kinases including SRC,Citation28 lck,Citation27 Syk,Citation26 ZAP-70,Citation26 or PI3 kinase.Citation40 Similar or as of yet, undefined signaling cascades may lead to the lintuzumab-mediated reduction in cytokine and chemokine production by AML cells. Elevated circulating levels of cytokines and chemokines including IL-6, MCP-1, IL-8 and TNF-α have been reported in AML and MDS patients,Citation30,Citation31,Citation41–Citation43 and linked to quality-of-life and treatment issues, such as fatigue and poor prognosis.Citation43,Citation44 Long term exposure to high TNF-α levels is associated with immunosuppression, tumor growth, and cachexiaCitation32,Citation33,Citation45 as a result of TNF-α-induced production of cytokines and chemokines such as MCP-1, RANTES and IL-8. These factors in turn can promote tumor growth or function as chemoattractants for immune cells that secrete factors supporting immunosuppression, tumor angiogenesis, and tumor metastases.Citation32,Citation33,Citation45,Citation46 Therefore, therapies that can decrease levels of tumorigenic factors may provide clinical benefit and improve overall survival in leukemia patients. The findings here suggest that lintuzumab may serve in this capacity. In vitro, lintuzumab significantly decreased the TNF-α-induced production of IL-8, MCP-1, IP-10 and RANTES from KG-1 cells. In vivo, lintuzumab appeared to reduce the circulating levels of IL-6, IL-8 and MIP1-α and prolonged the survival of mice in disseminated models of AML. These findings are in line with those reported earlier, where JTE-607, a small molecule multiple cytokine inhibitor, has been shown to increase survival in a mouse model of AML.Citation47 Additional appropriate in vivo AML tumor models will need to be developed to test the hypothesis that modulation of cytokine production contributes to the therapeutic efficacy of lintuzumab.
A second potential mechanism for lintuzumab to exert its anti-tumor activity is through interactions with the immune system to mediate effector functions. Previous investigators have reported that lintuzumab exhibited ADCC activity against HL60 cells and a mouse fibroblast cell line expressing human CD33.Citation18 The current study has extended these findings to show that lintuzumab mediated effective tumor cell killing against a panel of MDR− and MDR+ AML cell lines as well as primary cells from AML patients. Clinically, it is conceivable that enhancing NK function or NK cell numbers may improve ADCC and hence, the anti-tumor activity of lintuzumab. Indeed, IL-2 in combination with rituximabCitation48 and IL-2 with low doses of lintuzumabCitation49 have been reported to demonstrate some clinical benefit.
The ability of lintuzumab to interact with macrophages through Fcγ receptors to mediate ADCP and tumor cell killing is a novel finding, as this has not been reported previously for anti-CD33 antibodies. The importance of macrophages in mediating the anti-leukemic effect of lintuzumab in vivo was demonstrated using antibodies and other reagents to ablate effector cells. Depletion of Fcγ receptor-expressing cells required for the mediation of effector function, in particular macrophages and neutrophils, eliminated the protective effect of lintuzumab in mouse xenograft models. In light of the reports demonstrating an essential role played by the reticulo-endothelial system in anti-CD20-mediated B cell depletion in mice,Citation35,Citation50 the opsinization of AML cells to enhance their phagocytosis may be an important mechanism of action for lintuzumab. In addition to removing tumor cells, ADCP may play a role in the development of an adaptive anti-tumor immune response by promoting antigen presentation. Phagocytosis of antibody-bound tumor cells by recruited macrophages may result in the presentation of AML-associated antigens to the host immune system, thereby stimulating the development of antibody- and/or cell-mediated anti-tumor responses.
Additionally, extrapolation of these findings to the clinic could include retrospectively and prospectively assessing the outcome of trials with respect to Fcγ receptor IIA and IIIA polymorphisms as has been reported for other antibodies including rituximab,Citation51,Citation52 and circulating levels of cytokines (pre- and post-treatment) in the patient population. The correlation of any these factors with trial outcome may identify them as useful pharmacodynamic (PD) biomarkers.
Lintuzumab and other anti-CD33 antibodies can induce tumor cell lysis through fixation of rabbit complement.Citation18,Citation36,Citation37 Interestingly, activity with lintuzumab and human serum was evident in the presence of blocking antibodies to CD46 and CD55. Although blocking antibodies to CD59 were not tested in this study, it is possible that anti-CD59 alone or in combination with anti-CD46 and anti-CD55 may yield greater activity. Enhancing antibody activity by modulating CRP levels has been tested with other antibodies including rituximab (reviewed in ref. Citation38). A correlation between the susceptibility of tumor cells to complement-mediated lysis and the presence of CRPs was also found for an anti-CD70 antibody.Citation34 Therefore the presence of CRPs on tumor cell lines, and not necessarily limiting levels of CD33 as reported previously,Citation18 could be a major factor affecting CDC activity with human serum and lintuzumab. Variations in the levels of CRPs on leukemic blasts were reported, with a trend for the levels of some CRPs to be lower in AML.Citation25,Citation38,Citation39 Correlating CRP levels on AML blasts to response in lintuzumab clinical trials may shed light on the importance of complement fixation for this antibody.
The development of better therapeutics requires reliable and valid preclinical disease models for the evaluation of the activity of the molecules. Numerous tumor models are available but drawbacks are associated with each. For example, subcutaneously implanted tumor modelsCitation53 do not realistically reflect disseminated disease whereas genetically engineered mice to model AML driven by gene mutationsCitation54 are not useful in the evaluation of lintuzumab since murine and human CD33 proteins differ in both expression profile and biology.Citation55 Mouse xenografts generated with primary human AML leukemic stem cellsCitation56,Citation57 may better simulate the human disease, but there are problems in obtaining sufficient numbers of leukemic patient stem cells and in the variability in engraftment of these cells in mice.Citation58 As part of the investigation into the activities of lintuzumab, disseminated models of MDR− and MDR+ AML were developed by intravenous administration of commercially available human AML cell lines into SCID mice. In all three models, lintuzumab unequivocally and significantly enhanced the survival of the animals, regardless of MDR status.
In summary, lintuzumab demonstrated significant anti-tumor activity through its abilities to mediate effector functions and to engage intracellular signaling processes associated with decreased production of tumorigenic and immunosuppressive factors. These activities may also promote the development of anti-tumor immune responses and correlate well with the significantly prolonged survival that was observed in preclinical models of AML. Our data clearly show that lintuzumab is a valid, targeted therapeutic, the activity of which is not affected by common mechanisms of MDR; clinical activity is expected under conditions where gemtuzumab ozogamicin (Mylotarg®) shows limited efficacy. Lintuzumab represents a potential treatment option for patients with AML, especially those unable to tolerate high-dose induction chemotherapy.
Materials and Methods
Cell lines and reagents. The human AML cell lines HL60, HEL9217, TF1-α and U937 cells were obtained from ATCC (Manassas, VA) while the KG-1 cell line was purchased from DSMZ (Braunschweig, Germany). The cells were grown in RPMI-1640 media (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine sera (Invitrogen). Primary normal human monocytes and primary AML patient bone marrow samples were obtained from AllCells (Emeryville, CA). Fluorochrome-conjugated antibodies against human CD33, P-glycoprotein (Pgp), CD56, CD46, CD55, CD59 and MHC Class I (HLA-A, B and C) were obtained from BD Pharmingen (San Diego, CA). The DAKO indirect immunofluorescence QiFiKit (DAKO A/S, Glostrup, Denmark) was used for the quantification of CD33 on AML cell lines. Flow cytometric analyses were conducted on a BD FACScan instrument (BD Biosciences, San Jose, CA). Lintuzumab (SGN-33) is a hIgG1κ that was constructed by grafting the complementarity-determining regions (CDRs) of the mouse M195 antibody onto a human framework.Citation18,Citation59
In vitro characterization of AML cell lines.CD33 and CRP levels, and MDR status. CD33 copy number was determined using the DAKO QifiKit, according to the manufacturer's directions. The expression of complement regulatory proteins (CRPs—CD46, CD55 and CD59) was determined by flow cytometry using commercially available antibodies. The expression of Pgp and the ability of cells to efflux Rhodamine-123 (Rh123, Sigma, St. Louis, MO) were used to categorize AML cell lines as either MDR− or MDR+. Cells that are MDR+ do not retain the Rh123 and efficiently pump it out, indicative of Pgp or other pump activity.Citation60 AML cells were incubated with Rh123 and the efflux measured by flow cytometry as previously described.Citation60
Saturation binding studies. To assess binding of lintuzumab to AML cells, the antibody was linked to Alexa Fluor 488 reactive dye (Molecular Probes, Eugene, OR). AML cells were incubated in RPMI growth media containing 100 µg/ml hIgG (Jackson ImmunoResearch, West Grove, PA) for 20 minutes prior to the addition of labeled lintuzumab. The cells were treated for 1 h on ice, washed with cold PBS, and the antibody binding assessed by flow cytometry. The apparent Kd values were determined using the one-site binding Prism algorithm (GraphPad Software, San Diego, CA).
Cytokine production. KG-1 tumor cells were plated at 8,000 cells per well in RPMI-1640 medium containing 10% heat-inactivated human AB serum (Gemini Biproducts, West Sacramento, CA) in 96-well dishes. Cells were pre-treated with 100 ng/ml to 10,000 ng/ml lintuzumab, hIgG1κ (10,000 ng/ml), or lintuzumab F(ab)2 fragment (1,000 ng/ml) for 1 to 2 h prior to a 18 h challenge with 1 ng/ml TNFα (PeproTech, Rocky Hill, NJ). Media were collected at the end of the incubation period and analyzed for cytokine production by chemiluminescent ELISA (Pierce Endogen, Rockville, IL) or by Bio-plex human cytokine assay (Bio-Rad Laboratories, Hercules, CA).
Signaling. HL60 cells and cells from AML patients were incubated with lintuzumab or hIgG1κ in growth medium. Cells were harvested after 15 minutes or up to 2 h. Cell lysates were processed for immunoprecipitation of CD33 using a rabbit polyclonal anti-CD33 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoprecipitates or cell lysates were run on gradient TRIS-Gly mini-gels (Invitrogen), transferred onto PVDF membranes, and blotted with an anti-Shp-1 or anti-Shp-2 antibody (Calbiochem, San Diego, CA).
Effector functions.Antibody-dependent cellular cytotoxicity (ADCC). ADCC activity was measured using the standard 51Cr-release assay as previously described.Citation34 Briefly, target tumor cells (KG-1, HEL9217 and TF1-α AML cell lines and cells from an AML patient with refractory disease) were labeled with 100 µCi Na51CrO4, washed, and pre-incubated with lintuzumab prior to addition of effector (natural killer, NK) cells. NK cells were prepared from non-adherent peripheral blood mononuclear cells (PBMCs) obtained from normal FcγRIIIA 158V/V donors (Lifeblood, Memphis, TN) with immunomagnetic beads (EasySep, StemCell Technologies, Vancouver, BC, Canada). Viable NK cells (CD16+ CD56+) were added to target cells at an effector to target cell ratio of 10:1 for the AML cell lines and 20:1 for the AML patient. A human IgG1κ (Ancell, Bayport, MN) was used as a negative control in this assay.
Antibody-dependent cellular phagocytosis (ADCP). ADCP was assessed using a previously described method.Citation34 Target AML cells were incubated with the fluorescent dye PKH26 (Sigma, St. Louis, MO) prior to addition of lintuzumab or hIgG1κ and primary human macrophages. Macrophages were generated from normal human PBMCs cultured for 10 to 14 days with 500 U/ml human GM-CSF (PeproTech, Rocky Hill, NJ). After 1 h at 37°C, the macrophages were labeled with a FITC-conjugated CD11b antibody (BD Pharmingen). To mitigate the possibility of lintuzumab binding to the macrophages and interfering with the assay, target AML cell lines were first coated with lintuzumab and washed several times before they were incubated with the macrophages. Uptake of the target cells by the macrophages (phagocytosis) was assessed by flow cytometry and visualized by immunofluorescence using a Carl Zeiss Axiovert 200M microscope.
Complement-dependent cytotoxicity (CDC). CDC activity with U937 cells was assessed in the presence of human serum or rabbit complement as described elsewhere.Citation34 Target cells and antibody (lintuzumab or hIgG1κ) were mixed in RPMI medium containing 10% non-heat-inactivated human serum (Lifeblood) or 12.5% rabbit complement (Cedarlane Laboratories, Burlington, NC) and incubated for 2 h at 37°C. The amount of lysed cells was assessed by flow cytometry in the presence of propidium iodide. In some experiments, U937 cells were pre-incubated with 6 µg/ml blocking antibodies to CD46 (Cell Sciences, Canton, MA) and CD55 (AbCam, Cambridge, MA) for 30 minutes prior to the addition of lintuzumab or hIgG1κ and human serum.
Efficacy of lintuzumab in In vivo models of disseminated human AML. All animal experiments were conducted in an AALAC (Association for Assessment of Laboratory Animal Care) accredited facility and under IACUC (Institutional Animal Care and Use Committee) guidelines and approval. Disseminated models of AML were established in female C.B-17 SCID mice (Harlan, Indianapolis, IN). HL60, HEL9217, and TF1-α AML tumor cells (5 × 106 or 7.5 × 106 cells/mouse) were administered intravenously. For the HEL9217 model, the mice were irradiated (1 Gy) one day prior to injection of the cells to improve tumor take. The animals were routinely monitored for signs of disease, including a significant weight loss of 15 to 20%, development of scruffy coats, hind limb paralysis, and presence of palpable tumors. The onset of disease occurred within 30 to 60 days after injection of the cells. Solid tumor masses found in about 40% of the mice were associated with bone and soft tissues including the front and hind limbs, lower mandible, kidney, ovary, face and intraperitoneal cavity.
Mice were euthanized upon onset of symptoms and tissues including blood, bone marrow, lymph node and tumor masses were harvested to assess the presence and distribution of human tumor cells by flow cytometry. Tissues were rinsed with cold PBS, minced with scissors, and incubated with 20 µg/ml DNAse and 100 µg/ml collagenase (Sigma) for 1 h at 37°C. Single cell suspensions, obtained upon filtering the digested tissues through 70-micron sterile Falcon cell strainers (BD Biosciences), were incubated with fluorochrome-labeled antibodies and analyzed by flow cytometry. CD33 expression in tumor tissues was also assessed by immunohistochemistry with a mouse monoclonal anti-human CD33 antibody (NovoCastra) on formalin-fixed, paraffin-embedded tissue sections. Tissue sections were processed using a BondMaX Autostainer and accompanying blocking buffer, EDTA retrieval buffer, and AP Red Detection kits (Leica Biosystems, Mount Waverly, Australia). Sections were examined using a Carl Zeiss Axiovert 200M microscope and showed cell surface expression of human CD33 on tumor cells in the tumor masses. Representative samples of tumor masses were placed in culture and analyzed at 6 days and 2 weeks after culture for the expression of CD33.
For efficacy experiments, mice were injected intravenously with tumor cells. The mice were then pooled, randomly distributed into treatment groups, and dosed intraperitoneally one day or 3 days later with a nonbinding control antibody or lintuzumab (3 to 30 mg/kg, dosed every 4 days x 4 doses). In some experiments, gemtuzumab ozogamicin (Mylotarg®, 3 mg/kg) was given once a week for 2 weeks. This dose of gemtuzumab ozogamicin is near the maximum tolerated dose (MTD) in SCID mice (between 5 and 9 mg/kg total dose, data not shown). Plasma was collected from mice 3 weeks after the final dose of lintuzumab for measurement of cytokines. Data were plotted and analyzed using the Kaplan Meier Survival Curves and logarithmic rank test in Prism (GraphPad Software).
Abbreviations
| ADC | = | antibody drug conjugate |
| ADCC | = | antibody-dependent cellular cytotoxicity |
| ADCP | = | antibody-dependent cellular phagocytosis |
| AML | = | acute myeloid leukemia |
| CDC | = | complement dependent cytotoxicity |
| CRP | = | complement regulatory proteins |
| FcγR | = | Fcγ receptor |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| mAb | = | monoclonal antibody |
| MDR | = | multi-drug resistance |
| MTD | = | maximum tolerated dose |
| PBMC | = | peripheral blood mononuclear cells |
| NK | = | natural killer cells |
Figures and Tables
Figure 1 MDR status of AML cell lines as determined by rhodamine efflux. The AML cell lines, HL60, TF1-α and HEL9217, were incubated with rhodamine and efflux was evaluated by flow cytometry, as described in the Materials & Methods. HL60 cells are MDR- while TF1-α and HEL9217 cells are MDR+.
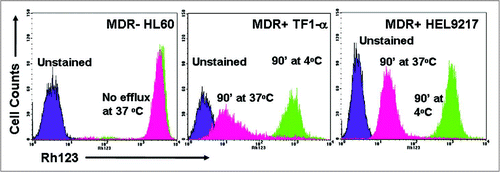
Figure 2 Lintuzumab (SGN-33) mediates signaling upon binding to CD33 and reduces cytokine and chemokine production. (A) HL60 cells and primary AML cells (#1 from a newly diagnosed patient and #2 from a patient with relapsed disease) were treated for 15 minutes (left) or various times (right) with 5 µg/ml lintuzumab or hIgG1κ. CD33 immunoprecipitates were prepared from cell lysates and analyzed by western blot analysis for tyrosine phosphorylation of CD33 and Shp-1 recruitment as indicated. Right panel represents a time course of Shp-1 recruitment to CD33 in lintuzumab-treated HL-60 cells. (B) KG-1 cells were treated with 2,500 ng/ml lintuzumab or 10,000 ng/ml hIgG1κ in the presence or absence 1 ng/ml TNF-α. Media were collected and assayed for cytokine or chemokine levels. Lintuzumab significantly reduced the production of IL-8, MCP-1 and IP-10. (C) Lintuzumab significantly reduced the production of IL-8 in a dose-dependent manner. Reduction of chemokine production was dependent upon binding to CD33 since the lintuzumab F(ab)2 fragment (1,000 ng/ml) was also effective. Data shown represent mean ± SD of duplicates from three separate experiments. **p < 0.01, and ***p < 0.0001 compared to hIgG1κ-treated cells by one-way ANOVA.
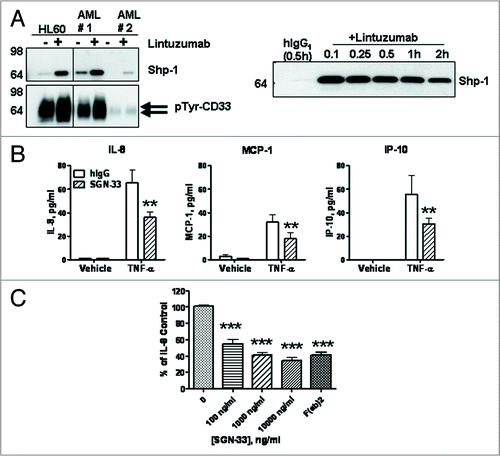
Figure 3 Lintuzumab (SGN-33) mediates effector functions. (A) ADCC activity with lintuzumab was evaluated in three AML cell lines and with cells from an AML patient with refractory disease using the standard 51Cr-release assay and NK cells isolated from FcγRIIIA 158V/V donors. (B) ADCP activity with lintuzumab was evaluated in 3 AML cell lines with primary human macrophages prepared from PBMCs. Phagocytosis (specific uptake of antibody-coated tumor cells by macrophages) was visualized by flow cytometry and by immunofluorescence (white arrows show uptake). (C) CDC activity with lintuzumab in U937 cells was measured with rabbit complement or human serum in the presence or absence of CRP blocking antibodies. Lintuzumab mediated CDC activity with rabbit complement or with human serum + CRP blocking antibodies. Data shown represent mean ± SD of triplicate values from three separate experiments.
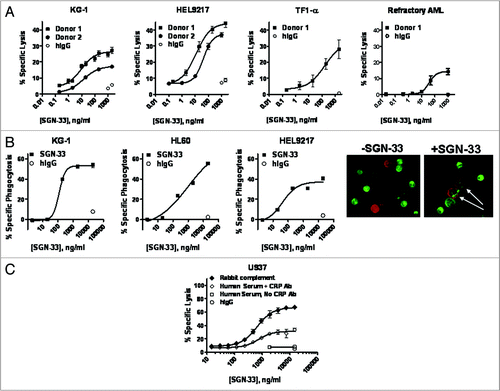
Figure 4 AML model development in SCID mice. Mice (n = 8 to 10 mice/group) were injected with HL60, TF1-α or HEL9217 AML cell lines and euthanized upon appearance of physical signs of disease development. Data shown are for HL60 model but similar results were found with the other models. (A) Expression of human CD33 in cells isolated from a solid tumor mass was maintained in culture when analyzed by flow cytometry after 6 days. The levels of CD33 in cells from the tumor masses were similar to those in the original tumor cell line. (B) Tissues were harvested from mice and the presence of human tumor cells (MHC-I+ CD33+) assessed by flow cytometry. Human CD33+ tumor cells were found in the blood, bone marrow, lymph nodes, and in solid tumor masses. (C) Human CD33 expression was detected by immunohistochemistry in a solid tumor mass isolated from bone.
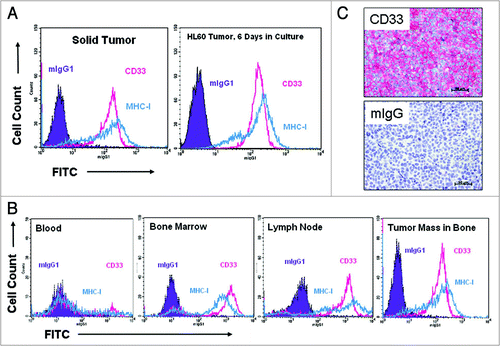
Figure 5 Efficacy of lintuzumab (SGN-33) in MDR− and MDR+ disseminated models of AML. Data shown are representative of findings in other studies. (A) In the MDR− HL60 model, mice (n = 8 to 10/group) were injected with 7.5 million cells and dosed one day or three days later with lintuzumab or hIgG1 (q4d × 4, arrows) or Mylotarg® (3 mg/kg, q7d × 2). Lintuzumab significantly enhanced survival of mice (p < 0.0001 to hIgG1-treated or untreated mice by log-rank test). (B) Plasma was collected from HL60 tumor-bearing mice three weeks after the last dose of lintuzumab and analyzed for cytokine levels. Data shown represent the individual and mean values obtained from 2 to 5 mice per treatment group. Lintuzumab appeared to significantly decreased the levels of IL-6 and IL-8 compared to untreated or hIgG1-treated mice. (C) In the MDR+ TF1-α model, mice were injected with 5 million cells and dosed 1 day or 3 days later with hIgG1, lintuzumab or Mylotarg®. Lintuzumab significantly enhanced survival of mice (p = 0.0002 to untreated or hIgG1-treated mice and p = 0.015 to Mylotarg®). (D) In the MDR+ HEL9217 model, mice were injected with five million cells and dosed 1 day or 3 days later with lintuzumab, hIgG1 or Mylotarg®. Lintuzumab significantly enhanced survival of mice compared to other treatments (p = 0.002 to hIgG1-treated group and p = 0.01 to Mylotarg®-treated or untreated mice).
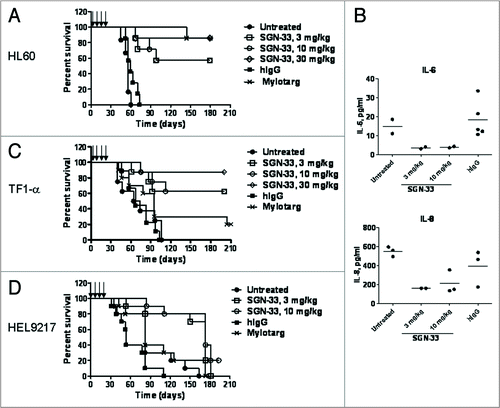
Table 1 Characterization of AML cell lines
Acknowledgements
We are grateful to Julie McEarchern, Kerry Klussman, Kristine Gordon and Weiping Zeng for their excellent technical assistance and helpful discussions and to Muriel Siadek for her help with reviewing the manuscript.
References
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood 2006; 107:3481 - 3485
- Frohling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Dohner K, Dohner H. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood 2006; 108:3280 - 3288
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med 1999; 341:1051 - 1062
- Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood 1997; 89:3323 - 3329
- Drach J, Zhao S, Drach D, Korbling M, Engel H, Andreeff M. Expression of MDR1 by normal bone marrow cells and its implication for leukemic hematopoiesis. Leuk Lymphoma 1995; 16:419 - 424
- Gekeler V, Beck J, Noller A, Wilisch A, Frese G, Neumann M, et al. Drug-induced changes in the expression of MDR-associated genes: investigations on cultured cell lines and chemotherapeutically treated leukemias. Ann Hematol 1994; 69:19 - 24
- Malayeri R, Filipits M, Suchomel RW, Zochbauer S, Lechner K, Pirker R. Multidrug resistance in leukemias and its reversal. Leuk Lymphoma 1996; 23:451 - 458
- Marie JP, Zittoun R, Sikic BI. Multidrug resistance (mdr1) gene expression in adult acute leukemias: correlations with treatment outcome and in vitro drug sensitivity. Blood 1991; 78:586 - 592
- Dinndorf PA, Andrews RG, Benjamin D, Ridgway D, Wolff L, Bernstein ID. Expression of normal myeloid-associated antigens by acute leukemia cells. Blood 1986; 67:1048 - 1053
- Jilani I, Estey E, Huh Y, Joe Y, Manshouri T, Yared M, et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol 2002; 118:560 - 566
- Tanimoto M, Scheinberg DA, Cordon-Cardo C, Huie D, Clarkson BD, Old LJ. Restricted expression of an early myeloid and monocytic cell surface antigen defined by monoclonal antibody M195. Leukemia 1989; 3:339 - 348
- Fenton C, Perry CM. Gemtuzumab ozogamicin: a review of its use in acute myeloid leukaemia. Drugs 2005; 65:2405 - 2427
- Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 2007; 21:66 - 71
- Matsui H, Takeshita A, Naito K, Shinjo K, Shigeno K, Maekawa M, et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia 2002; 16:813 - 819
- Naito K, Takeshita A, Shigeno K, Nakamura S, Fujisawa S, Shinjo K, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia 2000; 14:1436 - 1443
- Pagano L, Fianchi L, Caira M, Rutella S, Leone G. The role of Gemtuzumab Ozogamicin in the treatment of acute myeloid leukemia patients. Oncogene 2007; 26:3679 - 3690
- Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 2007; 109:4168 - 4170
- Caron PC, Co MS, Bull MK, Avdalovic NM, Queen C, Scheinberg DA. Biological and immunological features of humanized M195 (anti-CD33) monoclonal antibodies. Cancer Res 1992; 52:6761 - 6767
- Caron PC, Dumont L, Scheinberg DA. Supersaturating infusional humanized anti-CD33 monoclonal antibody HuM195 in myelogenous leukemia. Clin Cancer Res 1998; 4:1421 - 1428
- Caron PC, Lai LT, Scheinberg DA. Interleukin-2 enhancement of cytotoxicity by humanized monoclonal antibody M195 (anti-CD33) in myelogenous leukemia. Clin Cancer Res 1995; 1:63 - 70
- Feldman E, Kalaycio M, Weiner G, Frankel S, Schulman P, Schwartzberg L, et al. Treatment of relapsed or refractory acute myeloid leukemia with humanized anti-CD33 monoclonal antibody HuM195. Leukemia 2003; 17:314 - 318
- Raza A, Jurcic JG, Roboz GJ, Maris M, Stephenson J, Wood BL, et al. Complete Remissions Observed in Acute Myeloid Leukemia Following Prolonged Exposure to SGN-33 (lintuzumab), a Humanized Monoclonal Antibody Targeting CD33. Blood 2007; 110:159
- Raza A, Jurcic JG, Roboz GJ, Maris M, Stephenson JJL, Wood B, et al. Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 experience. Leukemia Lymphoma 2009; In Press
- Xu Y, Scheinberg DA. Elimination of human leukemia by monoclonal antibodies in an athymic nude mouse leukemia model. Clin Cancer Res 1995; 1:1179 - 1187
- Guc D, Canpinar H, Kucukaksu C, Kansu E. Expression of complement regulatory proteins CR1, DAF, MCP and CD59 in haematological malignancies. Eur J Haematol 2000; 64:3 - 9
- Balaian L, Zhong RK, Ball ED. The inhibitory effect of anti-CD33 monoclonal antibodies on AML cell growth correlates with Syk and/or ZAP-70 expression. Exp Hematol 2003; 31:363 - 371
- Paul SP, Taylor LS, Stansbury EK, McVicar DW. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 2000; 96:483 - 490
- Taylor VC, Buckley CD, Douglas M, Cody AJ, Simmons DL, Freeman SD. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J Biol Chem 1999; 274:11505 - 11512
- Ulyanova T, Blasioli J, Woodford-Thomas TA, Thomas ML. The sialoadhesin CD33 is a myeloid-specific inhibitory receptor. Eur J Immunol 1999; 29:3440 - 3449
- Bruserud O, Ryningen A, Olsnes AM, Stordrange L, Oyan AM, Kalland KH, Gjertsen BT. Subclassification of patients with acute myelogenous leukemia based on chemokine responsiveness and constitutive chemokine release by their leukemic cells. Haematologica 2007; 92:332 - 341
- Hsu HC, Lee YM, Tsai WH, Jiang ML, Ho CH, Ho CK, Wang SY. Circulating levels of thrombopoietic and inflammatory cytokines in patients with acute myeloblastic leukemia and myelodysplastic syndrome. Oncology 2002; 63:64 - 69
- Lowenberg B, Touw IP. Hematopoietic growth factors and their receptors in acute leukemia. Blood 1993; 81:281 - 292
- Yan L, Anderson GM, DeWitte M, Nakada MT. Therapeutic potential of cytokine and chemokine antagonists in cancer therapy. Eur J Cancer 2006; 42:793 - 802
- McEarchern JA, Oflazoglu E, Francisco L, McDonagh CF, Gordon KA, Stone I, et al. Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood 2007; 109:1185 - 1192
- Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 2004; 199:1659 - 1669
- Robertson MJ, Soiffer RJ, Freedman AS, Rabinowe SL, Anderson KC, Ervin TJ, et al. Human bone marrow depleted of CD33-positive cells mediates delayed but durable reconstitution of hematopoiesis: clinical trial of MY9 monoclonal antibody-purged autografts for the treatment of acute myeloid leukemia. Blood 1992; 79:2229 - 2236
- Stiff PJ, Schulz WC, Bishop M, Marks L. Anti-CD33 monoclonal antibody and etoposide/cytosine arabinoside combinations for the ex vivo purification of bone marrow in acute nonlymphocytic leukemia. Blood 1991; 77:355 - 362
- Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol 2003; 40:109 - 123
- Hara T, Kojima A, Fukuda H, Masaoka T, Fukumori Y, Matsumoto M, Seya T. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br J Haematol 1992; 82:368 - 373
- Lajaunias F, Dayer JM, Chizzolini C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur J Immunol 2005; 35:243 - 251
- Legdeur MC, Broekhoven MG, Schuurhuis GJ, Beelen RH, Ossenkoppele GJ. Monocyte-chemoattractant-protein-1-mediated migration of human monocytes towards blasts from patients with acute myeloid leukemia. Cancer Immunol Immunother 2001; 50:16 - 22
- Mazur G, Wrobel T, Butrym A, Kapelko-Slowik K, Poreba R, Kuliczkowski K. Increased monocyte chemoattractant protein 1 (MCP-1/CCL-2) serum level in acute myeloid leukemia. Neoplasma 2007; 54:285 - 289
- Stifter G, Heiss S, Gastl G, Tzankov A, Stauder R. Overexpression of tumor necrosis factor-alpha in bone marrow biopsies from patients with myelodysplastic syndromes: relationship to anemia and prognosis. Eur J Haematol 2005; 75:485 - 491
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005; 104:788 - 793
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004; 4:540 - 550
- Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res 2003; 5:31 - 36
- Uesato N, Fukui K, Maruhashi J, Tojo A, Tajima N. JTE-607, a multiple cytokine production inhibitor, ameliorates disease in a SCID mouse xenograft acute myeloid leukemia model. Exp Hematol 2006; 34:1385 - 1392
- Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, et al. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-hodgkin's lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res 2004; 10:2253 - 2264
- Kossman SE, Scheinberg DA, Jurcic JG, Jimenez J, Caron PC. A phase I trial of humanized monoclonal antibody HuM195 (anti-CD33) with low-dose interleukin 2 in acute myelogenous leukemia. Clin Cancer Res 1999; 5:2748 - 2755
- Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 2005; 174:817 - 826
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754 - 758
- Lin TS, Flinn IW, Modali R, Lehman TA, Webb J, Waymer S, et al. FCGR3A and FCGR2A polymorphisms may not correlate with response to alemtuzumab in chronic lymphocytic leukemia. Blood 2005; 105:289 - 291
- Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem 2002; 13:47 - 58
- McCormack E, Bruserud O, Gjertsen BT. Review: genetic models of acute myeloid leukaemia. Oncogene 2008; 27:3765 - 3779
- Brinkman-Van der Linden EC, Angata T, Reynolds SA, Powell LD, Hedrick SM, Varki A. CD33/Siglec-3 binding specificity, expression pattern and consequences of gene deletion in mice. Mol Cell Biol 2003; 23:4199 - 4206
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 2007; 25:1315 - 1321
- Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood 2005; 106:4086 - 4092
- Pearce DJ, Taussig D, Zibara K, Smith LL, Ridler CM, Preudhomme C, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood 2006; 107:1166 - 1173
- Co MS, Avdalovic NM, Caron PC, Avdalovic MV, Scheinberg DA, Queen C. Chimeric and humanized antibodies with specificity for the CD33 antigen. J Immunol 1992; 148:1149 - 1154
- Nelson EJ, Zinkin NT, Hinkle PM. Fluorescence methods to assess multidrug resistance in individual cells. Cancer Chemother Pharmacol 1998; 42:292 - 299