Abstract
We prepared and characterized golimumab (CNTO148), a human IgG1 tumor necrosis factor alpha (TNFα) antagonist monoclonal antibody chosen for clinical development based on its molecular properties. Golimumab was compared with infliximab, adalimumab and etanercept for affinity and in vitro TNFα neutralization. The affinity of golimumab for soluble human TNFα, as determined by surface plasmon resonance, was similar to that of etanercept (18 pM versus 11 pM), greater than that of infliximab (44 pM) and significantly greater than that of adalimumab (127 pM, p=0.018). The concentration of golimumab necessary to neutralize TNFα-induced E-selectin expression on human endothelial cells by 50% was significantly less than those for infliximab (3.2 fold; p=0.017) and adalimumab (3.3-fold; p=0.008) and comparable to that for etanercept. The conformational stability of golimumab was greater than that of infliximab (primary melting temperature [Tm] 74.8 °C vs. 69.5 °C) as assessed by differential scanning calorimetry. In addition, golimumab showed minimal aggregation over the intended shelf life when formulated as a high concentration liquid product (100 mg/mL) for subcutaneous administration. In vivo, golimumab at doses of 1 and 10 mg/kg significantly delayed disease progression in a mouse model of human TNFα-induced arthritis when compared with untreated mice, while infliximab was effective only at 10 mg/kg. Golimumab also significantly reduced histological scores for arthritis severity and cartilage damage, as well as serum levels of pro-inflammatory cytokines and chemokines associated with arthritis. Thus, we have demonstrated that golimumab is a highly stable human monoclonal antibody with high affinity and capacity to neutralize human TNFα in vitro and in vivo.
Introduction
The inflammatory cytokine tumor necrosis factor α (TNFα) is known to play a central role in several chronic immune-mediated inflammatory disorders.Citation1,Citation2 TNFα induces the production of other pro-inflammatory cytokines such as interleukin (IL)-1 and IL-6, increases endothelial layer permeability and expression of adhesion molecules, activates neutrophils and eosinophils and induces acute phase reactants and tissue-degrading enzymes produced by synoviocytes and chondrocytes.Citation1,Citation3 Biologic agents that target TNFα, such as infliximab, etanercept and adalimumab, have been approved for marketing in many countries. These agents are effective in the treatment of patients with immune-mediated inflammatory disorders including rheumatoid arthritis,Citation4 inflammatory bowel diseaseCitation5 and psoriasis,Citation6 thus expanding the treatment armamentarium for physicians and patients.
The marketed biologic agents have limitations with regard to affinity, stability, solubility and immunogenicity, all of which affect their route and frequency of administration.Citation7,Citation8 Etanercept is a fusion protein, comprising the Fc portion of IgG1 and the extracellular domain of the TNFR2 receptor, that is administered by subcutaneous (s.c.) injection once or twice per week.Citation9,Citation10 Adalimumab is a human IgG1 monoclonal antibody (mAb), derived by phage display, that is specific for human TNFα and is administered by s.c. injection once every 2 wks.Citation11,Citation12 Infliximab, a chimeric IgG1 mAb comprising human constant and murine variable regions, binds specifically to human TNFα and is administered by intravenous (i.v.) infusion every 4 to 8 wks.Citation13,Citation14
Here we report on the preclinical studies conducted to characterize golimumab (also referred to as CNTO148), a human anti-TNFα mAb that was approved in 2009 in both the US and Europe. Golimumab was derived from TNF-immunized transgenic mice engineered to express human IgGs. We show that golimumab is a highly stable human mAb with high affinity and capacity to neutralize human TNFα, attributes that have enabled less frequent dosing than the other currently available anti-TNFα agents.
Results
Hybridoma generation and protein characterization.
Mice modified to express human IgG transgenes that were immunized with recombinant human TNFα yielded 12 hybridoma cell lines secreting human mAbs that bound human TNFα with high affinity. Four of the antibodies were neutralizing, as shown by their ability to block the binding of human TNFα to recombinant human TNFR1 and inhibit human TNFα-mediated cell cytotoxicity (). GenTNV 148.26.12 (golimumab), the most potent of these antibodies, was selected for further characterization. The closest matching human germline sequences to golimumab were IGHV3-30.3 (95% identical) and IGKV3–11 (99% identical), both of which were present in the transgenic mice.Citation16
Differential scanning calorimetry (DSC) was used to evaluate the conformational and thermal stability of golimumab. As shown in , golimumab had the highest primary transition temperature (74.8°C), while values for infliximab and polyclonal human IgG1 (69.5°C and 70.6°C, respectively) were lower, suggesting that golimumab has greater stability compared with other IgGs of the same subclass. Further analysis using Fab fragments of golimumab and infliximab showed a similar difference in the transition temperatures (74.5°C vs. 69.8°C) within the variable and CH1 domains of these mAbs ().
A liquid formulation containing 100 mg/mL of golimumab was developed and further analyzed by size exclusion chromatography (SEC) for formation of dimers during long-term stability studies at various temperatures (). Under these conditions, golimumab remained more than 98% monomeric at 25°C and 5°C through 12 and 18 mo, respectively.
Affinity and in vitro potency.
Both transmembrane (tm) and soluble TNFα homotrimers are bioactive; therefore, binding and neutralization of both forms of TNFα were evaluated for golimumab in comparison with other TNFα antagonists.
Affinity. The dissociation equilibrium constant (KD) measured by surface plasmon resonance (SPR) for the binding of soluble TNFα to immobilized golimumab was 18 pM, compared with 11 pM for etanercept, 44 pM for infliximab and 127 pM for adalimumab (). The 2.4-fold difference between golimumab and infliximab, which was primarily related to a slower dissociation rate constant (kd) observed for golimumab, was not statistically significant. The affinity of adalimumab for soluble TNF was less than infliximab and significantly less than golimumab and etanercept, with the difference in KD ranging from 2.9- to 11.5-fold.
Assessment of golimumab, infliximab, etanercept and adalimumab binding to tmTNFα by radioimmunoassay () revealed that each protein bound with substantially less affinity to tmTNFα compared with soluble TNFα. The affinities of golimumab and infliximab for tmTNFα were similar and somewhat greater than that of adalimumab. The affinity of all three anti-TNFα mAbs for tmTNFα was significantly greater than the affinity derived for etanercept.
In vitro bioassays. The cytotoxic effect of TNFα on the human rhabdomyosarcoma cell line KYM-1D4 was used to compare the neutralization of soluble TNFα and tmTNFα by golimumab with that of infliximab, adalimumab and etanercept. For soluble TNFα (), the overall ranking was similar to that observed for the affinity for soluble TNFα, with etanercept having a half maximal inhibitory concentration (IC50) value of 0.8 ng/mL, which was significantly lower than that for golimumab (6.5 ng/mL, p < 0.001). The IC50 values for both golimumab and etanercept were significantly lower than those for infliximab (24.2 ng/mL, p < 0.001) and adalimumab (36.4 ng/mL, p < 0.001). With regard to the neutralization of tmTNFα (), the IC50 values were similar for all four TNFα antagonists, ranging from 162 ng/mL for golimumab to 303 ng/mL for infliximab.
Adhesion proteins have been shown to be upregulated on the joint vasculature of patients with rheumatoid arthritis;Citation17 therefore, we also compared the potency of TNF antagonists using primary human umbilical vein endothelial cells (HUVEC) stimulated with soluble TNFα (). The ranking of IC50 values was similar to the cytotoxicity assay, with the mean IC50 value observed with etanercept (2.5 ng/mL) being comparable to that for golimumab (3.9 ng/mL), both of which were significantly lower than mean IC50 values for adalimumab (12.7 ng/mL; p = 0.008 vs. golimumab and p < 0.001 vs. etanercept) and infliximab (12.3 ng/mL; p = 0.017 vs. golimumab; p < 0.001 vs. etanercept).
Fc-mediated effects.
As expected for human IgG1 mAbs, we could detect golimumab and infliximab binding to human neonatal Fc receptor (FcRn) and Fcγ receptors (data not shown). The functional consequences of golimumab binding to FcγR, in addition to tmTNFα (), were evaluated in an antibody-dependent cellular cytotoxicity (ADCC) assay using lipopolysaccharide (LPS)-stimulated human monocytes. While LPS-stimulated human peripheral blood mononuclear cells (PBMCs) secreted large amounts of soluble TNFα, very little cell-surface TNFα could be detected on CD14+ monocytes using biotinylated golimumab (). In contrast, golimumab binding to K2 cells expressing the Δ1–12 variant of human TNFα that remains cell-associated was clearly evident (). No increase in the lysis of LPS- stimulated human monocytes was detected in the presence of golimumab or infliximab when compared with a negative control mAb (), while efficient cell lysis was demonstrated for both golimumab and infliximab using the K2 cell line (). Similar results were obtained with golimumab using LPS-stimulated PBMCs and flow cytometry to examine human complement-mediated cell lysis and apoptosis (data not shown). These results suggest that while golimumab has the inherent capacity to induce antibody-dependent and complement-mediated cell lysis, the density of tmTNFα on the cell surface of LPS-stimulated human monocytes may be insufficient for the binding of golimumab to mediate cell lysis.
In vivo bioactivity: Tg197 transgenic mouse model.
Due to their limited species cross-reactivity, golimumab and infliximab were assessed and compared in vivo in the Tg197 transgenic mouse. Constitutive expression of human TNFα in this mouse leads to a chronic progressive polyarthritis with histopathologic features that resemble rheumatoid arthritis.Citation18 A single, 10 mg/kg intraperitoneal injection of golimumab or infliximab significantly reduced the arthritic index at weeks 3–7 compared with transgenic mice that received vehicle (, p < 0.05). Golimumab was also effective at a dose of 1 mg/kg (p < 0.05), while the effect of infliximab at this dose was not significantly different from the control group (). The effect of 1 mg/kg golimumab on the arthritic index was also significantly greater than that of 1 mg/kg infliximab at weeks 3, 4 and 6 (p < 0.05).
The effect of golimumab on joint pathology and serum biomarkers was evaluated in a second study in which a single s.c. dose ranging from 1–30 mg/kg was administered. Compared with the vehicle-treated transgenic mice ( and D), stained tissue sections from the golimumab 30 mg/kg treatment group showed evidence of reduced synovitis, bone erosions () and articular cartilage loss (). However, compared with disease-free non-transgenic mice ( and F) the golimumab-treated mice still showed signs of chondrocyte hyperplasia and cartilage thickening, which are characteristic of joint changes seen in Tg197 mice. The reductions in overall disease severity () and cartilage degradation () scores following treatment with golimumab were dose-dependent and statistically significant for the 30 mg/kg treatment group compared with the vehicle control group (p < 0.01 and p = 0.025, respectively). The delay in disease progression observed with s.c. administration of golimumab (data not shown) was similar to that seen with intraperitoneal administration of golimumab.
Serum samples collected 23 days post-treatment from these mice were analyzed in a multiplex assay for mouse cytokines and chemokines. Based on the panel tested, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, granulocyte colony-stimulating factor (G-CSF), keratinocyte chemoattractant (KC) and interferon-inducible protein-10 (IP-10) showed significant reductions in the combined golimumab-treated mice (3, 10 and 30 mg/kg) versus untreated controls (). The remaining 17 cytokines and chemokines in the panel were either undetectable or showed no significant differences between the disease-free, non-transgenic animals and the phosphate-buffered saline (PBS)-treated transgenic animals.
Discussion
The data presented here describe the molecular properties of golimumab, a human IgG1 anti-TNFα monoclonal antibody that received US Food and Drug Administration and European Medicines Agency approval in 2009 for treatment of moderate-to-severe rheumatoid arthritis, active psoriatic arthritis and active ankylosing spondylitis. Golimumab was initially selected for development due to a combination of molecular attributes that enabled less frequent (i.e., every 4 wks) s.c. dosing.Citation19 These attributes include sequences that match human germline sequences, high affinity and neutralization capacity for human TNFα, conformational stability and high solubility.
The generation of human monoclonal antibodies has focused on three methodologies: humanization of murine mAbs (e.g., certolizumab), phage display libraries of human antibodies (e.g., adalimumab), and immunization of mice transgenic for human immunoglobulin genes.Citation20 We have successfully generated golimumab, a high affinity mAb, from human Ig transgenic mice without the need for further in vitro affinity maturation. In contrast, mAbs developed by humanization or from phage display libraries often require additional changes in the complementarity determining region sequences to improve affinity for the antigen.Citation21
As part of the characterization of golimumab we included side-by-side comparisons of affinity and potency with other TNFα antagonists that have well-defined preclinical and clinical properties, including infliximab,Citation3,Citation22 adalimumabCitation8 and etanercept.Citation23 The affinity of golimumab for soluble TNFα was similar to that of etanercept () and greater than those of infliximab and adalimumab (2.4-fold and 7.1-fold, respectively). A similar pattern was observed regarding golimumab neutralization of soluble TNFα in the cytotoxicity () and endothelial cell activation () assays. The IC50 values for golimumab were comparable to those for etanercept and ranged from 2.5- to 5.7-fold lower than those for infliximab and adalimumab. These in vitro bioassays suggest that a lower serum concentration of golimumab, compared with infliximab or adalimumab, would provide similar pharmacological effects in patients.
Confirming previous observations,Citation8 the affinity of each TNFα antagonist for tmTNFα was approximately 20- to 1,400-fold lower than their affinities for soluble TNFα, and the neutralization of tmTNFα-mediated cytotoxicity was similar for all four TNFα antagonists (). Reduced affinity for tmTNFα may reflect a steric or charge interference with mAb binding due to the close proximity of the cell membrane. Mutant mice that produce only tmTNFα have been shown to be as resistant as normal mice to intracellular bacterial infections and autoimmune demyelination, suggesting that selective targeting of soluble TNFα may be superior to complete blockade of TNFα.Citation24
The binding of golimumab to Fc receptors mirrored that seen for other human IgG1 antibodies,Citation25,Citation26 which suggests the antibody has a long terminal half-life due to FcRn binding at low pH and the potential to mediate cell lysis via complement-dependent cytotoxicity (CDC), ADCC, or apoptotic mechanisms. We confirmed that golimumab and infliximab could mediate lysis of mouse myeloma cells that overexpress cell-surface Δ1–12 human TNFα, which was previously reported for infliximab.Citation27 However, no contribution of golimumab or infliximab to cell lysis was detected using LPS-stimulated human monocytes that were actively secreting TNFα. A similar result was recently reported for adalimumab.Citation8 While infliximab-mediated apoptosis has been proposed as an explanation for reduced numbers of synovial macrophages in rheumatoid arthritis patients,Citation28 others have reported no increase in apoptosis after comparing synovial biopsies collected before and 48 h after treatment with infliximab.Citation29 One possible explanation for our results is that circulating monocytes or macrophages from healthy subjects may be less sensitive to anti-TNFα-induced cell lysis,Citation28,Citation30 which may be due in part to the low levels of tmTNFα expressed on these cells.
In the mouse model of arthritis, both golimumab and infliximab were effective at an intraperitoneal dose of 10 mg/kg, while only golimumab was effective at a dose of 1 mg/kg (). Hence, the greater potency of golimumab observed from in vitro bioassays was also evident in vivo. We have also observed that s.c. injection of golimumab suppressed histological evidence of disease progression relative to vehicle-treated mice (), as well as reduced the serum levels of pro-inflammatory cytokines and chemokines (). These results were similar to those previously reported for infliximab.Citation33
Our studies also demonstrate that the golimumab IgG and papain-derived Fab fragment maintain conformational stability at higher temperatures, as measured by DSC, relative to infliximab and polyclonal human IgG1 antibody ( and B). This inherent stability of golimumab in solution allowed development of a liquid formulation containing 100 mg/mL of golimumab that remained monomeric at 5°C for 18 mo (). A comparison with infliximab stability was not included in the SEC analysis of protein stability because infliximab is prepared as a lyophilized powder for reconstitution to 25 mg/mL.Citation14 Thus, a direct comparison to the high concentration liquid formulation for golimumab would not be meaningful. The excellent stability profile of golimumab as a 100-mg/mL liquid formulation allowed for the development of a prefilled syringe for s.c. administration, which is now the approved dosage form.
The early clinical experience with golimumab in patients with rheumatoid arthritis showed pharmacokinetic characteristics typical of an endogenous IgG following both i.v. and s.c. administration, with a half-life of 2–3 weeks.Citation19,Citation34 A positive correlation between serum golimumab trough concentration and improvement in clinical efficacy measurements was observed.Citation35 Based on an exploratory exposure-response analysis, a trough serum concentration of ≤1 µg/mL could significantly improve composite measures of disease activity. In a previous phase 2 study, golimumab in combination with methotrexate was found to be well-tolerated and reduced the signs and symptoms of rheumatoid arthritis.Citation19 All four golimumab dosage regimens tested (50 or 100 mg every 4 weeks or every 2 weeks) had a greater effect than placebo in suppressing levels of C-reactive protein (CRP), although the magnitude of the effect for the lowest dose level tested (i.e., 50 mg every 4 weeks) was smaller than for the other higher doses. Serum CRP concentration is believed to be related to the structural damage in rheumatic diseases.Citation36 Based on the collective considerations of the efficacy, safety, pharmacokinetics and biomarker results, golimumab 50 mg every 4 weeks was selected in the phase 3 studies as the lowest efficacious dose.
In summary, we have demonstrated that golimumab has higher affinity, more capacity to neutralize TNF and greater conformational stability compared with infliximab. This combination of properties in a human mAb suggested that less frequent dosing may be possible. Indeed, results of Phase 3 pivotal studies demonstrated the efficacy and safety of golimumab as a once-monthly subcutaneous injection for the treatment of patients with moderate-to-severe rheumatoid arthritis, active psoriatic arthritis, or active ankylosing spondylitis.
Materials and Methods
Hybridoma generation.
A mouse modified to express human IgG transgenes was employed for hybridoma generation.Citation37 An F2 hybrid mouse (CBA/J x C57BL/6J), containing human variable and constant region antibody transgenes for both heavy and light chains (HuMab-Mouse®; Medarex, Princeton, NJ), was immunized with 100 µg of recombinant human TNFα emulsified in an equal volume of TiterMax® Gold adjuvant (TiterMax, Norcross, GA, USA) on days 0, 12 and 28. The human TNFα used for immunization and characterization studies was expressed and purified as previously described.Citation38 The mouse was rested for 7 weeks, and then a final i.v. injection of 50 µg of recombinant human TNFα in physiological saline was administered and splenocytes were harvested 3 days later. A cell fusion was then performed with mouse myeloma P3X63Ag8.653 cells using conventional methods. Hybridomas secreting fully human IgGs against human TNFα were detected using an enzyme immunoassay and were subcloned twice by limiting dilution. The antibodies secreted into the cell supernatant were purified by protein A affinity chromatography.
TNFα receptor binding.
Human TNFα (25 µg) was iodinated in a final volume of 150 µL PBS in an Iodogen-coated tube (Pierce). The reaction was initiated by adding 6.44 µL (625 µCi) of radioactive sodium iodide (125I; Amersham Biosciences, Buckinghamshire, England), with subsequent incubation for 10 min at room temperature. The iodinated protein was desalted using a PD-10 column (Amersham Biosciences), and the pooled fractions (23 µCi per µg TNFα; 7.14 µg/mL) were stored at 4°C in PBS-0.1% bovine serum albumin.
Microtiter plates (MaxiSorp, Nunc, Rochester, NY, USA) were coated with goat anti-human IgG Fc (Jackson ImmunoResearch, West Grove, PA; 50 µL/well at 10 µg/mL in PBS) at 4°C overnight, washed and then incubated with human TNFR1 or TNFR2 receptor fusion protein (50 µL at 5 µg/mL) for 1 h at 37°C. Serial dilutions of each test antibody and an isotype-matched, negative control human IgG1 mAb (CNTO6234; used as negative control antibody for all assays unless otherwise specified) were pre-incubated with 125I-TNFα (30 ng/mL) for 30 min at 37°C. The antibody-TNFα dilutions were added to triplicate wells and incubated for 2 h at 37°C. Plates were washed three times and the wells were counted in a gamma counter (Perkin-Elmer model 1470, Waltham, MA, USA).
Cytotoxicity assays.
Human KYM-1D4 rhabdomyosarcoma cells or murine WEHI-164 fibrosarcoma cells (both obtained from Dr. Marc Feldmann, Kennedy Institute, London, UK) were seeded in RPMI 1640 (Invitrogen, Carlsbad, CA, USA; supplemented with 2 mM glutamine and 10% fetal bovine serum) containing 2 µg/mL of actinomycin D and incubated at 37°C and 5% carbon dioxide for 4 h. Serial dilutions of each antibody were pre-incubated with human TNFα for 30 min. The cells were then incubated for 16 h at 37°C. 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-tetrazolium bromide (MTT), at a final concentration of 0.5 mg/mL, was added and incubation continued for 3 h. The medium was removed, and the insoluble MTT metabolic product was solubilized by adding 100 µL of dimethyl sulfoxide. Optical densities of 550–650 nm were used as an indicator of cell viability.
Protein stability.
To compare the overall stabilities of the intact IgG and Fab fragments for golimumab, infliximab (Remicade®; Centocor Ortho Biotech, Malvern, PA, USA), and human polyclonal IgG1 (Sigma-Aldrich, St. Louis, MO, USA), DSC was employed. Fab fragments were generated by incubation of intact IgG with immobilized papain for 5–16 h at 37°C. Infliximab and polyclonal human Fabs were purified by affinity chromatography using Protein-A Sepharose (Immunopure Fab Purification kit, Pierce, Rockford, IL, USA). Golimumab Fab was purified by ion exchange using a DEAE-Sepharose column equilibrated with 50 mM Tris pH 9.0, 5 mM sodium chloride and eluted with a 5-to-50 mM sodium chloride gradient. Intact antibody and Fab fragments were dialyzed into PBS, degassed, and injected into the sample chamber of a Nano DSC II (Calorimetry Sciences Corp., now TA Instruments, New Castle, DE, USA), while the corresponding degassed buffer dialysate was placed in the reference compartment. Protein-buffer pairs were heated from 25–105°C at a rate of 1.0°C per minute under a constant pressure of 3.0 atm while the system differential power was monitored. Typically, samples were run at concentrations between 0.1 and 1.0 mg/mL. Buffer-buffer scans run independently were used for data baseline subtraction.
High performance liquid chromatography of golimumab formulated at 100 mg/mL and stored at 5°C, 25°C, 37°C and 45°C was performed with a TSK-GEL G3000SWXL SEC column (Sigma-Aldrich, St. Louis, MO). A 20-µL (8 µg golimumab) sample was injected and eluted at a flow rate of 1 mL/min using 0.2 M sodium phosphate, pH 6.8, as the mobile phase. The eluted protein was detected using a diode array detector (Agilent model 1100/1200; Santa Clara, CA, USA) set at 214 and 280 nm.
Affinity.
Affinity for the soluble human TNFα trimer was determined by SPR using a Biacore 3000 (Biacore AB, Piscataway, NJ, USA). For binding of human TNFα trimer to the antibodies, mouse anti- human IgG Fc-specific antibody was covalently immobilized on a CM5 chip (Biacore AB). Approximately 71–107 response units of golimumab, infliximab, etanercept (Enbrel®; Immunex, Thousand Oaks, CA, USA) or adalimumab (Humira®; Abbott Laboratories, Chicago, IL, UDA) were captured, followed by injection of 0.18–15 nM of TNFα trimer. A low protein density on the chip was chosen to minimize crosslinking. Flow cells injected with buffer alone and flow cells that did not contain TNFα or immobilized anti-IgG antibody were used as references for background subtraction. Global analysis was performed with a 1:1 binding model using the BIAevaluation software version 4.0.1 (Biacore AB). All experiments were performed in Dulbecco's PBS at 25°C.
Binding to tmTNFα was measured using iodinated antibodies and K2 murine myeloma cellsCitation38 engineered to express a Δ1–12 deletion mutant of human TNFα that is resistant to protease cleavageCitation39 and thus remains anchored on the cell surface. Golimumab, infliximab, etanercept and adalimumab were iodinated with sodium iodide (125I; GE Healthcare, Piscataway, NJ, USA) using Iodogen-coated tubes (Pierce, Rockford, IL, USA), and their specific activities were 6.93, 6.93, 7.25 and 8.11 µCi/µg, respectively. Serial dilutions of each iodinated protein were incubated with the K2 cells overnight at 4°C in buffer/medium, and were then washed and counted. Sp2/0 cells that did not express tmTNFα were tested in parallel using the same assay method and used to subtract background binding.
Human endothelial cell assay.
Mouse anti-human E-selectin (Jackson ImmunoResearch) was iodinated by mixing 50 µg of antibody with 6–8 µL (∼725–825 µCi) of 125I sodium iodide (Amersham Biosciences) in an Iodogen-coated tube (Pierce). The final specific activity of 125I-mouse anti-human E-selectin was 9.25 µCi/µg (10.6 µg/mL).
Subsequently, HUVEC (passage 2) were thawed, washed with EGM®-2 medium (Lonza, Basel, Switzerland) and dispensed into 96-well microtiter plates (5,000 cells/well). After 3 days in culture at 37°C, the cells were confluent and ready for assay. Serial dilutions of golimumab, infliximab, etanercept, adalimumab and negative control mAb were prepared in HUVEC medium containing 1 ng/mL human TNFα and pre-incubated for 20 min at 37°C. The medium was removed from the microtiter wells seeded with cells and the antibody dilutions dispensed in duplicate (50 µL per well). The cells were incubated at 37°C for 4 h and then washed with RPMI 1640 medium (Invitrogen). Wells were then incubated with 125I-anti-E-selectin (0.1 µg/mL, 50 µL/well) for 1 h at 37°C, washed three times and counted in a gamma counter (Perkin Elmer model 1470).
Fc-mediated cell lysis.
Human PBMCs were cultured at 37°C overnight prior to analysis of tmTNFα expression. The PBMCs were incubated for 2 h in the absence or presence of 1 µg/mL of LPS (Escherichia coli 0111:B4, Sigma-Aldrich, St. Louis, MO, USA). Polyclonal human IgG (Jackson ImmunoResearch) at a final concentration of 100 µ/mL was added, and the cells were subsequently seeded at 1 × 105 cells per well in a 96-well microtiter plate. The PBMCs were then incubated with biotinylated golimumab or biotinylated negative control antibody at 10 µg/mL on ice for 30 min. The PBMCs were then centrifuged, washed with culture medium and incubated with anti-human CD14-APC as a marker for monocytes and streptavidin-phycoerythrin to detect bound biotinylated proteins. Unstimulated murine K2 cells expressing elevated levels of the non-cleavable, Δ1–12 deletion mutant of human tmTNFα served as a positive control for golimumab binding to tmTNFα. The cells were then analyzed by flow cytometry (FacsCalibur™ with FlowJo 4.6.2 software; BD Biosciences and Tree Star Inc.).
ADCC assays were performed using a DELFIA® EuTDA-based assay (PerkinElmer; Waltham, MA, USA) to quantitate cytotoxicity as indicated by the manufacturer's instructions, with minor modifications. Human monocytes (target cells) were isolated from PBMCs by negative selection using a monocyte isolation kit II (Miltenyi Biotec; Auburn, CA, USA) and resuspended in culture medium. Freshly reconstituted LPS ( E. coli 0111:B4, List Biologicals, Campbell, CA, USA) was added to a final concentration of 1 µg/mL, and the monocytes were incubated for 4 h at 37°C. During the last 30 min of incubation, fluorescence-enhancing agent was added to label the monocytes. Monocytes were washed three times in buffer containing 1 mg/mL LPS and resuspended at 2 × 105 cells/mL in culture medium. As a positive control, murine K2 cells expressing elevated levels of the non-cleavable Δ1–12 deletion mutant of human tmTNFα were also loaded with fluorescence-enhancing agent for 30 min at 37°C, then washed twice with culture medium and resuspended at 2 × 105 cells/mL.
Serial dilutions of golimumab, infliximab and negative control mAb (golimumab deglycosylated enzymatically with PNGase F) were prepared in culture medium; 100 µL of each dilution was added to replicate wells in a round-bottom 96-well microtiter plate. All wells then received 50 µL of PBMCs (effector cells) and 50 µL of LPS-stimulated monocytes or K2 cells as the target cells (final effector:target cell ratio of 50:1). The plate was briefly centrifuged to maximize effector and target cell contact, then incubated for 2 h at 37°C. After incubation, a 20-µL sample was removed from each well and added to a 96-well plate containing 200 µL of DELFIA® europium solution. The plate was shaken for 15 min at room temperature, and fluorescence was measured using a plate reader (EnVision™; PerkinElmer, Waltham, MA, USA).
Tg197 transgenic mouse model.
Four-week-old, female, hemizygous mice obtained from the litters of homozygous Tg197 males bred with F1 (non-transgenic) females (obtained from Dr. George Kollias, Hellenic Pasteur Institute, Athens, Greece) were assigned to one of five treatment groups (n = 10) so that the average body weight was similar across groups. Study groups received a single intraperitoneal bolus of golimumab 1 mg/kg, golimumab 10 mg/kg, infliximab 1 mg/kg, infliximab 10 mg/kg or vehicle (PBS). Mice were weighed and assessed weekly for disease activity using a modified scoring system,Citation40 whereby 0 = normal, 1 = edema or distortion of paw or ankle joints, 2 = distortion of paw and ankle joints and 3 = ankylosis of wrist or ankle joints. The arthritic index was defined as the sum of scores from all four paws. The experimental protocols were approved by Centocor's Institutional Animal Care and Use Committee.
Histology was assessed in a second experiment in which five groups of Tg197 mice (n = 8) received a single s.c. injection of golimumab (1, 3, 10 or 30 mg/kg) or PBS. Four-week-old non-transgenic F1 females served as the control group. All mice were euthanized at day 23, rather than at the planned week 4 time point for ethical reasons related to the severity of disease in the vehicle control group. Both hind limbs were fixed intact by immersion in 10% neutral buffered formalin, decalcified, dehydrated and embedded in paraffin. Mid-sagittal sections (5 µm were cut through the tibio-tarsal and metatarsal joints and stained with hematoxylin and eosin. Sections were evaluated in a blinded fashion and ranked in order of global disease severity (synovitis, cartilage destruction and bone erosion) with the highest score representing the worst disease. The blind was broken, the mice grouped according to treatment and the ranks from each treatment compiled. Evaluation of cartilage degradation was performed on sections stained with Toluidine Blue. Proteoglycan depletion and matrix erosion are associated with cartilage matrix destruction and are accompanied by a loss of Toluidine Blue staining. Cartilage destruction was scored from 0–4 using the system described by Douni and colleagues,Citation41 whereby 0 = intact, 1 = minor (<10%), 2 = moderate (10–50%), 3 = high (50–80%), and 4 = severe (80–100%). Several tibio-talus joints could not be scored for cartilage damage due to insufficient material or irregular staining in the tissue section (four each from PBS and golimumab 1 mg/kg, 10 mg/kg; five from golimumab 3 mg/kg; and one each from golimumab 30 mg/kg and F1 non-transgenic controls).
Representative images were collected using a Nikon Eclipse E800 (Nikon Corp., Tokyo, Japan) microscope equipped with an Evolution™ MP 5.0 RTV color camera (Media Cybernetics, Inc., Silver Spring, MD, USA). Images were captured and analyzed using Image-Pro Plus software version 5.1 (Media Cybernetics, Inc.,).
The cytokine profile was determined using a mouse cytokine/chemokine premixed 22-plex kit (Lincoplex MCYTO-70K-PMX; Linco Research-Millipore; St. Charles, MO, USA). Sera collected from euthanized mice were tested in duplicate, undiluted, as described in the kit instructions and then analyzed on a Bio-Plex™ instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A five- parameter logistic regression algorithm was used to determine the concentration of analyte in each serum sample based on a 6-point standard curve.
Data analysis.
Individual KD (affinity) or IC50 values with standard error (potency) comparisons used log scaling for the metric of statistical analysis since it facilitates comparisons in terms of fold-difference, ratios, or percent difference. Pairwise comparisons are made across the four test TNF-antagonists where possible. p values of ≤0.05 were deemed significant unless noted otherwise. Comparisons of chemokine/cytokines differences were made between the combined 3, 10 and 30 mg/kg golimumab dose groups and the control group using similar methods.
For the animal studies, an analysis of variance (ANOVA) and Tukey post-test were used to compare arthritic index between groups at each time point. Treatment differences in histological scores for global disease severity and cartilage damage were assessed with the Cochran-Mantel-Haenszel ANOVA statistic using ranked scores for each group. Step-down analyses were conducted for each endpoint; pairwise comparisons versus no treatment were carried out for each dose if the overall test for differences was significant.
Previous publication
Data presented in this manuscript were previously published in the following abstracts: (1)Shealy D, et al. Characterization of golimumab (CNTO148), a novel fully human monoclonal antibody specific for human TNFα. Ann Rheum Dis 2007; 66:151 and (2) Shealy D, et al. Characterization of golimumab (CNTO 148), a novel monoclonal antibody specific for human TNFα. Arthritis Rheum 2007; 56:155, Abstract no. 274.
Abbreviations
| ADCC | = | antibody-dependent cellular cytotoxicity |
| ANOVA | = | analysis of variance |
| CDC | = | complement-dependent cytotoxicity |
| CRP | = | C-reactive protein |
| DSC | = | differential scanning calorimetry |
| FcRn | = | neonatal Fc receptor |
| G-CSF | = | granulocyte colony-stimulating factor |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| HUVEC | = | human umbilical vein endothelial cells |
| IC50 | = | half maximal inhibitory concentration |
| IL | = | interleukin |
| IP-10 | = | interferon-inducible protein-10 |
| i.v. | = | intravenous |
| ka | = | association rate constant |
| KC | = | keratinocyte chemoattractant |
| KD | = | dissociation equilibrium constant |
| kd | = | dissociation rate constant |
| LPS | = | lipopolysaccharide |
| LTα | = | lymphotoxin alpha |
| mAb | = | monoclonal antibody |
| MTT | = | 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide |
| PBMC | = | peripheral blood mononuclear cells |
| PBS | = | phosphate-buffered saline; s.c., subcutaneous |
| SA-PE | = | streptavidin-phycoerythrin SEC, size exclusion chromatography |
| SPR | = | surface plasmon resonance |
| tm | = | transmembrane |
| Tm | = | melting temperature |
| TNFα | = | tumor necrosis factor alpha |
| TNFR2 | = | tumor necrosis factor 2 |
Figures and Tables
Figure 1 Stability of golimumab in solution. Intact antibodies (A) and Fab fragments (B) were dialyzed, degassed and analyzed by differential scanning calorimetry with dialysate in the reference cell. Buffer-buffer scans run independently were used for data baseline subtraction. (C) golimumab formulated at 100 mg/mL and stored at the indicated temperatures was analyzed by size exclusion chromatography (SEC) at each time point to determine the % monomeric golimumab.
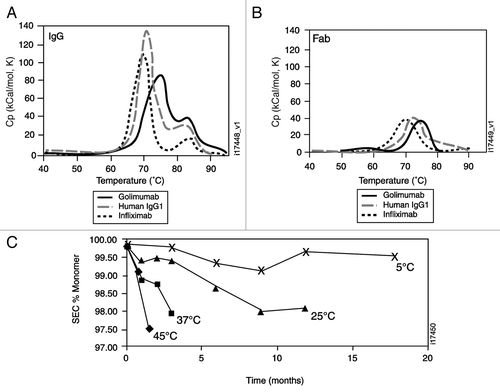
Figure 2 Golimumab neutralization of soluble TNFα and tmTNFα compared with other TNFα antagonists. Neutralization of cell cytotoxicity was compared using serial dilutions of golimumab (solid circles), infliximab (solid triangles), etanercept (open circles), adalimumab (open triangles) or negative control mAb (open squares) pre-incubated with 0.1 ng/mL of soluble TNFα (A) or murine K2 cells expressing human tmTNFα (B), followed by overnight incubation with KYM target cells. Each data point represents the mean of duplicate wells, and the error bars represent the range of the duplicate values. (C) Serial dilutions of the same proteins listed above were pre-incubated with 1 ng/mL of soluble TNFα followed by incubation for 4 hours on human umbilical vein endothelial cells. Iodinated anti-E-selectin antibody was used to detect expression of E-selectin on the cell surface. The data points represent the mean of duplicate wells and the error bars show the range.
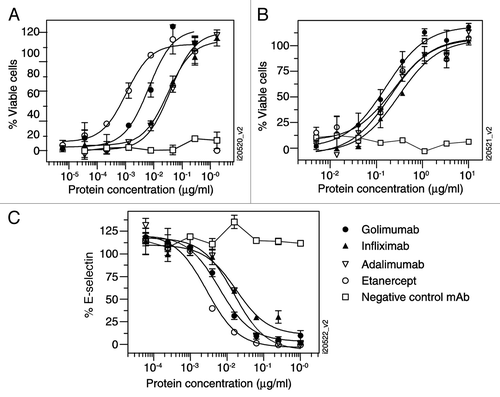
Figure 3 Contribution of golimumab and infliximab to lysis of LPS-stimulated human PBMCs. Human PBMCs treated +/−1 µg/mL of LPS for 2 hours (A) or murine K2 cells expressing tmTNFα (B) were incubated with biotinylated golimumab (shaded area, no LPS; dark line, LPS-stimulated) or negative control mAb (light gray line, LPS-stimulated) and detected with streptavidin-phycoerythrin. PBMCs were gated on CD14+ monocytes and human IgG (100 µg/mL) was added to block FcγRs. Cell lysis using LPS-stimulated human monocytes (C) or murine K2 cells expressing tmTNFα (D) was determined following the addition of the indicated concentration of golimumab (solid circles), infliximab (solid triangles), or negative control antibody (open squares) followed by the addition of human PBMC as the source of immune effector cells. The extent of target cell lysis was quantitated after 2 hours. The data shown are the mean ±SEM of replicate wells (n = 2–6).
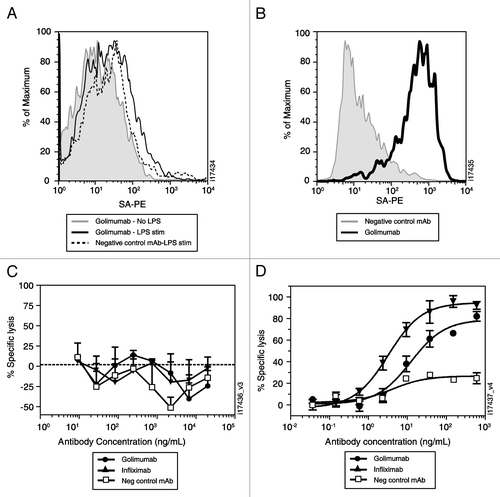
Figure 4 Golimumab and infliximab suppress disease activity in the human TNFα transgenic mouse model of arthritis. The change in arthritic index was monitored weekly following a single intraperitoneal injection at week 0 of 1 mg/kg (A) or 10 mg/kg (B) of golimumab (solid circles) and infliximab (solid triangles). Results for the vehicle control group (open squares) are shown on both graphs. Each data point is the mean (n = 10) ±SEM. The symbols indicate p < 0.05 compared with vehicle (*) or golimumab compared to the same dose of infliximab (#).
Figure 5 Golimumab preserves joint architecture. Representative images of the tibia-talus joint stained with hematoxylin and eosin (A–C) or Toluidine Blue (bottom, D–F) are shown from Tg197 transgenic mice treated with a single s.c. injection of PBS (A and D) or 30 mg/kg golimumab (B and E) alongside an untreated F1 non-transgenic mouse (C and F). All images are 20x magnification. (G) Hematoxylin and eosin-stained tissue sections from transgenic mice treated with PBS or with golimumab doses ranging from 1 to 30 mg/kg were ranked in order of severity (highest score representing the worse disease), then the ranks were grouped by treatment. The golimumab 30-mg/kg group was significantly different from the PBS group (p < 0.01). (H) The tibia-talus joints were scored for the extent of cartilage matrix degradation in mice treated with PBS (n = 4) or with golimumab doses of 1 mg/kg (n = 4), 3 mg/kg (n = 4), 10 mg/kg (n = 4), 30 mg/kg (n = 7). The 30-mg/kg group achieved a statistically significant reduction in cartilage damage compared with the PBS-untreated animals (p = 0.025). Overall severity and cartilage destruction are shown as mean ± SD, with arrows indicating articular cartilage.
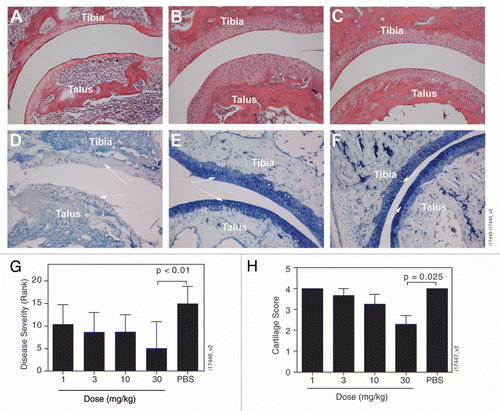
Figure 6 Golimumab reduces serum level of proinflammatory cytokines and chemokines. Samples collected 23 d posttreatment were analyzed for granulocyte-macrophage colony-stimulating factor (GM-CSF; A), interleukin-6 (IL-6; B), granulocyte colony-stimulating factor (G-CSF; C), keratinocyte chemoattractant (KC; D), and interferon-inducible protein-10 (IP-10; E). Treatment groups included TNF transgenic mice treated with PBS or golimumab and non-transgenic wild type mice treated with PBS. The plotted values represent the mean +/− SEM.
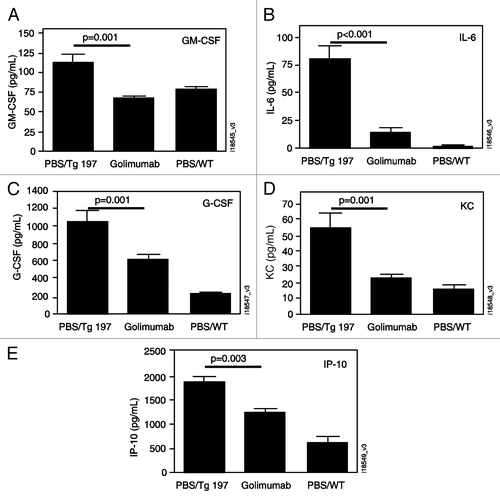
Table 1 Comparison of human monoclonal anti-TNF± antibodies derived from transgenic mice
Table 2 Affinity parameters for golimumab, infliximab, etanercept and adalimumab binding to human TNFα
Acknowledgements
The authors would like to thank Patricia Rafferty, Rose Mary Decker, Devon Egenolf and Tom Nesspor for their technical expertise and Michelle Perate, MS and Dr. Mary Whitman of Centocor Ortho Biotech, Inc. for assistance with the preparation of the manuscript.
We are dedicating this manuscript to the memory of Michael Brigham-Burke as a tribute to his contribution to the molecular recognition field. Michael was dedicated to biomolecular interactions analysis, being one of the pioneers in the area of surface plasmon resonance on the Biacore and a specialist in analytical ultracentrifugation analysis. He made significant scientific contributions that advanced the development of pharmaceutical sciences and several biotherapeutics.
This work was supported by Centocor Research and Development. All authors are employees of Centocor Research and Development and hold an equity stake in Johnson & Johnson (J&J), the parent company of Centocor Research and Development. Johnson & Johnson has a commercial interest in drugs mentioned in this article.
Funding
Centocor Research and Development, Inc., Malvern, PA, USA funded this work.
References
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117:244 - 279
- Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, et al. TNFα blockade in human diseases: mechanisms and future directions. Clin Immunol 2008; 126:121 - 136
- Feldmann M, Brennan FM, Williams RO, Woody JN, Maini RN. The transfer of a laboratory based hypothesis to a clinically useful therapy: the development of anti-TNF therapy of rheumatoid arthritis. Best Pract Res Clin Rheumatol 2004; 18:59 - 80
- Ackermann C, Kavanaugh A. Tumor necrosis factor as a therapeutic target of rheumatologic disease. Expert Opin Ther Targets 2007; 11:1369 - 1384
- Chang JT, Lichtenstein GR. Drug insight: antagonists of tumor-necrosis factor-alpha in the treatment of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol 2006; 3:220 - 228
- Myers WA, Gottlieb AB, Mease P. Psoriasis and psoriatic arthritis: clinical features and disease mechanisms. Clin Dermatol 2006; 24:438 - 447
- Nestorov I. Clinical pharmacokinetics of TNF antagonists: how do they differ?. Semin Arthritis Rheum 2005; 34:12 - 18
- Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian DK, et al. Comparisons of affinities, avidities and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol 2009; 131:308 - 316
- Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, et al. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2004; 50:353 - 363
- Immunex Corporation. Enbrel® (etanercept) package insert 2000; Thousand Oaks, CA
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004; 50:1400 - 1411
- Abbott Laboratories. Humira® (adalimumab) package insert 2009; Abbott Park, IL
- Maini R, St. Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor a monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999; 354:1932 - 1939
- Centocor. Remicade® (infliximab) package insert 2009; Malvern, PA
- Lefranc MP. IMGT-ONTOLOGY and IMGT databases, tools and Web resources for immunogenetics and immunoinformatics. Mol Immunol 2004; 40:647 - 660
- Fishwild DM, O'Donnell SL, Bengoechea T, Hudson DV, Harding F, Bernhard SL, et al. High-avidity human IgG1 monoclonal antibodies from a novel strain of minilocus transgenic mice. Nature Biotechnology 1996; 14:845 - 851
- Paleolog E. Target effector role of vascular endothelium in the inflammatory response: insights from the clinical trial of anti-TNFαlpha antibody in rheumatoid arthritis. Mol Pathol 1997; 50:225 - 233
- Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 1991; 10:4025 - 4031
- Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Arthritis Rheum 2008; 58:964 - 975
- Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother 2006; 29:1 - 9
- Lonberg N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol 2008; 20:450 - 459
- Shealy DJ, Visvanathan S. Anti-TNF antibodies: lessons from the past, roadmap for the future. Handb Exp Pharmacol 2008; 181:101 - 129
- Arora T, Padaki R, Liu L, Hamburger AE, Ellison AR, Stevens SR, et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine 2009; 45:124 - 131
- Alexopoulou L, Kranidioti K, Xanthoulea S, Denis M, Kotanidou A, Douni E, et al. Transmembrane TNF protects mutant mice against intracellular bacterial infections, chronic inflammation and autoimmunity. Eur J Immunol 2006; 36:2768 - 2780
- Dijstelbloem HM, van de Winkel JG, Kallenberg CG. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol 2001; 22:510 - 516
- Roopenian DS, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715 - 725
- Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNFα monoclonal antibody cA2 binds recombinant transmembrane TNFα and activates immune effector functions. Cytokine 1995; 7:251 - 259
- Catrina AI, Trollmo C, Klint EA, Engstrom M, Lampa J, Hermansson Y, et al. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints. Arthritis Rheum 2005; 52:61 - 72
- Smeets TJ, Kraan MC, van Loon ME, Tak PP. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum 2003; 48:2155 - 2162
- Ringheaunu M, Daum F, Markowitz J, Levine J, Katz S, Lin X, et al. Effects of infliximab on apoptosis and reverse signaling of monocytes from healthy individuals and patients with Crohn's disease. Inflamm Bowel Dis 2004; 10:801 - 810
- Wollin M, Abele S, Bruns S, Weyand M, Kalden JR, Ensminger SM, et al. Inhibition of TNFαlpha reduces transplant arteriosclerosis in a murine aortic transplant model. Transplant Int 2009; 22:342 - 349
- Deveci F, Muz MH, Ilhan N, Kirkil G, Turgut T, Akpolat N. Evaluation of the anti-inflammatory effect of infliximab in a mouse model of acute asthma. Respirology 2008; 13:488 - 497
- Shealy DJ, Wooley PH, Emmell E, Volk A, Rosenberg A, Treacy G, et al. Anti-TNFα antibody allows healing of joint damage in polyarthritic transgenic mice. Arthritis Res 2002; 4:7
- Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNFαlpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol 2007; 47:383 - 396
- Zhou H, Xu Z, Rahman MU, Wagner C, Pendley C, Han J, et al. Exposure-efficacy assessment of golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: application of population pharmacokinetic/pharmacodynamic modeling. Arthritis Rheum 2006; 54:5418
- Emery P, Gabay C, Kraan M, Gomez-Reino J. Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatol Int 2007; 27:793 - 806
- Taylor LD, Carmack CE, Huszar D, Higgins KM, Mashayekh R, Sequar G, et al. Human immunoglobulin transgenes undergo rearrangement, somatic mutation and class switching in mice that lack endogenous IgM. Int Immunology 1993; 6:579 - 591
- Scallon BJ, Cai A, Solowski N, Rosenberg A, Song X-Y, Shealy D, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002; 301:418 - 426
- Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell 1990; 63:251 - 258
- Miotla J, Maciewicz R, Kendrew J, Feldmann M, Paleolog E. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab Invest 2000; 80:1195 - 1205
- Douni E, Sfikakis PP, Haralambous S, Fernandes P, Kollias G. Attenuation of inflammatory polyarthritis in TNF transgenic mice by diarecein: comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis Res Ther 2004; 6:65 - 72