Abstract
G protein-coupled receptors (GPCRs) are one of the most important classes of targets for small molecule drug discovery, but many current GPCRs of interest are proving intractable to small molecule discovery and may be better approached with bio-therapeutics. GPCRs are implicated in a wide variety of diseases where antibody therapeutics are currently used. These include inflammatory diseases such as rheumatoid arthritis and Crohn disease, as well as metabolic disease and cancer. Raising antibodies to GPCRs has been difficult due to problems in obtaining suitable antigen because GPCRs are often expressed at low levels in cells and are very unstable when purified. A number of new developments in over-expressing receptors, as well as formulating stable pure protein, are contributing to the growing interest in targeting GPCRs with antibodies. This review discusses the opportunities for targeting GPCRs with antibodies using these approaches and describes the therapeutic antibodies that are currently in clinical development.
Introduction
G protein-coupled receptors (GPCRs) represent one of the largest and most important classes of proteins for drug discovery. There are over 350 non-olfactory GPCRs that are the receptors for a very diverse range of ligands ranging in size from small ions, e.g., calcium, neurotransmitters, metabolites, to peptides and glycoprotein hormones. GPCRs have long been exploited for treating disease; some of the oldest known drugs, including opioids and alkaloids derived from mandrake and foxglove that were used by the Romans and Egyptians, mediate their activity through GPCRs. In the last century, interaction with GPCRs has accounted for the mode of action of around 30% of small molecule marketed drugs, but in the last decade the success of targeting these receptors has diminished, with small molecule drugs directed at only eight new GPCR targets gaining marketing approval. This has occurred despite a huge growth in target biology demonstrating new links between GPCRs and disease. One reason for this low success rate is that many GPCRs of interest are proving to be surprisingly intractable to modern methods in small molecule drug discovery. An alternative approach is to target GPCRs with antibody therapeutics. This review discusses the benefits of antibody therapeutics over small molecule approaches for GPCRs and examines possible targets. The technical challenges of raising antibodies to membrane spanning proteins are reviewed together with mention of receptor breakthrough technologies that may facilitate the discovery of antibody therapeutics for GPCRs. Case studies of GPCR-targeting antibodies that are progressing to clinical trials are also discussed.
GPCR Families
GPCRs are characterized by having an extracellular N-terminus, 7-transmembrane spanning domains (TMDs) and an intracellular C-terminus. Within the TMD, there are a number of characteristic motifs that are highly conserved within the subfamilies; however, the homology between subfamilies is very limited. There are around 800 known GPCRs, but over half of these are olfactory receptors or sensory receptors, leaving approximately 370 that can be considered drug targets.Citation1,Citation2 The Rhodopsin family or Family A, is the largest family with the most diverse set of ligands, including peptides, amines and purines. This family, which includes histamine, dopamine and the adrenergic receptors, comprises the largest set of targets to existing drugs. The family also includes receptors for small neuropeptides such as the neurokinins and opioids, as well larger peptides such as the chemokines. The majority of Family A receptors have short N-termini and their ligands act either directly within the transmembrane domains (TMDs) or through an interaction with the extracellular loops. An exception to this are the Family A receptors that have a leucine-rich repeat region, such as the receptors for the glycoprotein hormones, e.g., follicle stimulating hormone receptor.
Family B is subdivided into the Secretin and Adhesion subfamilies. The Secretin family has 15 members that are all receptors for large peptide ligands such as glucagon-like peptide receptor and parathyroid hormone. This family has a large extracellular domain that is involved in ligand binding. Many of these receptors are clinically validated using the endogenous ligands or related peptides, e.g., calcitonin, amylin and PTH;Citation3 however, to date there are no small molecule marketed drugs for this family. The Adhesion family contains 33 members that consist of a TMD related to the Secretin family linked to very large multi-domain N-termini. The majority of the Adhesion family ligands have yet to be identified, but those that are include extracellular matrix proteins. This family undergoes a novel proteolytic cleavage between the N-terminal extracellular domain and the TMD, although the two remain closely associated at the plasma membrane.
The third major class of GPCRs is the Family C or metabotropic Glutamate family. These bind their small ligands such as glutamate or Ca2+ in their large bilobular extracellular domain. These receptors appear to function mainly as homo- or hetero-dimers and ligand activation involves interactions between the extracellular domains and TMDs.Citation4
A final class of GPCRs that are potentially of interest as targets for therapeutic antibodies is the Frizzled family. This consists of ten frizzled receptors and the smoothened receptor. Some of these receptors have been shown to couple to G proteins and there are some structural similarities with other Family A and B GPCRs.
Across all the families, many receptors have been identified through sequence identity, but the ligand, and in many cases the function, of the GPCR is still unknown. There are over 100 of these so-called orphan receptors. If functional antibodies could be raised to such receptors, this would provide a useful route to target validation.
Advantages of Targeting GPCRs with Antibody Therapeutics
General properties.
There are many differences between antibody and small molecule therapeutics that are widely known. While it is worth briefly mentioning some of these here, we have focused on the aspects that particularly apply to therapeutics directed at GPCRs. Although the costs of antibody development and manufacture are higher than small molecules, in general they have higher approval success rates compared with new chemical entities.Citation5 Antibodies usually have a much longer duration of action than small molecules and show less inter-patient variability in plasma concentration at a given dose. Disadvantages of antibodies include the potential for an immunogenic response; this is substantially reduced in humanized or human mAbs, but can vary with the route of administration.
Drugability.
The majority of small molecule drugs directed at GPCRs are simple analogs of their natural ligands, e.g., beta-blockers are related in structure to adrenaline. Many current GPCRs of interest as therapeutic targets have peptide or large protein ligands, such as the chemokine receptors or the Family B peptide receptors, including CGRP or GLP1. High throughput screening of very large compound libraries has become the method of choice to identify starting points for small molecule drug discovery; however, success rates are poor. The hit rate for Family A GPCRs evaluated by high throughput screening (HTS) is around 50%,Citation6 but the number of these molecules actually progressing to the clinic is around 10%.Citation6 This is often due to the poor drug-like properties, such as molecular weight and pharmacokinetics, of small molecules directed at GPCRs. In the case of Family B receptors, the hit rate in HTS is extremely low or non-existent.Citation6 The subfamilies of Adhesion receptors and the Frizzled receptors have a very interesting and emerging biology that implicates them in inflammation, tumor growth and metastasis. Conventional small molecule drug discovery methods have generally failed to identify agonists or antagonists for these receptors. Orphan GPCRs across any of the families are difficult to screen for interactions with small molecules because it can be difficult to configure a suitable assay in the absence of a ligand and knowledge of the relevant signaling pathways to measure function. Therefore, the GPCRs of current interest are poorly tractable to existing small molecule drug discovery approaches. For many of these targets, a functional monoclonal antibody (mAb) would provide a useful alternative approach.
Selectivity.
The orthosteric binding site of GPCRs, which is the usual site of action of small molecule drugs directed at these receptors, is highly conserved across members of a single subfamily, making it difficult to obtain selectivity for specific receptors. This has been a particular problem for receptors in the monoamine family against which drugs on the market, e.g., clozapine, have activity at a dozen or more receptors.Citation7 Drugs that bind to allosteric sites that are less conserved between receptors are more likely to show complete selectivity. Indeed, many companies are now trying to identify small molecule allosteric modulators for this reason. The greatest diversity in GPCR structures lies in the N-terminus and loop regions such that even receptors that bind the same peptide ligands can have very diverse extracellular domains. Since antibodies to GPCRs will target these regions, they have an opportunity to show unique specificity not possible in small molecule modulators. Nevertheless, care is needed since many GPCR antibodies developed primarily as research tools have been shown to react across many GPCRs. For example, antibodies raised against β-adrenergic receptors were shown to bind as many as nine different adrenergic receptor subtypes.Citation8 Although there is diversity in sequence between receptors, three-dimensional structural similarity may remain, resulting in a lack of selectivity even for antibodies.Citation9
Distribution.
Drug discovery targeted at GPCRs expressed in the periphery is often hampered by serious side effects mediated by receptors within the central nervous system (CNS). Although there are examples of small molecule drugs that are excluded from the CNS, it can be difficult to balance other physicochemical properties to give good pharmacokinetics with lack of CNS penetration. In addition, it is difficult to predict or indeed measure absolute CNS penetration in man.Citation10 Since antibodies can be easily designed to have limited CNS exposure, this provides a significant advantage for antibody therapeutics that target peripheral receptors. For example, µ opioid receptors are the site of action of morphine, and analgesic effects are mediated by both central and peripheral receptors. Side effects such as tolerance and dependence are mediated by central receptors only, and it is known that peptide agonists that do not access the CNS are able to produce robust analgesic effects in the absence of CNS side effects.Citation11 Histamine receptors for allergyCitation12 and cannabinoid receptors for painCitation13 and obesityCitation14 are other examples of targets where lack of CNS activity would be a significant benefit. The lung also represents a privileged site for the treatment of respiratory disease, and it may be possible to administer antibody therapeutics that have limited systemic exposure while targeting GPCRs in lung tissue.Citation15
Pharmacology of GPCR-Targeting Antibodies
Target overview.
Out of the 370 non-olfactory GPCRs, just over 60 are already targeted by marketed small molecule drugs, while approximately 25 are targeted by bio-therapeutics, most of which are derivatives of the natural peptide ligand (data from Thomson Pharma database). A further 100 receptors are strongly implicated in disease, and the remainder are mainly orphan receptors with unknown biology. We have carried out an analysis of the targets against which antibody therapeutics could have utility. We excluded targets that would require CNS penetration for efficacy or where there are already optimized small molecule drugs available or in development. We estimate that antibody therapeutics could be valuable against around 80 GPCR targets. Of these, approximately 25% would require activating or agonistic antibodies. The majority of the opportunities are in the cancer, inflammation or metabolic disease areas ().
GPCRs in cancer.
There is growing awareness of the diverse roles of GPCRs in cancer biology.Citation16 GPCRs are expressed on many tumors where they play a role in proliferation, metastasis, survival and angiogenesis. Several GPCRs have been identified as oncogenes, some of which are encoded by viruses. For example, Kaposi sarcoma-associated herpes virus (KSHV) genome includes a chemokine like receptor that has pro-survival and pro-angiogenic activity.Citation17
GPCRs are upstream of many signaling pathways strongly implicated in cell proliferation in tumors. This includes mitogen-activated protein (MAP) kinase cascades, Rho, Ras and Rac signaling, as well as PI 3-kinase and transactivation of cytokine receptors.Citation16 Many GPCR ligands are potent mitogens, such as, lysophosphatidic acid (LPA) and prostaglandins. Neuropeptides, such as neurotensin, endothelin and gastrin-releasing peptide (GRP), promote tumor growth through autocrine and paracrine routes. The receptors for these peptides represent attractive targets for diagnostics or therapeutics. Since receptors to these peptides are often highly upregulated on tumor cells, it is possible to use them as a carrier mechanism for targeted toxins or targeted radiotherapy with labeled peptides. For example, the somatostatin analogue 111In-octreotide is used for localization and staging of neuro-endocrine tumors that express somatostatin receptors and modified somatostatin analogues are successfully being used for tumor imaging and radionuclide therapy.Citation18
The role of chemokines and chemokine receptors is to orchestrate the movement of immune cells to sites of inflammation. Aberrant expression of chemokine receptors on tumor cells can result in tumor metastasis to secondary organs that release chemokine ligands.Citation19 A chemokine receptor of particular interest for cancer therapy is the CXCR4 receptor. Chemokine receptors, including CXCR4, CXCR2 and other receptors, such as adrenomedullin and sphingosine phosphate receptors, are involved in tumor angiogenesis either directly or through the release of other mediators, such as vascular endothelial growth factor (VEGF).Citation20
Antibodies directed at GPCRs thus may have utility in the diagnosis, prognosis and treatment of cancers either directly by blocking pathways involved in proliferation, metastasis and angiogenesis or indirectly through facilitating the action of tumor targeted chemo- and radiotherapies.
GPCRs in inflammation.
GPCRs play a role in innate, adaptive and pathological responses of the immune system. GPCRs are the site of action of a diverse set of inflammatory mediators, including prostaglandins, leukotrienes and histamine. Small molecule antagonists are effective treatments for a range of inflammatory diseases, but may have limitations. For example, many drugs directed at prostaglandin receptors lack specificity and are chemically unstable. Histamine antagonists are hampered by their CNS mediated side effects such as sedation. Therefore, antibodies directed at such receptors may offer some advantages.
Chemokine and chemo-attractant receptors through their role in attracting leukocytes to sites of inflammation have been implicated in a wide variety of autoimmune diseases, including rheumatoid arthritis, asthma, inflammatory bowel disease and multiple sclerosis. Despite substantial efforts from pharmaceutical companies working on small molecule chemokine receptor antagonists, there have been many failures during clinical trials.Citation21 While some of these are likely to be due to the target biology, which would equally apply to antibody therapeutics, in some cases small molecule antagonists had unacceptable pharmaceutical properties or insufficient selectivity, and for these targets an antibody may prove to be better treatments. For example, many of the chemokine antagonists that entered development had activity at other GPCRs and issues with high plasma protein binding, poor pharmacokinetics or off-target toxicities.Citation21
Lysophospholipids including LPA and sphingosine 1 phosphate (S1P) mediate their effects through a family of 8 GPCRs (LPA1-3, S1P1-5). These receptors are widely expressed on lymphocytes, macrophages, endothelial and neuronal cells. Lysophospholipids can regulate immune responses through effects on cell migration, adhesion, apoptosis and survival, as well as activation and cytokine secretion.Citation22 To date, it has been very difficult to discover potent and selective inhibitors of this family of receptors. Fingolimod (FTY720) is a derivative of the fungal metabolite myrocin and has potent immunosuppressive activity. This is an analogue of sphingosine and is thought to mediate its activities through the S1P1 receptor. Fingolimod and related molecules are in clinical development for multiple sclerosis and transplant. Antibodies with improved potency and selectivity at these receptors offer an alternative therapeutic route that may have an improved side effect profile.
Targeting the receptor can be more successful than targeting the ligand with an antibody. This is due to the redundancy of some GPCRs for multiple ligands or because the levels of ligands can be upregulated to overcome antibody blockade more easily than the receptor.Citation23 This can be exemplified by the observed lack of efficacy when targeting MIP1-α or RANTES as opposed to targeting the receptors CCR1 and CCR5,Citation24 or CXCL8 (IL-8) and its receptors CXCR1 and CXCR2.Citation25 The chemokine ligand IP10 (CXCL10) and its receptor CXCR3 are one exception to this observation, where IP10 appears to be a more selective ligand and the receptor exists in different isoforms. In 2009, an anti-IP10 therapeutic neutralizing antibody (MDX-1100) successfully attained the study primary endpoint in a Phase 2 trial for rheumatoid arthritis patients receiving methotrexate (clinicaltrials. gov identifier NCT01017367). The same antibody holds promise in studies for other indications, such as ulcerative colitis.
GPCRs in metabolic disease.
GPCRs are widely expressed on tissues and cells that regulate diverse processes, such as appetite, digestion, energy expenditure, fat metabolism and storage and glucose homeostasis and, as such, represent important targets in metabolic diseases, such as type II diabetes and obesity.Citation26 Metabolic diseases represent one of the fastest growing challenges in healthcare. In the United States alone, the number of people with obesity (BMI ≥40 kg/m2) has increased 4-fold in the last 10 years.
GPCRs involved in the regulation of insulin and glucagon secretion are current targets of significant interest to the pharmaceutical industry. Several of these are expressed on pancreatic β cells, such as the free fatty acid receptors, FFA 1, 2 and 3 (previously known as GPR40, 41 and 43).Citation27 FFA1 is involved in the regulation of FFA-potentiated glucose stimulated insulin secretion, but is also involved in deleterious effects of fatty acids on β-cell function. Since FFA1 knockout mice are protected against the lipotoxic effects caused by a high fat diet, a FFA1 antibody could have a similar effect. GPR119 and GPR120 are other fatty acid binding proteins that can regulate islet function. GPR119 has a very selective expression on pancreatic β cells and activation by its ligands, such as oleoylethanolamide, stimulates insulin secretion.Citation28 GPR120 is expressed in the intestine where its activation by fatty acids releases glucagon-like peptide (GLP)-1.Citation29
The GPCR target that has attracted the most interest for diabetes is the glucagon-like peptide receptor, GLP1R. GLP1 acts on receptors expressed on a number of islet cell types resulting in increased insulin secretion and decreased glucagon secretion. A peptide analogue of GLP1, exenatide (Byetta) is approved for the treatment of diabetes. This is given by injection and has a relatively short half-life. To date it has proved impossible to find effective small molecule agonists of this receptor.
Although many of the metabolic GPCR targets require activation, it may be possible to identify an activating antibody for these receptors. Indeed, an antibody that potentiated the activity of the endogenous agonists would be particularly interesting.
Approaches to Raising Antibodies Against GPCRs
General background.
The generation of antibodies against GPCRs is associated with several problems that arise from the lack of suitable antigen reagents. Ideally, the receptor should be in a pure, homogenous and conformationally stable form that is still relevant to the native receptor structure, but this is hampered by the requirement for GPCRs to be within the plasma membrane. GPCRs and other membrane proteins are also problematical for raising antibodies since the majority of the receptor protein is embedded in the lipid bilayer. For GPCRs, only the N-terminal domain and the extracellular loop regions are accessible as immunogenic epitopes. Antibodies have been raised successfully against the large extracellular domains (ECDs) of Family B and C GPCRs, but success is limited when small N-terminal epitopes of the ECD are targeted. It is common to obtain antibodies that bind to the extracellular domains, but have no effect on receptor function and, therefore, are of limited utility as therapeutic agents. A goal with regard to immunogen format is to produce a generic technology applicable across different GPCR classes. To date, although several of the formats described here have yielded GPCR-targeting antibodies, the success has not been reproducible across multiple receptors.
Low cell surface expression is another limiting factor and an obstacle for obtaining sufficient quantities of protein for purification.Citation30 Another problem is the instability of protein when purified. Purified receptors have been produced for a limited number of GPCRs and display functional activity as determined by radioligand binding;Citation31 however, this may be due to a small percentage of functional receptor in a larger quantity of unfolded protein.
The difficulties in production of GPCR protein in a relevant conformation also affect the quality of the GPCR antigen in question. Unless purified in the presence of a stabilizing ligand or in a conformationally stabilized form, it is very likely that such receptor proteins will display several different conformations. This may generate antibodies against these different forms, but more often results in antibodies that bind to linear epitopes present in the denatured receptor protein, e.g., CXCR4.Citation32,Citation33 Antigenic heterogeneity can also result from post-translational modifications such as N-glycosylation,Citation34 tyrosine and serine sulphation,Citation35 N-terminal processing,Citation36 conformational fluctuations, receptor oligomerization and G-protein coupling.Citation37
Another problem is the requirement for detergents to maintain protein folding in a functionally active form following purification of membrane proteins. Long chain detergents that confer stability can mask extracellular epitopes. The overall implication for subsequent identification of functionally relevant mAbs is the requirement for a very large number of clones to be screened to identify those that bind native epitopes and in many cases none are identified. Because of the problems associated with the different formats, a useful approach in both immunizations and panning may be to alternate between peptides or recombinant proteins and whole cell antigens. A summary of the key features of antigen formats that have been used to generate anti-GPCR antibodies is presented in .
Once an antibody has been raised to a GPCR, it is important to characterize its pharmacological properties. In many cases this is not done, but instead direct binding assays such as fluorescence-activated cell sorting (FACs) or enzyme-linked immunosorbent assay (ELISA) are used. Antibodies directed at GPCRs may bind to extracellular epitopes to sterically hinder ligand binding without altering GPCR signalling; alternatively, they may bind selectively to stabilize different conformations of the receptor, thereby either activating or inactivating signaling. Functional cell-based assays would be required to evaluate such activities. This can be a challenge, even for small molecules, since cell-based assays do not always reflect the activity of GPCRs in vivo. An interesting potential effect of antibodies is to modulate heterodimers of GPCRs.Citation38 There is emerging evidence that heterodimerization of GPCRs in specific tissues can offer alternative therapeutic targets. For example, heteromers of mu and delta opioid receptors may represent a novel pain target.Citation39
Immunogen Formats Used for GPCRs
Peptides.
The least expensive and simplest antigen to generate is a synthetic peptide. Peptides may be considered a pure source of antigen, but these rarely reflect the native protein conformation, with the resulting antibodies showing a lack of specificity, functional activity or suitability for receptor mapping.Citation40 This has been a problem for commercial GPCR antibodies to dopamine receptors,Citation41 muscarinic,Citation8 β-adrenergic receptors,Citation9 α1-adrenergic receptorsCitation42 and galanin receptors.Citation43 Most antibodies obtained via this route react only with the immunizing peptides and not with the native proteins in which the peptide will often have a different conformation.
Compared with synthetic peptides, larger protein fragment antigens have a higher success rate at producing antibodies that recognize native protein. They are easier to generate than correctly folded native protein and allow for selection of an area of the protein to be targeted. Some functionally relevant antibodies against GPCRs have been obtained by this approach, but the majority of these are against a peptide receptor where steric blockade of the ligand/receptor interaction is more likely to be achieved than for small molecule ligand receptors. For example, the N-terminal domain of the µ-opioid receptor was used to generate antibodies that were able to distinguish between inactive and agonist bound receptors, but no functional properties were reported.Citation44 Peptides corresponding to the second extracellular loop of the human β2 adrenoceptor have been used to produce agonist-like mAbs.Citation45
In order to obtain information about orphan GPCRs, peptide immunization has generated a broad panel of research tools. Immunization via an N-terminal peptide of LGR5 led to a mAb that specifically binds to the receptor on transfected cells with high affinity, but does not bind to the related LGR4 or LGR6. Furthermore, this antibody shows potent complement-dependent cytotoxicity in vitro and strong anti-tumor activity in vivo.Citation46 These receptors are unusual in having a large extracellular domain that includes a leucine-rich repeat motif.
Antibody binders have been identified via phage display technology against the chemo-attractant receptors C5aR and C3aR by using either an N-terminal peptide of the receptorCitation47 or the second extracellular loop. This led to two distinctive antibody groups that recognize two immunodominant domains within the N-terminal region of the loop. The antibodies were specific over C5aR, able to detect C3aR on the surface of human cells such as leukocytes or monocytes, but did not interfere with the binding of the receptor ligand indicating that the immunodominant loop regions are not essential for ligand binding.Citation48 The N-terminus of CCR5 has also been used to raise antibodies in rabbits.Citation49 Peptides were linked with a T-cell epitope from tetanus toxin in order to enhance the immune-response. Polyclonal antisera or purified antibodies showed inhibition of HIV infection in cellular assays.
Recent advances in the structure determination of GPCRs by X-ray crystallography have provided information for homology modeling of GPCRs.Citation50 Such structural information has been used to design constrained peptides with more “native-like” structures to create an improved format of antigen.Citation51 Initially such technologies were used to generate antibodies against GPCR peptide ligands,Citation51 but have been expanded to generate peptido-mimetics for extracellular epitopes of GPCRs. A cyclic dodeca-peptide mimicking the conformation specific domain of the extracellular loop-2 of CCR5 was prepared using MAP-conjugate as a carrier.Citation52 The antibodies raised against this peptide reacted with human CCR5 and potently suppressed infection by the R5 HIV-1 virus in cells. Cyclic peptides have also been successful in the isolation of scFv phage antibodies targeted to the extracellular loops of CCR5.Citation53 Chemical Linkage of Peptides onto Scaffolds (CLiPS) technology has also been used successful to generate functional antibodies to CXCR7.
The chemical conjugation of constrained antigens to virus-like particles (VLPs) was described in 2009.Citation54 Many viral structural proteins can self-assemble into virus-like particles (VLPs) that resemble infectious virus, but lack a viral genome and are therefore non-infectious. Heterologous antigens displayed in a highly dense, multivalent format on the surface of the VLP are extremely immunogenic and can induce high-titer antibody responses at low doses even in the absence of exogenous adjuvants.Citation55 VLP display can also be used to induce an antibody response against self-antigens, essentially abrogating the mechanisms of B-cell tolerance.Citation56
This approach has been used to target two domains (N-terminal via a 21 amino acid peptide and a 12 amino acid domain of the ECD2) of the HIV co-receptor CCR5 that are involved in HIV binding. Anti-CCR5 antibodies that bind to native CCR5 and inhibit SIV infection in vitro were induced in rats.Citation57 The polyclonal sera were tested in ELISA assays with CCR5 transfected HEK cells, but no other member of the CCR family was tested against the polyclonal antibodies.
Despite the success in the generation of blocking antibodies that target peptide GPCRs, antibodies useful for therapeutic purposes are rare and data for only a few have been published, e.g., peptides corresponding to the extracellular regions or the N-terminal domain of human GCGR were fused to an Fc fragment and used to generate and identify human mAbs.Citation58 Antibodies were identified that antagonized glucagon-induced cyclic adenosine monophosphate (cAMP) production and effectively prevented glucagon-induced GCGR endocytosis. A fully human blocking IgG2 antibody candidate with picomolar affinity was isolated and demonstrated prolonged in vivo efficacy in mice and cynomolgous monkeys. Furthermore, the antibody was evaluated in a diabetic ob/ob mouse model for pharmacokinetic and pharmacodynamic characteristics.Citation59 The results indicate that such an antibody could represent an effective new therapeutic for the treatment of type 2 diabetes by reducing blood glucose levels and inhibiting GCGR activity.
These examples clearly indicate the potential of anti-GPCR antibodies as therapeutics in clinical applications, but the success rate using synthetic peptides as antigens is low and thereby limits a general approach towards therapeutically relevant antibodies that target GPCRs.
Whole cell antigens.
To obtain antibodies reactive to the native extracellular structure of membrane proteins, immunization by injection of cultured cells expressing the antigen has been used.Citation60 But this approach has drawbacks, e.g., large numbers of cells are usually required, modifications may be necessary to obtain higher titers, elimination of a high non-specific background is necessary.
Transfected cell lines expressing a high level of receptors have been used successfully in generating mAbs against GPCRs. Functional rat mAbs were isolated against the human sphingosine-1 phosphate (S1P1) receptor expressed in rat hepatoma cells.Citation61 The antibody bound specifically to native receptor in membranes, but not to solubilized and denatured protein. Specificity over other S1P receptors was not determined.Citation62
Anti-CXCR4 antibodies were raised following immunization of Balb/c mice with recombinant NIH3T3-CXCR4 cells. One antibody (Mab 414H5) was able to bind and induce conformational changes of both CXCR4 homodimers and CXCR4/CXCR2 heterodimers. Mab 414H5 has strong anti-tumor activities both in mice xenograft and survival models. Such antibody properties should be of interest for cancer therapy application given the important roles of these two chemokine receptors in cancer.
Whole cell antigen presentation approaches have also been used for screening phage libraries. For example, a large library of human phage antibodies was exposed to mammalian cells displaying the GLP-1 receptor. Phage antibodies that triggered an agonist response were internalized with the receptor while inert phage antibodies were washed away. Positive antibodies were screened for agonist or allosteric modulatory activity against cells expressing the human GLP-1 receptor (US2006/0275288 A1). Antibodies obtained from this approach were able to magnify the cAMP response to GLP-1 by 75–100%, while other antibodies showed equivalent binding determined by fluorescence-activated cell sorting (FACS), but failed to show such an allosteric modulation. Such antibodies may therefore have utility in the treatment of metabolic disorders such as diabetes.
Dendritic cells (DCs) are potent antigen presenting cells and a detailed description of the cellular pathways involved in mechanisms that lead to the immune response generated by DCs has been published.Citation63 Antibodies against the human prostate specific GPCR (PSGR) were raised after intravenous injection of receptor-expressing mouse bone marrow derived dendritic cells or direct injection into the spleen of the mice.Citation64 Antibodies were analyzed by flow cytometry, immunofluorescent staining and western blotting, but no further characterization was performed.
DCs can also release exosomes, containing intact antigen, which induce activation of the antigen-specific B-cell antibody response.Citation65 These dendritic-cell-derived exosomes have drawn interest because of their immunological propertiesCitation66 and two Phase 1 clinical trials in autologous dexosome therapy have been described.Citation67,Citation68 Exosome vesicles (50–100 nm) are formed in intracellular vesicular bodies of most cells and released in the extracellular milieu.Citation69 These particles are capable of containing GPCRs from overexpressing cell lines, as described for CXCR4 and CCR7.Citation70 Sequence modification of the receptors is not necessary to target the protein towards the exosome particles; however, subcellular distribution of unmodified membrane proteins can be found in the plasma membrane of the host cell. This technology was recently applied to the somatostatin receptor SSTR-2.Citation71 Antibodies raised in mice injected with SSTR-2 containing exosomes were identified by ELISA.
A common expression system for the generation of GPCR protein is the Baculovirus/Sf9 insect cell system. Good expression levels can be achieved in the membranes of the insect cells for a number of GPCRs. Recent investigations have also shown that the recombinant, extracellular baculovirus itself also can contain functional GPCR protein in a budded virus form.Citation72 Such viruses carrying functional active receptor protein might also be of use as antigen carrier for immunization approaches.
Cell membranes.
Approaches to use of GPCRs in membrane fractions have been described.Citation73 This strategy is more likely to provide correctly folded GPCR protein than using purified protein or peptides, but this also creates a high background of irrelevant epitopes. Cell membranes from stable cell lines expressing the human glucagon receptor (GCGR) were used to generate human mAbs to hGCGR from the transgenic XenoMouse. The lead candidate mAb displayed potent antagonistic activity with a single injection able to reduce the blood glucose levels in a mouse model for several days.Citation58 The single injection lowered dose-dependent fasting blood glucose levels without inducing hypoglycemia, improved glucose tolerance and might therefore be an effective new therapeutic for the treatment of type 2 diabetes. This was strengthened by evidence that the antibody effectively normalized non-fasting blood glucose levels in a 5-week treatment of diet-induced obese mice.Citation74 Similar treatment also reduced fasting blood glucose without inducing hypoglycemia or other undesirable metabolic effects. Importantly, pancreatic β-cell function was preserved, as demonstrated by improved glucose tolerance throughout the 18-week treatment period. This study supports the concept that long-term inhibition of GCGR signalling by a mAb could be an effective approach for controlling diabetic hyperglycemia.
Purified protein.
Purified functional protein provides one of the best regents for generating antibodies, but there are problems: GPCRs are generally very unstable when purified from the plasma membrane into detergent. Solubilization often results in protein unfolding or aggregation. As a result, functionally relevant epitopes are disordered and antibodies raised to such protein are usually non-functional. For GPCRs that are more stable, such as the chemokine receptors CXCR4 and CCR5, purification of functional protein is possible, although even in these cases the resultant protein will contain a mixture of folded and unfolded receptor. For GPCRs with very large extracellular domains, such as the family B receptors, it is possible to obtain purified soluble N-termini that can be used to raise antibodies. In most cases, these will be antagonistic, i.e., acting to sterically hinder peptide binding. Secondly, GPCRs must be formulated in detergents or similar reagents, and this can cause irritation when used for in vivo approaches or high non-specific binding when used for in vitro screening. In many cases, the use of long chain detergents required for unstable GPCRs actually masks epitopes that would normally be exposed in cells. Finally, the use of purified protein can result in antibodies to intracellular epitopes, such as the intracellular loops and C-terminus. Such antibodies would not be suitable as therapeutic agents, but can be of interest for immunohistochemistry or crystallography.
A few examples of functional antibodies raised to purified GPCR protein have been reported. For example, a mAb was described against the vasoactive intestinal peptide/pituitary adenylyl cyclase-activating peptide (VPAC1) receptor that specifically binds to an extracellular domain (extracellular loops 2/3) of the receptor.Citation75 The IgG1 mAb was raised against purified human VPAC1 receptor. The antibody was capable of binding to the VPAC1 receptor, decreasing cAMP levels in cells and enhanced the maturation of in vitro cultured immature megakaryocyte cells. The inhibition of cAMP levels is probably not related to the prevention of ligand binding as PACAP ligand binding was not affected by the presence of the antibody. A patent has been filed for the treatment of thrombocytopenia (WO/2009/000894) and clinical trials are expected to commence in 2011.
A new approach to generate stable GPCRs for structural studies also has utility for raising functional antibodies. StaR® GPCRsCitation76 have been engineered to include a small number of point mutations that greatly increase their thermostability and allows generation of a GPCR that is stable enough for large scale purification and milligram-scale protein production of homogenous material. This approach has been used for a diverse set of GPCRs, including the β1-adrenergic receptor, the adenosine A2A receptor and the neurotensin peptide receptor NTS1.Citation77–Citation79 Another interesting aspect of this approach is that the thermostabilization process drives the receptor into a specific chosen conformational state, for example, agonist or antagonist. This may present the immune system with conformationally specific epitopes, thereby allowing the selection of antibodies that will selectivity bind to and stabilize specific conformations. Such antibodies would be expected to have functional agonist or antagonist activity as a result.
Conformational coupling between the extracellular domain and orthosteric binding site has recently been demonstrated for the wild-type β2-adrenergic receptor.Citation80 Nuclear magnetic resonance (NMR) spectroscopy was utilized to elucidate the role of the extracellular domain and investigate ligand-specific conformational changes of the receptor, specifically the salt bridge linking extracellular loops 2 and 3 that is a key structural feature of the extracellular domain of the β2-adrenergic receptor. This finding implies that drugs targeting this diverse surface could function as allosteric modulators with high subtype selectivity, such as conformationally-sensitive antibodies. Antibodies have also provided utility in crystallography studies, as exemplified by the mAb generated to intracellular loop 3, where the role of the antibody was to stabilize non-covalent interactions between the transmembrane segments while providing a polar surface for crystal lattice contacts.Citation81
In an attempt to stabilize unstable GPCR proteins, a number of formulations have been developed that seek to mimic the environment of the plasma membrane. These include liposomes made up of mixtures of detergents and lipids. The human orphan receptor RAI3, also known as GPRC5A and RAIG-1, was overexpressed in bacteria and, after purification, reconstituted into liposomes to raise mAbs using classical hybridoma techniques.Citation82
Preparations of CXCR4-containing paramagnetic proteoliposomes have been used to screen a large, non-immune human antibody phage library.Citation83 This approach produced a panel of mAbs that exhibited unique structural and biochemical features and the resulting mAbs were able to inhibit chemotaxis induced by the CXCR4 ligand SDF-1a in Jurkat cells. Antibodies to CCR5 have also been raised using a similar approach.Citation84
After purification, GPCRs have also been reconstituted into phospholipid bilayers using a method based on partial vesicle solubilisation.Citation85 The method generated vesicles that showed homodimerization of the neurotensin 1 receptor and might therefore provide a method to generate mixed antigens of receptor homo- or heterodimers. This approach could allow the generation of dual antibodies directed against two interacting GPCRs, which has been described against CXCR4/CXCR2 (WO 2010/037831 A1).
The reconstitution of a purified GPCR into high density lipoprotein (HDL) particles has been described for the β2 adrenergic receptor.Citation86 The reconstitution in combination with a hetero-trimeric G protein allowed the investigation of a receptor/G-protein complex.Citation87 This provides an antigen source of GPCRs in an agonist-like conformation in order to generate conformational-sensitive mAbs. This so-called nanodisc approach has been successfully used to raise antibodies against haemagglutininCitation88 and thereby proves that such a system can be used for in vivo immunization experiments.
Several new developments in polymer chemistry have led to compounds that could overcome the detergent issue of purified GPCRs for immunization. Amphipols have been described to stabilize GPCRs in a functional active form with only traces of the Amphipol present in the buffer.Citation89 Commercially available polymers like the Amphipol (A8–35) might be useful to protect GPCRs long enough after injection into an animal for the required immune response.
DNA.
Unlike other antigen production methods, DNA immunization has the unique advantage that the antigen is produced in the natural environment of the host animal. This maximizes the probability of the protein forming its true native structure via intracellular synthesis with correct posttranslational modifications and three-dimensional folding. One disadvantage has been that it may only produce low levels of antibodies, which severely limits the success rate. One standard method of DNA delivery is the bombardment of skin with DNA-coated microparticles, known as the Gene Gun approach. The approach of DNA-immunization is now used commercially, and has been successfully applied to the isolation of a blocking antibody against FPRL-1.Citation90 Antibodies generated against the receptor were screened for anti-FPRL-1 activity by FACS and for receptor blocking activity using fluorometric imaging plate reader (FLIPR) technology. Pre-incubation with the selected antibody showed an 80–90% reduction in calcium mobilization in a FPRL-1 expressing cell line after activation of the receptor with the ligand sCKβ8-1.
Plasmid DNA can also be injected via the intramuscular route followed by in vivo electroporation. Strong IgG antibody responses to the chemokine receptors CXCR4, CCR3 and CCR5 receptors were induced after repeated injections with plasmids encoding the receptors into transgenic mice. Here, the GPCR of interest was fused to the E. coli chaperone GroEL at its C-terminus or, alternatively, the co-transfection of plasmids encoding GPCR and GroELCitation91 was performed. The antiserum containing human polyclonal antibodies against native human CXCR4 was capable of detecting endogenously expressed CXCR4, but no functional activity of these antibodies has been reported yet. A similar approach was successful after injection of plasmid DNA into Lewis rats generating polyclonal antibodies against the viral GPCRs pBILF1 and pR78.Citation92 Again, no functional activity of these antibodies has been described so far.
GPCR-Targeting Antibodies in Development
As yet, no GPCR-targeting antibody drugs have been approved, whereas there are over 400 approved new chemical entity drugs that target these receptors. There is growing interest from pharmaceutical and biotechnology companies in GPCR-targeting therapeutic antibodies and a number of agents are now in clinical trials. MAb therapeutics showing promise at an early stage of drug development target receptors such as the chemo-attractant receptor C5aR and the angiogenesis/tumor metastasis associated receptor CXCR4. There is limited data from peer-reviewed articles on the progress of GPCR-targeting mAbs in preclinical and clinical evaluations,Citation93 therefore we also collected information from sources such as company web sites, press releases, investor-corporate presentations, patent databases and the clinicaltrials.gov web site. The most advanced mAb therapeutics are in Phase 2 studies, including, PRO140, which targets the chemokine receptor CCR5, and is currently studies of HIV patients, MLN1201, which blocks CCR2 and is in studies of rheumatoid arthritis patients and KW-0761/AMG761, which is a depleting anti-CCR4 antibody that is in studies as a treatment for cancer. Examples of mAbs at various stages of development are summarized in .
Case Studies in GPCR-Targeting Therapeutic Antibodies
Anti-complement C5aR.
Complement C5aR is an inflammatory chemo-attractant receptor implicated in the pathogenesis of several disease indications, including rheumatoid arthritis, sepsis, systemic lupus erythematosus, stroke, amyotrophic lateral sclerosis, Alzheimer disease and macular degeneration.Citation94 Consequently, C5aR presents a highly competitive area in which there are several small molecule, peptide and mAb candidates in development targeting either the ligand or the receptor. For example, G2 Therapies' humanized anti-C5aR mAb antagonist, NeutrazumabCitation95 is in Phase 1 studies for rheumatoid arthritis and lupus.
Anti-CXCR4.
CXCR4-associated angiogenesis and tumor metastasis has led to a number of antibody development programs, such as MDX-1338 (Medarex; WO2008060367) a fully human anti-CXCR4 antibody currently in Phase 1 studies for acute myelogenous leukemia (AML); NorthWest Biotherapeutics have progressed an anti-CXCR4 antibody project to late-preclinical development where the resulting antibody panel has demonstrated efficacy. Subsequent development will include humanization and toxicity studies in preparation for Phase 1 clinical trials.
CXCR4 antibodies have also been described in early stage studies for immunotherapy in breast cancer. Treatment of CXCR4-expressing breast cancer cells with a neutralizing anti-CXCR4 reduced metastasis to the lungs in an intravenous tail-injections model and an orthotopic implantation disease model.Citation96 In addition, neutralization of CXCR4 with blocking antibodies has also shown a delay in tumor formation by CXCR4-expressing Colon38 tumor cells,Citation97 as well as presenting a potential therapeutic treatment for non-Hodgkin lymphoma.Citation98
In preclinical studies, MDX-1338 has been shown to effectively block the binding of CXCR4 to CXCL12, thereby inhibiting chemotaxis and migration responses. In addition, MDX-1338 also reduced tumor growth in acute myelogenous leukemia and lymphoma xenograft models. MDX-1338 can block the growth of selected human tumor cells. The Phase 1, dose-escalation study planned for 2009 will assess MDX-1338 as a monotherapy with chemotherapy and is expected to enroll up to 34 patients with relapsed/refractory AML. Additionally, the trial will also establish and evaluate the safety, tolerability and maximum tolerated dose, as well as preliminary pharmacodynamics and efficacy of MDX-1338.
CXCR4 also presents a unique opportunity not only where the CXCR4-CXCL12 (SDF-1) biological axis is implicated in the latter stages of HIV infection, but also as a chemo-attractant receptor where antagonizing the receptor has been shown to mobilize CD34+ stem cells from the bone marrow. Precedence for employing this approach is exemplified and the target clinically validated by plerixafor (Mozobil), which selectively blocks CXCR4 signaling and mobilizes neutrophils and hematopoietic progenitor cells to the peripheral blood.Citation99 This small molecule drug was approved by the United States Food and Drug Administration at the end of 2008.Citation100
Another example of this strategy for mobilization of stem cells is provided in the emerging field of alternative antibody formats, where ALX-0651 (Ablynx; WO2010043650) is a biparatopic nanobody antagonist in preclinical development with investigational new drug application filing scheduled for 2011. In vivo studies have demonstrated a single, intravenous administration of formatted Nanobody that resulted in rapid mobilization of stem cells in an animal cancer model.
ALX-0651 comprises two humanized and sequence optimized Nanobodies that bind to different epitopes on CXCR4. This biparatopic Nanobody is a potent antagonist of the CXCR4/CXCL12 axis. The non-humanized and non-sequence optimized form of ALX-0651 has been shown to potently inhibit the interaction of CXCR4 with CXCL12 in a number of in vitro assays, including ligand binding and cell migration. This parental molecule has also been evaluated for its ability to mobilize stem cells in vivo. In a non-human primate model, a single-dose of Nanobody resulted in stem cell mobilization. Based on these results, ALX-0651 has been selected for pre-clinical development initially for the mobilization of stem cells prior to transplantation.
Anti-CCR2 MLN1202.
Monocyte chemo-attractant protein-1 (MCP-1, also known as CCL2) is a chemokine that attracts monocytes, dendritic cells, memory T lymphocytes and natural killer cells by virtue of binding to its receptor CCR2. In fact, CCR2 ligands comprise CCL2, CCL7, -8 and -13 (MCP-1, MCP-3, -2 and -4, respectively); however, CCL2 is somewhat different in that it is one of only a few chemokines that binds primarily to one receptor, i.e., CCR2.
Knock-out studies have demonstrated that loss of MCP-1 effector function is enough to impair monocyte trafficking in inflammation.Citation101 CCR2-deficient mice exhibit altered inflammatory and physiological responses in some allergy/asthma models, but the role of this receptor appears to vary between disease models. CCR2 has been identified as a determinant in athlerosclerosisCitation102 and expression of MCP-1/CCL2 has been found to be increased in disease conditions characterized by chronic inflammation and large number of infiltrating monocytes. Hence, inhibition of MCP-1/CCR2 interaction by a CCR2 antagonist is expected to prevent recruitment of monocytes/macrophages into the tissues where the disease manifests, thereby reducing or suppressing the inflammatory response. This would be particularly advantageous for the therapeutic treatment or management of chronic diseases, particularly autoimmune disease.
Prostate cancer frequently metastasizes to bone resulting in a mixture of osteolytic and osteoblastic lesions. Elevated levels of CCL2 in prostate cancer patients with bone metastases compared to those with localized tumors and CCL2 knockdown that diminished tumor growth in bone have been observed in studies, which implicates the CCR2-CCL2 axis in prostate cancer metastasis.Citation103 Breast cancer also metastasizes to bone (and lung) facilitated by CCL2 interaction with the CCR2+ stromal cells of monocytic origin, including macrophages and pre-osteoclasts.Citation104
MLN1202 is a humanized mAb specifically targeting CCR2 antagonism that has undergone evaluation or is currently being studied in Phase 2 trials for atherosclerosis, multiple sclerosis, ulcerative colitis and diabetes.Citation105 Recruitment is ongoing for a Phase 2 study in bone metastasis (Clinicaltrials.gov identifier NCT01015560) where the effect of MLN1202 on tumor cell proliferation, monocytes/macrophage trafficking and osteoclast maturation will be assessed. MLN1202 has also been reported as in preclinical development for scleroderma/systemic sclerosis. The Phase 2 trials for multiple sclerosis and atherosclerosis have been successfully completed,Citation105 but a recent publication noted that further development by a commercial sponsor has been discontinued.Citation106 MLN1202 failed to exhibit sufficient efficacy at Phase 2 in a clinical trial for rheumatoid arthritis.Citation107
Antagonism by MLN1202 was associated with a reduction in gadolinium (GD)-enhancing lesions on brain resonance scans in a multi-centre Phase 2 study of 50 patients with relapsing-remitting multiple sclerosis.Citation93 One of the key issues for the progression of CCR2 therapeutic antibodies is the low sequence homology of the receptor and ligand between human and other species. This makes preclinical evaluation of anti-human receptor antibodies in animal models of the disease questionable. Therefore, it may be necessary to evaluate CCR2 candidate antagonists in non-human primates and humanized mice.
Anti-CCR4 KW0761 (AMG761)
CCR4, a GPCR for C-C chemokines such MIP-1, RANTES, thymus and activation-regulated chemokine (TARC) and MCP-1, is expressed mainly on Th2-type helper T cells and regulatory T cells. Platelet activation is induced by the ligands TARC and macrophage-derived chemokine (MDC), mediated through CCR4. CCR4+ T cells are implicated in the pathology of asthma and other inflammatory diseases and T-cell malignancies. CCR4, also known as CD194, is overexpressed on adult T-cell lymphoma (ATL) and peripheral T-cell lymphoma (PTCL) cells.
KW-0761 is a defucosylated humanized IgG1 antagonist targeting CCR4 that was derived from the POTELLIGENT® technology platform (Kyowa Hakko Kirin) for the development of antibody-dependent cell-mediated cytotoxicity (ADCC) enhanced antibodies.Citation108 The enhanced ADCC is engineered via defucosylation of the KM2760 anti-CCR4 chimeric human-mouse antibody Fc regionCitation109 using the original murine anti-CCR4 antibody KM2160.Citation110 KW-0761 selectively binds to and blocks the activity of CCR4, consequently inhibiting CCR4-mediated signal transduction pathways and subsequent chemokine-mediated cellular migration, proliferation of T cells and chemokine-mediated angiogenesis.
Potent anti-tumor activity has been observed in vivo where KW-0761 was shown to mediate ADCC activity in vitro against primary adult T cell leukemia/lymphoma (ATLL) cells in an autologous setting. The degree of ADCC observed was related to the level of effector NK cells rather than the amount of target molecule on the ATLL cell surface.Citation109
Several clinical trials have been completed or are ongoing for this therapeutic candidate: Phase 1/2 studies in ATLL and peripheral T cell lymphoma PTCL (Clinicaltrials.gov identifier NCT00888927; NCT00355472)Citation111 where KW-0761 is the first defucosylated therapeutic mAb used clinically in cancer patients; and a Phase 2 study (NCT00920790) currently being conducted for relapsed/refractory ATLL after in vitro and in vivo efficacy demonstrated clinical application against ATLL.Citation109,Citation112 A marketing application filing is planned in 2011 and approval is anticipated in 2012 in Japan.
CCR4 expression is also associated with TH17 cells (IL-17-producing CD4+ T cells) that present a distinct T helper cell lineage involved in the pathogenesis of a number of autoimmune diseases.Citation113 Accordingly, KW-0761 is currently being studied in allergic rhinitis and has completed Phase 1 studies of KW-0761 in healthy volunteers and allergic rhinitis patients.
In 2008, Amgen acquired rights in all non-oncology indications and Kyowa Hakko Kirin will continue its own development activities in oncology until the completion of Phase 2a. At that time, Amgen may elect to reimburse Kyowa Hakko Kirin for its oncology-related development costs, expand its license to include oncology and assume the development and commercialization of KW-0761 in oncology settings. At Amgen, AMG761 is undergoing Phase 1 studies for the management/treatment of asthma where the mechanism of action is to deplete the build-up of Th2 cells responsible for the allergic immune response in conditions such as asthma. Preliminary results have been reported as favorable where potent depletion at low doses has been observed in the clinic; a long-lasting effect would mean dose administration could be quite infrequent.
Conclusions
GPCRs have long been recognized as important therapeutic targets, but it is only recently that their potential as antibody targets has been explored. There are many technical hurdles to overcome in raising antibodies to membrane proteins such as GPCRs and ion channels, but developments in stabilizing receptors using mutagenesis or additives, as well as methods to overexpress receptors in cell systems, is enabling the identification of functional antibodies. Such agents have great potential in the treatment of chronic diseases such as rheumatoid arthritis, diabetes and cancer.
Figures and Tables
Figure 1 Opportunities for G protein-coupled receptor-targeted antibody therapeutics. Therapeutic areas in which G protein-coupled receptors (GPCRs) suitable as targets for antibody-based drugs have been identified. In total, 88/370 GPCRs have strong disease rationale and a profile suitable for targeting with an antibody.
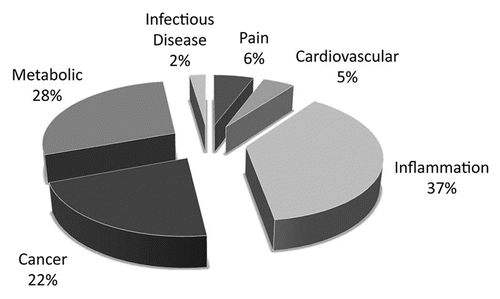
Table 1 Antigen formats used to generate G protein-coupled receptor targeted antibodies
Table 2 G protein-coupled receptor-targeted antibody candidates
References
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev 2005; 57:279 - 288
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 2008; 7:339 - 357
- Tyndall JD, Sandilya R. GPCR agonists and antagonists in the clinic. Med Chem 2005; 1:405 - 421
- Pin JP, Kniazeff J, Goudet C, Bessis AS, Liu J, Galvez T, et al. The activation mechanism of class-C G-protein coupled receptors. Biol Cell 2004; 96:335 - 342
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol 2005; 23:1073 - 1078
- Macarron R. Critical review of the role of HTS in drug discovery. Drug Discov Today 2006; 11:277 - 279
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996; 14:87 - 96
- Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 2009; 379:397 - 402
- Hamdani N, van der Velden J. Lack of specificity of antibodies directed against human beta-adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 2009; 379:403 - 407
- Jeffrey P, Summerfield S. Assessment of the blood-brain barrier in CNS drug discovery. Neurobiol Dis 2010; 37:33 - 37
- Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 2009; 9:3 - 8
- Welch MJ, Meltzer EO, Simons FE. H1-antihistamines and the central nervous system. Clin Allergy Immunol 2002; 17:337 - 388
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 2007; 10:870 - 879
- Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 2008; 57:2977 - 2991
- Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal 2006; 18:2105 - 2120
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer 2007; 7:79 - 94
- Montaner S, Sodhi A, Ramsdell AK, Martin D, Hu J, Sawai ET, et al. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor as a therapeutic target for the treatment of Kaposi's sarcoma. Cancer Res 2006; 66:168 - 174
- de Jong M, Breeman WA, Kwekkeboom DJ, Valkema R, Krenning EP. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc Chem Res 2009; 42:873 - 880
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004; 4:540 - 550
- Richard DE, Vouret-Craviari V, Pouyssegur J. Angiogenesis and G-protein-coupled receptors: signals that bridge the gap. Oncogene 2001; 20:1556 - 1562
- Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov 2009; 8:23 - 33
- Graler MH, Goetzl EJ. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta 2002; 1582:168 - 174
- Hancock WW. Chemokines and transplant immunobiology. J Am Soc Nephrol 2002; 13:821 - 824
- Gao W, Faia KL, Csizmadia V, Smiley ST, Soler D, King JA, et al. Beneficial effects of targeting CCR5 in allograft recipients. Transplantation 2001; 72:1199 - 1205
- Das AM, Brodmerkel CM. Chemokines and chemokine receptors: Large and small therapeutic strategies for inflammatory diseases. Drug Discovery Today: Therapeutic Strategies 2006; 3:381 - 388
- Winzell MS, Ahren B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther 2007; 116:437 - 448
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003; 278:11312 - 11319
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 2006; 3:167 - 175
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005; 11:90 - 94
- McCusker EC, Bane SE, O'Malley MA, Robinson AS. Heterologous GPCR expression: a bottleneck to obtaining crystal structures. Biotechnol Prog 2007; 23:540 - 547
- Grisshammer R. Purification of recombinant G-protein-coupled receptors. Methods Enzymol 2009; 463:631 - 645
- Baribaud F, Edwards TG, Sharron M, Brelot A, Heveker N, Price K, et al. Antigenically distinct conformations of CXCR4. J Virol 2001; 75:8957 - 8967
- Carnec X, Quan L, Olson WC, Hazan U, Dragic T. Anti-CXCR4 monoclonal antibodies recognizing overlapping epitopes differ significantly in their ability to inhibit entry of human immunodeficiency virus type 1. J Virol 2005; 79:1930 - 1933
- Sloane AJ, Raso V, Dimitrov DS, Xiao X, Deo S, Muljadi N, et al. Marked structural and functional heterogeneity in CXCR4: separation of HIV-1 and SDF-1alpha responses. Immunol Cell Biol 2005; 83:129 - 143
- Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 1999; 96:667 - 676
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines and stromal cells. Exp Hematol 2002; 30:973 - 981
- Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci 2004; 25:186 - 192
- Hipser C, Bushlin I, Gupta A, Gomes I, Devi LA. Role of antibodies in developing drugs that target G-protein-coupled receptor dimers. Mt Sinai J Med 2010; 77:374 - 380
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal 2010; 3:54
- Kirkpatrick P. Specificity concerns with antibodies for receptor mapping. Nat Rev Drug Discov 2009; 8:278
- Bodei S, Arrighi N, Spano P, Sigala S. Should we be cautious on the use of commercially available antibodies to dopamine receptors?. Naunyn Schmiedebergs Arch Pharmacol 2009; 379:413 - 415
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol 2009; 379:409 - 412
- Lu X, Bartfai T. Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol 2009; 379:417 - 420
- Gupta A, Decaillot FM, Gomes I, Tkalych O, Heimann AS, Ferro ES, et al. Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem 2007; 282:5116 - 5124
- Lebesgue D, Wallukat G, Mijares A, Granier C, Argibay J, Hoebeke J. An agonist-like monoclonal antibody against the human beta2-adrenoceptor. Eur J Pharmacol 1998; 348:123 - 133
- Sasaki Y, Kosaka H, Usami K, Toki H, Kawai H, Shiraishi N, et al. Establishment of a novel monoclonal antibody against LGR5. Biochem Biophys Res Commun 2010; 394:498 - 502
- Huang L, Sato AK, Sachdeva M, Fleming T, Townsend S, Dransfield DT. Discovery of human antibodies against the C5aR target using phage display technology. J Mol Recognit 2005; 18:327 - 333
- Hawlisch H, Frank R, Hennecke M, Baensch M, Sohns B, Arseniev L, et al. Site-directed C3a receptor antibodies from phage display libraries. J Immunol 1998; 160:2947 - 2958
- Chain BM, Noursadeghi M, Gardener M, Tsang J, Wright E. HIV blocking antibodies following immunisation with chimaeric peptides coding a short N-terminal sequence of the CCR5 receptor. Vaccine 2008; 26:5752 - 5759
- Li YY, Hou TJ, Goddard WA 3rd. Computational modeling of structure-function of g protein-coupled receptors with applications for drug design. Curr Med Chem 2010; 17:1167 - 1180
- Timmerman P, Puijk WC, Meloen RH. Functional reconstruction and synthetic mimicry of a conformational epitope using CLIPS technology. J Mol Recognit 2007; 20:283 - 299
- Misumi S, Nakayama D, Kusaba M, Iiboshi T, Mukai R, Tachibana K, et al. Effects of immunization with CCR5-based cycloimmunogen on simian/HIVSF162P3 challenge. J Immunol 2006; 176:463 - 471
- Zhang Y, Pool C, Sadler K, Yan HP, Edl J, Wang X, et al. Selection of active ScFv to G-protein-coupled receptor CCR5 using surface antigen-mimicking peptides. Biochemistry 2004; 43:12575 - 12584
- Sommerfelt MA. Circular CCR5 peptide conjugates and uses thereof (WO2008074895). Expert Opin Ther Pat 2009; 19:1323 - 1328
- Spohn G, Bachmann MF. Exploiting viral properties for the rational design of modern vaccines. Expert Rev Vaccines 2008; 7:43 - 54
- Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol 2008; 180:5816 - 5825
- Hunter Z, Smyth HD, Durfee P, Chackerian B. Induction of mucosal and systemic antibody responses against the HIV coreceptor CCR5 upon intramuscular immunization and aerosol delivery of a virus-like particle based vaccine. Vaccine 2009; 28:403 - 414
- Yan H, Gu W, Yang J, Bi V, Shen Y, Lee E, et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther 2009; 329:102 - 111
- Lau YY, Ma P, Gibiansky L, Komorowski R, Wang J, Wang G, et al. Pharmacokinetic and pharmacodynamic modeling of a monoclonal antibody antagonist of glucagon receptor in male ob/ob mice. AAPS J 2009; 11:700 - 709
- Ohno Y, Suda K, Masuko K, Yagi H, Hashimoto Y, Masuko T. Production and characterization of highly tumor-specific rat monoclonal antibodies recognizing the extracellular domain of human L-type amino-acid transporter 1. Cancer Sci 2008; 99:1000 - 1007
- Graeler M, Shankar G, Goetzl EJ. Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J Immunol 2002; 169:4084 - 4087
- Goetzl EJ, Dembrow D, Van Brocklyn JR, Graler M, Huang MC. An IgM-kappa rat monoclonal antibody specific for the type 1 sphingosine 1-phosphate G protein-coupled receptor with antagonist and agonist activities. Immunol Lett 2004; 93:63 - 69
- Breckpot K, Escors D. Dendritic cells for active anti-cancer immunotherapy: targeting activation pathways through genetic modification. Endocr Metab Immune Disord Drug Targets 2009; 9:328 - 343
- Tamura T, Chiba J. Production of antibodies against multipass membrane proteins expressed in human tumor cells using dendritic cell immunization. J Biomed Biotechnol 2009; 2009:673098
- Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J Immunol 2006; 177:3757 - 3762
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999; 147:599 - 610
- Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005; 3:9
- Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 2005; 3:10
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2:569 - 579
- Delcayre A, Shu H, Le Pecq JB. Dendritic cell-derived exosomes in cancer immunotherapy: exploiting nature's antigen delivery pathway. Expert Rev Anticancer Ther 2005; 5:537 - 547
- Estelles A, Sperinde J, Roulon T, Aguilar B, Bonner C, LePecq JB, et al. Exosome nanovesicles displaying G protein-coupled receptors for drug discovery. Int J Nanomedicine 2007; 2:751 - 760
- Loisel TP, Ansanay H, St-Onge S, Gay B, Boulanger P, Strosberg AD, et al. Recovery of homogeneous and functional beta2-adrenergic receptors from extracellular baculovirus particles. Nat Biotechnol 1997; 15:1300 - 1304
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA 1999; 96:5215 - 5220
- Gu W, Yan H, Winters KA, Komorowski R, Vonderfecht S, Atangan L, et al. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther 2009; 331:871 - 881
- Freson K, Peeters K, De Vos R, Wittevrongel C, Thys C, Hoylaerts MF, et al. PACAP and its receptor VPAC1 regulate megakaryocyte maturation: therapeutic implications. Blood 2008; 111:1885 - 1893
- Robertson N, Jazayeri A, Errey J, Baig A, Hurrell E, Zhukov A, et al. The properties of thermostabilised G protein-coupled receptors (StaRs) and their use in drug discovery. Neuropharmacology 2010; In press
- Serrano-Vega MJ, Magnani F, Shibata Y, Tate CG. Conformational thermostabilization of the β1-adrenergic receptor in a detergent-resistant form. Proc Natl Acad Sci USA 2008; 105:877 - 882
- Magnani F, Shibata Y, Serrano-Vega MJ, Tate CG. Co-evolving stability and conformational homogeneity of the human adenosine A2a receptor. Proc Natl Acad Sci USA 2008; 105:10744 - 10749
- Shibata Y, White JF, Serrano-Vega MJ, Magnani F, Aloia AL, Grisshammer R, et al. Thermostabilization of the neurotensin receptor NTS1. J Mol Biol 2009; 390:262 - 277
- Bokoch MP, Zou Y, Rasmussen SG, Liu CW, Nygaard R, Rosenbaum DM, et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature 2010; 463:108 - 112
- Day PW, Rasmussen SG, Parnot C, Fung JJ, Masood A, Kobilka TS, et al. A monoclonal antibody for G protein-coupled receptor crystallography. Nat Methods 2007; 4:927 - 929
- Jorissen H, Bektas N, Dahl E, Hartmann A, ten Haaf A, Di Fiore S, et al. Production and characterisation of monoclonal antibodies against RAI3 and its expression in human breast cancer. BMC Cancer 2009; 9:200
- Xu C, Sui J, Tao H, Zhu Q, Marasco WA. Human anti-CXCR4 antibodies undergo VH replacement, exhibit functional V-region sulfation and define CXCR4 antigenic heterogeneity. J Immunol 2007; 179:2408 - 2418
- Mirzabekov T, Kontos H, Farzan M, Marasco W, Sodroski J. Paramagnetic proteoliposomes containing a pure, native and oriented seven-transmembrane segment protein, CCR5. Nat Biotechnol 2000; 18:649 - 654
- Harding PJ, Attrill H, Boehringer J, Ross S, Wadhams GH, Smith E, et al. Constitutive dimerization of the G-protein coupled receptor, neurotensin receptor 1, reconstituted into phospholipid bilayers. Biophys J 2009; 96:964 - 973
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA 2007; 104:7682 - 7687
- Yao XJ, Velez Ruiz G, Whorton MR, Rasmussen SG, DeVree BT, Deupi X, et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc Natl Acad Sci USA 2009; 106:9501 - 9506
- Bhattacharya P, Grimme S, Ganesh B, Gopisetty A, Sheng JR, Martinez O, et al. Nanodisc-incorporated hemagglutinin provides protective immunity against influenza virus infection. J Virol 2010; 84:361 - 371
- Dahmane T, Damian M, Mary S, Popot JL, Baníres JL. Amphipol-assisted in vitro folding of G protein-coupled receptors. Biochemistry 2009; 48:6516 - 6521
- Elagoz A, Henderson D, Babu PS, Salter S, Grahames C, Bowers L, et al. A truncated form of CKbeta8-1 is a potent agonist for human formyl peptide-receptor-like 1 receptor. Br J Pharmacol 2004; 141:37 - 46
- Fujimoto A, Takatsuka S, Ishida I, Chiba J. Production of human antibodies to native cytokine receptors using the genetic immunization of KM mice. Hum Antibodies 2009; 18:75 - 80
- Kaptein SJ, Jungscheleger-Russell J, Martinez-Martinez P, Beisser PS, Lavreysen H, Vanheel A, et al. Generation of polyclonal antibodies directed against G protein-coupled receptors using electroporation-aided DNA immunization. J Pharmacol Toxicol Methods 2008; 58:27 - 31
- Kalinowska A, Losy J. Investigational C-C chemokine receptor 2 antagonists for the treatment of autoimmune diseases. Expert Opin Investig Drugs 2008; 17:1267 - 1279
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol 2005; 23:821 - 852
- Lee H, Zahra D, Vogelzang A, Newton R, Thatcher J, Quan A, et al. Human C5aR knock-in mice facilitate the production and assessment of anti-inflammatory monoclonal antibodies. Nat Biotechnol 2006; 24:1279 - 1284
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410:50 - 56
- Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res 2005; 65:5864 - 5871
- Bertolini F, Dell'Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, et al. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma. Cancer Res 2002; 62:3106 - 3112
- Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother 2000; 44:1667 - 1673
- Proudfoot AE, Power CA, Schwarz MK. Anti-chemokine small molecule drugs: a promising future?. Expert Opin Investig Drugs 2010; 19:345 - 355
- Conrad SM, Strauss-Ayali D, Field AE, Mack M, Mosser DM. Leishmania-derived murine monocyte chemoattractant protein 1 enhances the recruitment of a restrictive population of CC chemokine receptor 2-positive macrophages. Infect Immun 2007; 75:653 - 665
- Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998; 394:894 - 897
- Lu Y, Chen Q, Corey E, Xie W, Fan J, Mizokami A, et al. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin Exp Metastasis 2009; 26:161 - 169
- Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem 2009; 284:29087 - 29096
- Xia M, Sui Z. Recent developments in CCR2 antagonists. Expert Opin Ther Pat 2009; 19:295 - 303
- Ribeiro S, Horuk R. The clinical potential of chemokine receptor antagonists. Pharmacol Ther 2005; 107:44 - 58
- Vergunst CE, Gerlag DM, Lopatinskaya L, Klareskog L, Smith MD, van den Bosch F, et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum 2008; 58:1931 - 1939
- Ito A, Ishida T, Utsunomiya A, Sato F, Mori F, Yano H, et al. Defucosylated anti-CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi-scid, IL-2R gamma(null) mice in vivo. J Immunol 2009; 183:4782 - 4791
- Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res 2010; 16:1520 - 1531
- Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 2003; 9:3625 - 3634
- Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol 2010; 28:1591 - 1598
- Yano H, Ishida T, Imada K, Sakai T, Ishii T, Inagaki A, et al. Augmentation of antitumour activity of defucosylated chimeric anti-CCR4 monoclonal antibody in SCID mouse model of adult T-cell leukaemia/lymphoma using G-CSF. Br J Haematol 2008; 140:586 - 589
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol 2007; 19:652 - 657