 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We report preclinical data for CEP-37247, the first human framework domain antibody construct to enter the clinic. At approximately 11 – 13kDa, domain antibodies or dAbs are the smallest antibody domain able to demonstrate the antigen-recognition function of an antibody, e.g. high selectivity and affinity for target antigen. CEP-37247 is a bivalent anti-tumor necrosis factor (TNF)α domain antibody protein construct combining the antigen-recognition function of a dAb with the pharmacological advantages of an antibody Fc region. As a homodimer, with each chain comprising VL dAb, truncated CH1, hinge, CH2 and CH3 domains, CEP-37247 has a molecular mass of approximately 78kDa, which is about half the size of a conventional IgG molecule. Surface plasmon resonance data demonstrate that CEP-37247 possesses high selectivity and affinity for TNFα. CEP-37247 is a potent neutralizer of TNFα activity in vitro in the L929 TNF-mediated cytotoxicity assay. In a human TNFα-over-expressing mouse model of polyarthritis, CEP-37247 prevents development of disease, and is at least as effective as the marketed product etanercept. Fc functionality is intact – CEP-37247 is capable of mediating antibody-dependent cell-mediated cytotoxicity and has a circulating half-life of approximately 4.5 days in cynomolgus macaques. Given the favorable properties outlined above, and its high expression levels (approaching 7 g/L) in a CHOK1 based-expression system, CEP-37247 is progressing into the clinic, where other potential advantages such as enhanced efficacy due to improved tissue distribution, and beneficial immunogenicity profile, will be evaluated.
Introduction
Antigen binding by antibodies typically occurs via paired heavy (VH) and light (VL) chain variable domains. Ward et al.Citation1 demonstrated that isolated variable domains, i.e., only half of the typical antigen binding site, that display nanomolar binding affinities could be generated. They named these single domain antibodies or dAbs. This finding heralded a new class of smaller antibody-based proteins, with potential advantages over conventional antibodies, including enhanced tissue distribution, reduced immunogenicity and lower production costs.
The dAb component of the CEP-37247 domain antibody construct was generated using phage display technology. A TNFα-binding VL dAb was isolated from a library based on the human germline DPK9 framework as described by Holt et al.Citation2 and was affinity matured by diversification of a selected subset of residues. While the dAb alone bound and neutralized TNFα, it was unsuitable for development as a therapeutic targeting inflammatory diseases driven by chronic overexpression of TNFα because of its short serum half-life of less than 1 hour in mice.Citation2 Hence, we formatted the domain antibody into an Fc construct (homodimer with each chain comprising VL dAb, truncated CH1, hinge, CH2 and CH3 domains) to improve its therapeutic potential. We included a single CH1 residue (truncated CH1) to provide flexibility around the hinge region and for increased homology.
Tumor necrosis factor (TNF)α is a pro-inflammatory cytokine that is a principle mediator in a number of autoimmune and inflammatory diseases such as rheumatoid arthritis, Crohn disease, psoriasis, psoriatic arthritis and ankylosing spondylitis. The key role of TNFα in the progression of these diseases is highlighted by the therapeutic success of anti-TNF agents, which have demonstrated superiority over existing therapeutic modalities.Citation3,Citation4 For example, in rheumatoid arthritis, anti-TNF therapy represents a significant step forward, because, unlike disease-modifying anti-rheumatic drugs (DMARDs) that alleviate symptoms but do not stop joint destruction, anti-TNF agents prevent radiological progression.Citation5,Citation6 There are multiple registered anti-TNF agents serving this large (greater than $16Bn in 2008)Citation7 and growing market: infliximab (Remicade™), etanercept (Enbrel™), adalimumab (Humira™), certolizumab pegol (Cimzia™) and golimumab (Simponi™). Still, there is scope for improved anti-TNF agents with better safety and efficacy profiles.Citation8
We describe here the development of the human framework anti-TNFα domain antibody construct CEP-37247. The specificity, binding affinity, in vitro and in vivo potencies were assessed. Analysis of the ability of CEP-37247 to promote antibody-dependent cell-mediated cytotoxicity (ADCC) and measurement of circulatory half-life following single dose administration to cynomolgus macaques are reported. Our data indicate that, although CEP-37247 is approximately half the size, it demonstrates the functionality expected of a full-size anti-TNF monoclonal antibody (mAb). In addition, it has been manufactured, in fed-batch CHO cell culture, at industry-leading levelsCitation9,Citation10 and its potential for reduced immunogenicity and increased tissue penetration will be explored in ongoing preclinical and clinical studies.
Results
Novel construction of CEP-37247.
Initially, a panel of anti-TNFα domain antibodies was identified by phage display technology by panning against TNFα and screening for high affinity, TNFα-neutralizing binders. A VL dAb based on the human germline DPK9 framework was chosen and affinity matured by further rounds of selection and screening from libraries diversified in CDR1 and CDR3 residues known to be diversified in the mature human Ig repertoire and framework residues observed to produce functional proteins after mutagenesis in related dAbs.
We then genetically fused the lead domain antibody onto a human IgG1 Fc region to create CEP-37247 (previously known as ART621). A schematic representation of the structure of CEP-37247 (∼78 kDa) in comparison to a full antibody (∼150 kDa) and a domain antibody (∼12 kDa) is shown in .
Each subunit of CEP-37247 has a theoretical molecular weight of 38 kDa based upon the amino acid sequence. Following gene synthesis and cloning of the gene encoding the CEP-37247 protein sequence into a proprietary mammalian expression vector, a protein of approximately 78 kDa in size was expressed from transiently transfected CHOK1 cells. Consistent with the predicted molecular weight, the 78 kDa protein was found to reduce to two ∼40 kDa subunits when analyzed by reduced SDS PAGE analysis (data not shown). The slight increase in size over the theoretical molecular weight is due to the presence of N-linked glycan structures at the Asn191 located within the Fc portion of the molecule.
The lead domain antibody (prior to fusion to Fc) was found to have a TNFα binding affinity of 2.1 nM as assessed by surface plasmon resonance analysis (SPR; ). It was also found to inhibit the cytotoxicity of human TNFα in a dose-dependant fashion in the L929 assay (). The Fc-linked version of the dAb, CEP-37247 was also assessed for its ability to bind human TNFα and inhibit its activity. As shown in and C we found that by dimerizing the dAb into the Fc-linked construct CEP-37247, the binding affinity increased 6.6-fold to 325 pM. The Fc-linked construct was found to be approximately 200 times more potent than the un-linked version in TNFα inhibition, as determined using the L929 cell based assay.
Expression of CEP-37247.
Cell lines stably expressing CEP-37247 were created by transfecting CHOK1 host cells that were pre-adapted to suspension growth in a commercially available, fully chemically defined, protein-free, animal component-free medium. The mammalian expression vector was linearized and mixed with cells in exponential growth phase before electroporation. Following transfection, cells were selected for growth rate and productivity levels, resulting in the identification of a lead cell line. To date, a range of fermentation runs, both at laboratory 10 L scale and at 200 L GMP scale, have been undertaken using a fed batch process, with harvest titers approaching 7 g/L obtained.
Following fermentation, CEP-37247 was affinity purified via Protein A binding to the Fc region and then further purified by ion exchange before concentration to 50 mg/ml in a final formulation buffer. Following production, CEP-37247 was stored at 2–8°C.
CEP-37247 displays high specificity in binding to TNFα.
Binding kinetics of CEP-37247 to a range of TNF superfamily member ligands were determined by a SPR approach. CEP-37247 was captured on the CM5 chip surface via immobilized Protein A using a Biacore 3000. Each of nineteen TNF superfamily member ligands, including both TNFα and lymphotoxin (TNFβ), were passed over the captured CEP-37247, and binding was detected by change in response units. As shown in , of the nineteen TNF superfamily member ligands tested, CEP-37247 shows high affinity only for TNFα. In contrast, when the anti-TNF Fc receptor construct etanercept was captured by immobilized Protein A in a similar experiment (), binding to TNFα, lymphotoxin and the lymphotoxin heterotrimers LTα1β2 and LTα2β1 was detected.
CEP-37247 binds to membrane-associated TNFα and mediates antibody dependant cell-mediated cytotoxicity.
As detailed above, we demonstrated by SPR that CEP-37247 is able to bind to TNFα in solution. Membrane-associated TNFα has been implicated to be therapeutically relevant.Citation11,Citation12 In vivo, TNFα is also found in a membrane-associated presentation on a range of immune system cells including activated T cells and monocytes, macrophages, dendritic and NK cells.Citation13–Citation15 To demonstrate the ability of CEP-37247 to bind to membrane-associated TNFα, we created a NS0 cell line transfected to stably express membrane-associated TNFα. As detected by flow cytometry using a fluorescently labeled Fc-specific secondary antibody, CEP-37247 was able to bind to NS0 cells stably expressing membrane-associated human TNFα, while an IgG1 isotype control full-size antibody was unable to bind ().
We also evaluated the ability of etanercept to bind to membrane-associated TNFα, and demonstrated that etanercept was able to bind in a manner similar to that seen for CEP-37247 (). CEP-37247, etanercept and the IgG1 isotype control full-size antibody were also tested in this flow cytometry assay against an untransfected NS0 host cell line that did not express membrane associated TNFα. No binding of any of these three proteins was detected (data not shown).
Having demonstrated that both CEP-37247 and etanercept are able to bind to membrane-associated TNFα, studies were performed to determine whether these molecules were able to activate effector functions as a result of this binding. This was investigated by incubating increasing levels of CEP-37247, etanercept or IgG1 isotype control with both effector cells isolated from individual human donors and the NS0 cell line stably expressing membrane-associated TNFα. A representative result using effector cells from one human donor is shown in . CEP-37247 triggered efficient killing of the NS0 cells in a dose-dependent manner while no cell-killing was detected for the IgG1 isotype control or etanercept. Comparable results were found for each of three individual human donors.
CEP-37247 is able to neutralize the cytotoxic effects of TNFα in vivo.
CEP-37247 was also found effectively to reduce both the onset and severity of polyarthritic symptoms in the Tg197 transgenic mouse model. These mice overexpress the human TNFα gene and have been shown to develop chronic polyarthritis with 100% incidence at 4–7 weeks of age.Citation16
Following intraperitoneal administration twice a week from 3 weeks of age, CEP-37247 effectively reduces the onset and severity of polyarthritis in a dose-responsive manner (). These findings are evidenced in the continuous assessments of both mean arthritic score () and mean body weight (data not shown), as well as in the mean histopathology score, which is calculated by analysis of the ankle joints following necropsy at 10 weeks of age (). Statistical analysis of the mean arthritic score and histopathology score data using ANOVA was performed to determine if these findings were significantly different from the results obtained for the mice treated with the IgG1 irrelevant specificity isotype control (palivizumab). Treatment of mice with CEP-37247 at 10 mg/kg and 3 mg/kg showed significant improvements in both mean arthritic scores (p < 0.001) and histopathology scores (p < 0.001). Treatment with 1 mg/kg showed a significant improvement in mean arthritic score only (p < 0.05).
For the calculation of mean arthritic score, both ankle joints on each animal were assessed weekly for appearance, stiffness in paw flexion and joint movement. The mean of two scores from each of the eight animals in each group was calculated. The onset of polyarthritic symptoms also affects the general well-being of the animals and their body weight.Citation16 When treatment with CEP-37247 and etanercept alleviated the symptoms of the polyarthritis, mice gained body weight in a manner not seen in the control animals (data not shown).
At the end of treatment at 10 weeks of age, the mice were sacrificed and histopathological analysis of the ankle joints was performed. Sections were scored based upon the prevalence of pathologies, including hyperplasia of the synovial membrane and presence of polymorphonuclear infiltrates (score = 1); pannus and fibrous tissue formation and focal subchondrial bone erosion (score = 2); cartilage destruction and bone erosion (score = 3); and extensive cartilage destruction and bone erosion (score = 4). Treatment with increasing doses of CEP-37247 was found to reduce the histopathological score with a 10 mg/kg dose reporting a score of 1.44 ± 0.20 (mean ± SEM for eight mice). This is in comparison to treatment with 10 mg/kg of the isotype control (palivizumab), where a histopathological score of 3.50 ± 0.13 was determined. Interestingly, the histopathological score of the highest CEP-37247 dose group at the completion of the study (1.44 ± 0.20) was very similar to the group sacrificed at 3 weeks, i.e., at the start of the onset of the polyarthritic symptoms (0.90 ± 0.23).
Example histopathology sections from mice dosed with IgG1 isotype control and CEP-37247 at 10 mg/kg are shown in and D, respectively. Following dosing with the IgG1 isotype control and the onset of polyarthritic symptoms, the ankle joints exhibited high levels of both cartilage destruction and bone erosion along with hyperplasia of the synovial membranes. There was evidence of polymorphonuclear cell infiltration and poor definition in the synovial joint space. In contrast, following treatment with CEP-37247, clear definition of the synovial joint space was still seen with no evidence of hyperplasia, cartilage destruction or bone erosion. Polymorphonuclear cell infiltration was present, but at very low levels.
We also conducted similar Tg197 experiments where we have demonstrated that etanercept was also able to reduce the onset and severity of polyarthritic symptoms in a dose-responsive manner. Significant improvements in both mean arthritic score (p < 0.001) and histopathology score (p < 0.001) were recorded at 10 mg/kg and 3 mg/kg dose levels. No significant improvements were recorded at the 1 or 0.3 mg/kg dose levels.
To date, we have undertaken three separate Tg197 transgenic mouse studies to evaluate the dose responsiveness of CEP-37247 and etanercept. Although the studies were well controlled, being an in vivo system, there was some degree of variation in the findings of each study. A comparison of the percentage response of terminal mean arthritic scores of groups of eight mice treated with either one of four doses of CEP-37247 or one of four doses of etanercept is shown in . The percentage response was calculated by comparing the terminal mean arthritic score for each group against the score achieved for the group of mice treated with the IgG1 isotype control in the same study. These data were calculated for each of the three studies we have undertaken and indicate that CEP-37247 was able to reduce the increase of mean arthritic score in the Tg197 transgenic mouse model at similar levels to etanercept.
CEP-37247 has antibody-like pharmacokinetics following administration to primates.
In previous experiments, we demonstrated that CEP-37247 binds with high affinity to cynomolgus macaque TNFα and is able to compete against the interaction of cynomolgus macaque TNFα against its cognate receptor (data not shown). Based on these findings, we selected cynomolgus macaques as a relevant species in pre-clinical safety and toxicology studies.
CEP-37247 was administered by intravenous bolus infusion to three male and three female cynomolgus macaques at a concentration of 50 mg/kg. Plasma samples were taken and analyzed with the levels of CEP-37247 present determined against a standard curve using a validated pharmacokinetic ELISA assay.
Analysis of the results indicated that maximum plasma concentrations (Cmax) generally occurred at the first sampling occasion (10 minutes post-dose), and then declined in a bi-phasic manner. The elimination half-lives calculated were in the range of 87 to 110 hours in individual male and female animals. The total plasma clearance was found to be slow and the apparent volume of distribution values suggested that the distribution of CEP-37247 was limited, and may mainly be confined, to the central compartment, i.e., to blood plasma and extracellular fluid.
The mean (±SD) plasma concentrations measured for each sex are presented in with the calculated pharmacokinetic parameters presented in .
The pharmacokinetics of CEP-37247 was also assessed following subcutaneous administration at 0.5, 5.0 and 50 mg/kg. Three male and three female cynomolgus macaques were dosed at each dose level and plasma samples were taken and analyzed, with the levels of CEP-37247 present determined against a standard curve using a validated pharmacokinetic ELISA assay.
Analysis of the results indicated that following single subcutaneous administration of CEP-37247, plasma drug concentrations increased slowly to reach maximum levels (Cmax) at the blood sampling times of 24–48 h (tmax) in the majority of animals. No change in tmax was noted with increasing doses. The calculated elimination half-lives ranged from 86.8–132 h in individual animals and were independent of dose.
The systemic exposure of macaques to CEP-37247 following subcutaneous administration was generally independent of sex with AUC0–336 h and Cmax values being similar in both sexes across the 0.5 to 50 mg/kg dose range.
The mean (±SD) plasma concentrations measured for each sex at each dose level are presented in with the calculated pharmacokinetic parameters presented in .
The bioavailability following subcutaneous administration at 50 mg/kg was calculated to be 90% for male and 75% for female macaques based upon the calculated AUC0–336 h.
Discussion
We described here the characterization and preclinical data for the first human framework domain antibody construct to enter clinical studies. CEP-37247 broadly resembles a conventional antibody in that it comprises moieties that mediate antigen recognition and effector functions; however, whereas each of the two antigen-recognition arms of a conventional antibody is comprised of four protein domains (VH, CH1, VL and CL), each of the two antigen-recognition arms of the CEP-37247 construct comprises only a single (VL) domain. Hence, CEP-37247 is significantly smaller and structurally simpler than a conventional antibody. To evaluate whether these characteristics would significantly affect CEP-37247's behavior and whether CEP-37247 has suitable properties to warrant further development as a therapeutic, we assessed multiple characteristics of CEP-37247; results are discussed here, particularly with respect to conventional antibodies.
Given that the objective of generating CEP-37247 was to create an anti-TNF therapeutic, a primary requirement was high affinity and selective binding of TNFα. Our data showed that the VL dAb component of this construct, isolated by phage display, was sufficient to mediate high affinity binding. This dAb (as an Fc-lacking monomer) was able to bind to TNFα with an equilibrium dissociation constant (KD) in the nanomolar range as determined by SPR analysis. When formatted as a bivalent Fc construct, we showed that affinity was enhanced 6.6-fold, presumably due to avidity effects. Our data also showed that this construct was sufficient to fulfill another key antigen-recognition function, i.e., selectivity. When analyzed by SPR against a panel of closely related TNF superfamily members, CEP-37247 was highly selective for TNFα. This result contrasts with that for etanercept, which bound to multiple members of the TNF ligand superfamily.
For therapeutic purposes, however, high affinity binding of TNFα is not enough; neutralization of the effects of TNFα is paramount. This consideration was built into our anti-TNF dAb isolation strategy through incorporation of the TNF-mediated L929 cytotoxicity assay as a screening step. Although the epitope footprint of a dAb is likely to be smaller than that of the VH-VL pair in a conventional antibody, it is clear that the isolated anti-TNF dAb component was sufficient to disrupt the binding of TNFα to its cognate receptor, as evidenced by inhibition of TNF-mediated cytotoxicity towards L929 cells (). Further, when formatted as a bivalent Fc construct (CEP-37247), antagonism of TNF activity was enhanced 200-fold.
For protein therapeutics, the elicitation of anti-drug antibodies is a major concern. It can reduce efficacy by altering pharmacokinetics or neutralizing target recognition, cause infusion- or hypersensitivity-reactions and lead to therapeutic failure or discontinuation of development.Citation17 Even with established anti-TNF therapies, 20–40% of patients fail to significantly respond to initial treatment.Citation18 When patients do respond, there is growing evidence of significant dosage increase of both the anti-TNF and disease-modifying anti-rheumatic drug co-therapies (DMARDs) being required over time due to the immunogenicity of the anti-TNF agent. This is particularly exemplified by treatment with infliximab, where average dose increases of 12 and 18% after 1 and 2 years treatment of rheumatoid arthritis have been reported.Citation19 These findings correlate well with the prevalence of anti-infliximab antibodies in rheumatoid arthritis patients over a number of studies being reported as between 12 and 44%. In these studies, the prevalence of anti-infliximab antibodies was found to be inversely proportional to therapeutic response.Citation20 Even with escalating doses to maintain response a subset of patients may start to fail to respond to treatment, thereby requiring either termination of therapy or transfer to another therapeutic option.Citation21
As antibody generation technologies have developed and the resulting therapeutics have evolved from murine to chimeric to humanized molecules, the levels of reported immunogenicity have fallen. This reduction in immunogenicity has been attributable largely to removal of non-human sequence from the antibody framework.Citation22 However, even fully human antibodies, such as the anti-TNF adalimumab, are associated with an anti-drug antibody response in patients,Citation20 which suggests that there is scope for other strategies to further reduce immunogenicity.
Anti-drug antibody responses, also known as HAHA (human anti-human antibody) responses are often directed against the complementarity determining regions (CDRs) or the junctions between the framework and CDRs. This is due to the hypervariable nature of the CDRs.Citation23 A conventional antibody has six unique CDRs (three on each of two VH and VL domains). CEP-37247 has just three unique CDRs (three on each VL domain). Thus, the overall risk of immunogenicity is theoretically reduced in a domain antibody construct. Together with its human germline framework, the probability of immunogenicity of CEP-37247 is reduced; however, the overall immunogenic profile of CEP-37247 is likely to be known only after accumulation of clinical data.
It has been speculated that Fc-mediated effector function such as ADCC may play a role in the clinical efficacy of anti-TNF antibodies.Citation24,Citation25 It has been demonstrated that infliximab and adalimumab, but not etanercept, act on cells expressing the cellular membrane-bound form of TNFα by binding to the TNFα on these cells via the variable regions of the antibody and recruiting effector cells through interaction of the Fc region of the antibody with FcγRII and FcγRIII receptors on effector cells.Citation26 We investigated whether CEP-37247 was capable of binding to membrane-associated TNFα. In a flow cytometric assay using a stably transfected membrane-associated TNF-expressing NS0 cell line, we were able to demonstrate binding of CEP-37247 to these cells. Further, utilizing the same NS0 cells as targets, we were able to demonstrate dose-dependent and highly active ADCC activity mediated by CEP-37247. In this respect, CEP-37247 behaves like a conventional anti-TNF antibody. This was not a foregone conclusion, given the observation that etanercept, a TNF-R2-Fc fusion, was reported by Arora et al.Citation26 to be less capable of inducing ADCC activity than conventional anti-TNF antibodies. The result is in accord with our own observation that, when used as a comparator, we were able to demonstrate binding of etanercept to the membrane-associated TNF-expressing NS0 cell line, but little ADCC activity.
In vivo efficacy of CEP-37247 was demonstrated in a well-validated model of polyarthritis. Tg197 mice are transgenic for and overexpress human TNFα. This leads to the onset of inflammation manifested in increased joint swelling and distortion associated with toe deformation and reduced strength on paw flexion. These symptoms are reminiscent of the clinical symptoms of rheumatoid arthritis.Citation16 When CEP-37247 was administered at various doses, given intraperitoneally twice a week from three weeks of age, both the speed of onset and severity of these symptoms were reduced.
The Tg197 model has been extensively used by a number of investigators as a robust way to explore the efficacy of potent anti-arthritic compounds, as well as to investigate the mechanisms that are involved in the pathogenesis of chronic inflammatory arthritis.Citation27–Citation29 The results we have presented demonstrate that CEP-37247 has anti-arthritic activity, preventing both the onset and suppressing the progression of joint pathology in a dose-dependant manner. We have demonstrated similar clinical and histopathologic results over three separate studies of groups of Tg197 mice treated with four different doses (0.3, 1, 3 and 10 mg/kg CEP-37247 twice weekly). We have shown that CEP-37247 is at least as potent as the marketed anti-TNF drug etanercept in this model.
These data therefore confirm that CEP-37247 is able to mediate anti-TNFα activity in vivo and domain antibody Fc constructs are a viable means by which to treat autoimmune, and potentially other, diseases.
We have reported the creation of a cell line for the commercial manufacture of CEP-37247 that has a fermentation productivity approaching 7 g/L. Based upon a fully chemically defined, animal component-free, fed batch process, we have successfully manufactured at both 10 L laboratory scale and 200 L GMP scale. These titers, which exceed the typical titers of 1–3 g/L observed for conventional antibodies, have been obtained through minimal process optimization. It is likely that these high titers have been achieved as a result of the simpler, homodimeric structural nature of CEP-37247 compared with the heterotetrameric structure of a conventional antibody. During the expression of a full-size mAb, individual light chains complex with individual heavy chains before these complexes form disulfide bridges and are secreted. This process requires four subunits to be assembled in a coordinated process prior to secretion. There is evidence that the levels of both heavy and light chains must be precisely balanced to ensure optimal expression.Citation30,Citation31 In contrast, secretion of CEP-37247 involves the disulfide bridging of only two subunits that are identical. Furthermore, as a result of the shorter length of the protein subunits required to produce CEP-37247, a lower metabolic load is experienced by the cells. At 78 kDa CEP-37247 is approximately half the size of a conventional mAb, and is composed of approximately half the number of amino acids. We therefore speculate that the high titer production process that we developed for CEP-37247 is a result of the characteristics of the molecule, and similar high expression levels may be achieved for other domain antibody constructs that may be developed.
Following production and purification, we have successfully formulated CEP-37247 at 50 mg/ml. Such high concentration formulations are required for mAb therapies to allow the required dose to be administered in a reasonable volume—usually not more than 1 ml. Domain antibodies have been reported to show high levels of aggregation upon concentration or prolonged standing at 4°C. This is thought to result from exposure of hydrophobic interfaces on the VL region that would normally be packed up against the VH region in a full antibody.Citation1,Citation32 In reformatting the lead domain antibody as CEP-37247, we experienced neither increased levels of aggregation even at 50 mg/ml nor instability in liquid formulation when stored at 4°C. As such, CEP-37247 and other domain antibody constructs exhibit characteristics that make them suitable for clinical application, i.e., formulation to high concentrations and storage at readily available temperatures in pharmacies, clinics and in the homes of patients.
The half-life of a domain antibody has been reported to be 42 min in miceCitation2 and thereafter the molecule is rapidly degraded and processed. A half-life in humans of this magnitude would limit use as systemic treatments, especially of chronic diseases. One example of a therapeutic recombinant protein with a short half-life is anakinra (Kineret™), an interleukin-1 receptor antagonist. With a serum half-life in humans of approximately 4 h, anakinra requires daily dosing at 100 mg for the effective treatment of rheumatoid arthritis.Citation33 By comparison, conventional mAbs have a half-lives in the order of 4–20 days in humans, with these half-lives represented amongst the different currently available anti-TNF therapies.Citation34
For domain antibodies to have competitive and patient-friendly treatment regimens, means of extending the half-life have been explored, with Fc fusion being the alternative we selected. The Fc region is responsible for the long half-life of conventional antibodies because it interacts with, and is recycled by, the FcRn receptor. A domain antibody with an Fc region should theoretically be recycled by the FcRn, and therefore provide increased residence time in the circulation.
We investigated the pharmacokinetics of CEP-37247 in the cynomolgus macaque after demonstrating that this primate was a relevant species (data not shown). CEP-37247 was given as either a single intravenous dose of 50 mg/kg or a single subcutaneous dose of 0.5, 5 and 50 mg/kg. The half-life of CEP-37247 of 3.5–5.5 days obtained for both routes of administration (range of 86–132 h) confirmed that addition of an Fc region dramatically increased the potential circulating half-life of the domain antibody. The half-life also appeared to be unaffected by dose level in the subcutaneous treatment groups.
By comparison, for etanercept half lives of 77, 50 and 46 h were reported for subcutaneous dosing of cynomolgus macaques at 1, 5 or 15 mg/kg.Citation35 For adalimumab, dosing cynomolgus macaques at 1, 3 or 10 mg/kg gave mean half lives of 76, 132 and 104 h, respectively.Citation36 These results indicate that the domain antibody construct CEP-37247 has a circulatory half-life in cynomolgus macaques around double that of etanercept and similar to that of adalimumab. Should the human circulatory half-life of CEP-37247 also be similar to that of adalimumab, which is reported to be in the range of 15–19 days, then two weekly dosing regimens can be considered for CEP-37247.Citation37 Thus, addition of the Fc region to our anti-TNF domain antibody has potentially transformed it from a molecule with a very short half-life to one that is competitive with other conventional biologics targeting TNFα.
Successful treatment of inflammatory diseases by TNF-targeting drugs is proposed to follow the therapeutic window model.Citation38 Patients suffering diseases such as rheumatoid arthritis or Crohn disease exhibit elevated levels of TNFα in both serum and synovial fluid or serum and gut mucosa, respectively. Administration of an anti-TNF therapeutic should eliminate these pathological TNFα levels; however, significant decreases in TNFα levels increases the risk of infection as TNFα is also a key mediator of host defense and homeostasis. It is therefore important to dose the therapeutic anti-TNF at such a level, i.e., the therapeutic window, where there are neither safety concerns from exceeding a maximum safe concentration nor efficacy concerns from falling below an effective drug level. The two weekly dosing interval of adalimumab, based upon its 15–19 day published half-life, provides a constant level of exposure to drug with no high peaks or low troughs in drug concentration. For CEP-37247, a human serum half-life in the range of 14 days would allow for two weekly dosing regimens to be considered, making CEP-37247 and other domain antibody constructs suitable candidates for further clinical development.
Half-life is a composite pharmacokinetic parameter determined by both the clearance and the volume of distribution. It is increased by an increase in volume of distribution or decrease in clearance, or vice versa. The half-life of CEP-37247 in cynomolgus macaques benefits from both the low level of clearance and the high volume of distribution seen. The volume of distribution (∼150 ml/kg) was found to be greater than the plasma volume (∼45 ml/kg in monkeys) and approximately equate to extracellular fluid (∼200 ml/kg in monkeys).Citation39 These findings support the hypothesis that CEP-37247, as a result of its smaller size, has a wider tissue distribution than a full antibody. This may provide the potential for better efficacy in therapeutic applications, especially where, e.g., in rheumatoid arthritis, the site of disease pathology is outside of the circulation.
Other pharmacokinetic parameters calculated from the single intravenous and single subcutaneous doses of CEP-37247 in cynomolgus macaques were as expected. Increasing subcutaneous doses led to approximate dose proportional increases in exposure and Cmax. Bioavailability following subcutaneous administration was found to be 90% for male and 75% for female macaques. These high levels of bioavailability indicate that CEP-37247 is suitable for subcutaneous administration.
In summary, we described CEP-37247, a novel anti-TNF domain antibody construct that, as a result of protein engineering of a dAb onto an antibody Fc region, incorporates affinity and selectivity for TNFα, in vitro TNFα antagonism, Fc receptor-mediated activities such as ADCC, desirable pharmacokinetic profile, and in vivo potency. At half the size of a conventional antibody, CEP-37247 has full antibody functionality, but the benefits of a simpler molecular structure, lower production costs and the potential for low immunogenicity and improved tissue distribution. Each of these characteristics will be further investigated and defined as CEP-37247 progresses through development in the clinic.
Materials and Methods
Surface Plasmon Resonance methods for analysis of binding to TNF and superfamily proteins.
The experiments were performed using a Biacore 3000 biosensor and reagents (GE Healthcare) at room temperature.
Analysis of TNFα binding to the dAb construct was performed through affinity capture of the His-tagged dAb to an NTA sensor chip. Briefly, the surface was primed with 0.01 M HEPES, 0.15 M NaCl, 50 µM EDTA, 0.005% Surfactant P20, pH 7.4 (running buffer) prior to saturation of the NTA by a 20 µl pulse of 500 µM NiCl2 in running buffer (20 µl/min). The surface was washed thoroughly with running buffer prior to injection of the His-tagged dAb (200 nM in running buffer) (2 µl/min). Once the baseline stabilized TNFα was injected (5 µl/min). The surface was regenerated between each analysis with repeat 20 µl injections of 0.01 M HEPES, 0.15 M NaCl, 0.35 M EDTA, 0.005% Surfactant P20, pH 7.4 (regeneration buffer). The typical loading level of Ni2+ was 100 RU consistent with saturating levels and capture of the His-tagged dAb was in the range 2,500–3,000 RU.
For immobilization of CEP-37247 and etanercept recombinant Protein A (Sigma) was immobilized onto a CM5 sensor chip (Certified grade) using the Biacore immobilization wizard program. Immobilization was carried out using a 20 µg/ml solution of Protein A in 10 mM sodium acetate, pH 4.5 for a target density of 2,000 RU. The carboxymethylated dextran surface of the sensor chip was activated with the NHS-EDC solution prior to the injection of the recombinant Protein A for covalent attachment. Following coupling to the sensor surface, residual activated ester groups were blocked and washed with injections of 1 M ethanolamine hydrochloride, pH 8.5.
CEP-37247 (50 mg/ml) and etanercept (25 mg/ml) were diluted to 5 µg/ml in HBS-P (10 mM HEPES, pH 7.4 containing 150 mM NaCl and 0.05% (v/v) Tween 20). The capture of CEP-37247 or etanercept onto the Protein A immobilized surface was performed at a flow rate of 20 µl/min for 2 min using HBS-P buffer. Typical captured levels of CEP-37247 and etanercept were ∼5,000 and ∼1,100 RU, respectively. Minimal dissociations of either of the two molecules were observed.
Binding to the following TNF super family member proteins was assessed: TNFα, APRIL, EDA-A2, LIGHT, EDA, OX40 Ligand, CD30L, βNGF, Lymphotoxin α1/β2, Lymphotoxin α2/β1 (all from R&D Systems), TNFβ, sFas Ligand, TL-1A, sRANKL, 4-1BBL, TWEAK, AITRL, BAFF, sCD40L and TRAIL (all from PeproTech Inc.). Each protein was diluted to 5 µg/ml in HBS-P before injection onto the CM5 surface previously primed with immobilized Protein A and captured CEP-37247 or etanercept. Injections were performed at a flow rate of 20 µl/min for 2 min.
Between measurements, the sensor chip surface was regenerated with two × one minute injections of 10 mM glycine pH 2.0 at a flow rate of 20 µl/min. The ability of the immobilized protein A to bind CEP-37247 or etanercept was found to be unaffected by the regeneration condition.
L929 TNFα-mediated cytotoxicity assay.
Serial half log dilutions of anti-TNF dAb and dAb-Fc in RPMI medium (containing 10% fetal bovine serum and 2 mM L-glutamine) were prepared in 50 µl volume ranging from 52.5-0.0005 µg/ml across eleven wells in 96-well flat bottom plates. To each of the test wells, 25 µl of human TNFα (1.5 ng/ml), 25 µl actinomycin D (40 µg/ml) and 50 µl L929 cells (5 × 105 cells/ml) were added. Controls included a TNFα standard curve ranging from 3,125 pg/ml to 0.172 pg/ml across eleven wells, wells containing no TNFα (100% viability) and no cells (background). Assay plates were incubated at 37°C in a 5% CO2 humidified incubator for 20 hours then a further 2 hours after the addition of 30 µl 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-terazolium (MTS)/phenazine ethosulfate (PES). Absorbance was read at 492 nm and viability curves calculated using the average absorbance of duplicate wells. Viability curves were fitted to a sigmoidal dose model using GraphPad software (Prism®).
Tg197 transgenic mouse model.
All studies were performed by Biomedcode Hellas SA, Vari, Greece.
Briefly, from 3 weeks of age, groups of eight mice were dosed twice weekly by intraperitoneal injections of pre-blinded samples of doses of CEP-37247, etanercept, palivizumab (irrelevant specificity isotype control) and saline. Once a week assessment of morphological changes of ankle joints and body weights were made. At 10 weeks of age all mice were sacrificed and ankle joints were preserved in formalin. Joints were then embedded in paraffin, sectioned and stained with hematoxylin and eosin stain before histopathological evaluation of disease progression. The assessments of both macroscopic and microscopic changes in joint morphology were performed by the same technician using established scoring systems. These assessments from both ankle joints from each of the eight mice in each group led to the calculation of mean arthritic scores and mean histopathology scores for each treatment group.
Stable membrane-associated TNF cell line.
The sequence for the expression of human TNFα was modified to omit amino acids 1 to 12 (VRS SSR TPS DKP) which incorporate the recognition sequence of the TNFα converting enzyme (TACE) which is responsible for cleaving the expressed TNFα and allowing the 17 kDa TNFα monomer to shed from the cell membrane at which point it spontaneously forms a trimer.Citation15,Citation40,Citation41 The modified sequence was gene synthesized and cloned into a proprietary mammalian expression vector and stable cell lines established following standard cell culture methods.
Flow cytometry methods.
NS0 cells stably transfected to express membrane associated human TNFα were washed and resuspended at 1 × 106 cells/sample of 1x PBS (containing 2% FCS). Cells were incubated alone or in the presence of 100 µg/ml IgG1 Kappa, etanercept or CEP-37247 for one hour at room temperature, washed and incubated with FITC-conjugated secondary Fc-specific antibody (Sigma Aldrich). After incubation for one hour, samples were washed before final re-suspension in buffer and analysis by flow cytometry (Cell Lab Quanta 488; Becton Coulter).
ADCC method.
Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat fraction of anticoagulated blood from a number of individual donors using Lymphoprep (Axis Shield) and repeated centrifugation at room temperature. Following isolation the PBMCs were washed and centrifuged three times at 4°C in the presence of 1x PBS containing 0.1% BSA and 2 mM EDTA pH7.4 (Sigma Aldrich). Finally the PBMCs were resuspended in assay media [phenol red free RPMI medium (Sigma Aldrich) containing 0.5% FCS (Bovogen)] at 1 × 107 viable cells/ml and allowed to rest at 37°C for 30 min before use.
NS0 cells stably transfected to express membrane associated human TNFα were used as target cells. Cells, detached from adherent cell cultures by tapping the flasks, were resuspended at 2 × 105 viable cells/ml in assay media.
Serial dilution of CEP-37247, etanercept and an IgG1 isotype control (Sigma Aldrich) in assay media were also prepared.
For the assay, 100 µl of target cells were mixed in wells of a round bottomed tissue culture 96-well plate with 50 µl PBMC effector cells and 50 µl of the diluted protein to be tested. Four control mixtures were also prepared:
(1) Background control-200 µl assay media only, (2) Low control-100 µl target cells and 100 µl assay media, (3) High control-100 µl target cells and 100 µl 2% Triton-X-100 in assay media and (4) Spontaneous lysis control-100 µl target cells, 50 µl PBMC effector cells and 50 µl assay media. Once a complete plate of samples had been prepared the plate was centrifuged at 1,500 rpm for 2 min and incubated at 37°C for 4 h. Following incubation plates were again centrifuged and 100 µl from each well was assessed for LDH release using the Cytotoxicity Detection Kit (LDH) from Roche Applied Science.
The percentage cytotoxicity was calculated was calculated as follows:
Pharmacokinetic analysis.
Following intravenous dosing plasma samples were taken 10 and 30 min post infusion then at 1, 4, 24, 48, 96, 168, 240 and 336 h. A pre-dose plasma sample was also taken. All samples were analyzed and the quantity of CEP-37247 present determined against a standard curve using a validated pharmacokinetic ELISA assay.
Following subcutaneous dosing plasma samples were taken 30 min post dose then at 1, 2, 6, 24, 48, 96, 168, 240 and 336 h. A pre-dose plasma sample was also taken. All samples were analyzed and the quantity of CEP-37247 present determined against a standard curve using a validated pharmacokinetic ELISA assay.
For the pharmacokinetic ELISA assay, ELISA plates were coated with a solution of recombinant human TNFα (PeproTech Inc.) and were stored at 4°C overnight. The plates were treated with assay diluent (phosphate buffered saline supplemented with 5% skimmed milk) to decrease non-specific binding, incubated for 1 h at 25°C and washed with wash buffer (phosphate buffered saline supplemented with 0.05% Tween-20). Plasma samples, QCs and CEP-37247 standards were added to the appropriate wells and the plates incubated for 1 h at 25°C. The plates were then washed to remove any unbound CEP-37247, treated with a 1 in 20,000 dilution of horse radish peroxidize conjugated goat anti-human IgG secondary antibody (Zymed Laboratories) and incubated for a further hour at 25°C. The plates were again washed, TMB substrate solution (Europa Bioproducts) added and incubated at 25°C for 20 min before the addition of stop solution (1.8% v/v sulfuric acid in water). Finally, within 20 min of the addition of stop solution plates were read at 450 nm (630 nm reference) on a Dynex MRX plate reader (Thermo Laboratories).
Pharmacokinetic analyses were conducted on the data obtained using a validated pharmacokinetic software package (WinNonlin Version 4.0.1 Enterprise, Pharsight Corporation). The following toxicokinetic parameters were, if possible, determined from the plasma concentration-time data of CEP-37247 using non-compartmental procedures: AUC0-336 h, area under the plasma concentration-time curve from time zero up to 336 h post-dose; Cmax, maximum observed plasma concentration; tmax, time of maximum observed plasma concentration; t½, apparent terminal elimination half-life; CL, total plasma clearance; Vz, apparent volume of distribution during the terminal phase.
Abbreviations
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| AUC | = | area under the curve |
| CDR | = | complementarity determining region |
| dAb | = | domain antibody |
| DMARD | = | disease modifying anti-rheumatic drug |
| ELISA | = | enzyme-linked immunosorbent assay |
| IgG | = | immunoglobulin G |
| Fc | = | fragment crystalizable |
| PBMCs | = | peripheral blood mononuclear cells; RU, response unit |
| SPR | = | surface plasmon resonance |
| TNF | = | tumor necrosis factor |
Ethical Statement
All animal studies reported herein were undertaken with adherence to the ethical frameworks prescribed for the performance of experiments on animals according to the country where the studies took place.
Figures and Tables
Figure 1 Schematic structures of a full-size antibody and a domain antibody in comparison to CEP-37247. Schematic representations of (A) A conventional full-sized monoclonal antibody of approximate size 150 kDa consisting of a dimer of variable heavy (VH) and variable light (VL) domains responsible for antigen recognition fused to four constant regions, one on the light chain (CL) and three on the heavy chain denoted CH1, CH2 and CH3. A hinge between the CH1 and CH2 constant regions provides flexibility and dimerization via disulfide bonds. (B) CEP-37247—a bivalent dAb-Fc construct approximately half the size of a conventional antibody (78 kDa) comprising a dimer of only the variable light (VL) domain fused to a truncated CH1, the hinge and CH2 and CH3 domains of the heavy chain. The disulfide bridges between the two heavy chains in both molecules and between the CH1 and CL domains of the full antibody are indicated as black lines. (C) The monomeric variable light chain domain antibody is, at approximately 12 kDa, the smallest antibody domain that is capable of high affinity binding of target antigen.
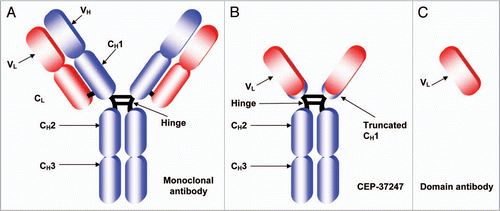
Figure 2 Fc-formatting of the anti-TNFα dAb increases ligand affinity and in vitro potency. (A) SPR analysis of the interaction of human TNFα with the lead domain antibody. Purified His-tagged domain antibody protein was immobilized on the sensor chip surface through metal chelation to a NTA surface. Human TNFα (4.5 nM black line, and then in ascending order 36, 72, 144, 288, 431 and 575 nM) was injected over at a flow rate of 5 µl/min. (B) SPR analysis of the interaction of human TNFα with CEP-37247. CEP-37247 was captured onto Protein A covalently immobilized to a CM5 sensor chip using NHS/EDC chemistry. CEP-37247 was captured with high affinity. Human TNFα (18 nM light blue line, and then in ascending order, 36, 72, 144, 288 and 575 nM) was injected over at a flow rate of 20 µl/min. Data was referenced with blank subtraction and fitted to a 1:1 Langmuirian model using BIAevaluation 3.0 BIAcore software. (C) The effect of Fc-formatting on in vitro potency was assessed using the TNFα sensitive L929 cell line. Increasing concentrations of either the lead anti-TNFα dAb (black circle) or CEP-37247 (red square) were incubated in the presence of human TNFα and the neutralization of TNFα-mediated cytotoxicity was measured.
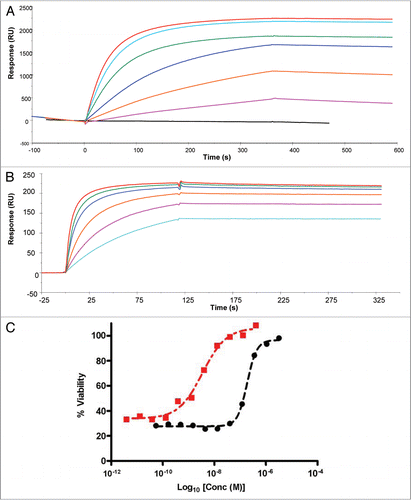
Figure 3 CEP-37247 displays high specificity for TNFα. CEP-37247 (A) or etanercept (B) were immobilized on the sensor surface using Protein A capture. Equal concentrations (5 µg/ml) of 19 different purified TNF super family member proteins were injected (20 µl/min for 2 min) over the surface and their ability to bind either CEP-37247 or etanercept was monitored by increase in response units. The surface was regenerated between injections with 10 mM Glycine pH 2.0. In (A) CEP-37247 is shown to be capable of binding to TNFβ (black line) alone. In (B) etanercept is shown to be capable of binding to TNFα (black line), TNFβ (dark red line), Lymphotoxin α2/β1 (orange line) and Lymphotoxin α1/β2 (green line) with different kinetics. TNFa is TNFα, TNFb is TNFβ, LTa2b1 is Lymphotoxin α2/β1 and LTa1b2 is Lymphotoxin β1/β2.
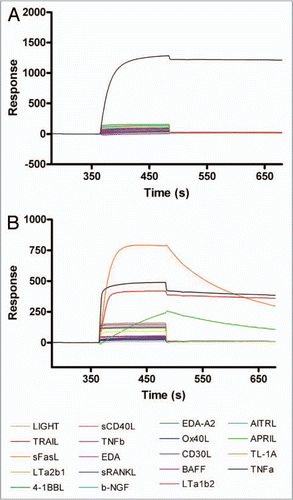
Figure 4 CEP-37247 mediates antibody-dependent cellular cytotoxicity. CEP-37247 was able to mediate ADCC of a membrane associated TNFα expressing target cell line in response to incubation with human peripheral blood mononuclear cells (PBMCs). (A) The target cells, stably transfected NS0 cells expressing membrane-associated TNFα were incubated with CEP-37247 (red line), etanercept (blue line) or IgG1 isotype control (black line) followed by fluorescein-conjugated anti-human IgG, Fc specific antibody. Following incubation, the cells were analyzed by flow cytometry. The population exhibited homogeneous fluorescence of similar intensities with both CEP-37247 and etanercept. The isotype control exhibited fluorescence comparable to background levels that were also seen for the incubation of CEP-37247, etanercept and the isotype control with untransfected NS0 host cells (data not shown). (B) The membrane associated TNFα expressing NS0 cells target cells (2 × 104 cells) were incubated with 5 × 105 PBMCs (effector cells) and increasing concentrations of either CEP-37247 (solid red circle), etanercept (solid blue square) or IgG1 isotype control (unfilled square). This is a representative dataset of three experiments using PBMCs from three different individual donors.
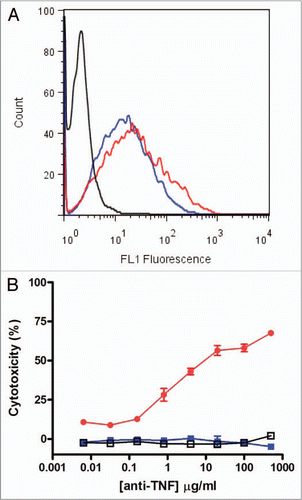
Figure 5 CEP-37247 has potent anti-arthritic properties in vivo in the Tg197 transgenic mouse model. (A) Weekly assessment of arthritic scores of groups of 8 Tg197 transgenic mice dosed with four different doses of CEP-37247 (green square, 0.3; red square, 1; blue triangle, 3; black circle, 10 mg/kg) or isotype control antibody (palivizumab, dark red diamond, 10 mg/kg) twice weekly from 3 weeks until 10 weeks of age. The score range runs from a base line of normal appearance where paw flexion is seen to a point where ankylosis is detected upon flexion and movement is severely impaired. Each ankle joint of each animal was scored weekly and the mean (±SEM) calculated. Significant result at week 10 as determined by ANOVA are indicated (**p < 0.001 or *p < 0.05) when compared to mean arthritic score of group dosed with IgG1 isotype control (palivizumab). (B) Mean histopathology scores (±SEM) after sacrifice at week 10 were calculated for groups of 8 Tg197 transgenic mice dosed with four different doses of CEP-37247 (0.3, 1, 3 or 10 mg/kg) or an isotype control (palivizumab 10 mg/kg) twice weekly from 3 weeks of age until 10 weeks of age. Fixed sections were scored based upon the prevalence of pathologies, including hyperplasia of the synovial membrane and presence of polymorphonuclear infiltrates (score = 1), pannus and fibrous tissue formation and focal subchondrial bone erosion (score = 2), cartilage destruction and bone erosion (score = 3), and extensive cartilage destruction and bone erosion (score = 4). Each ankle joint of each animal was scored and the mean ± SEM calculated. Significant result at week 10 as determined by ANOVA are indicated (**p < 0.001 or *p < 0.05) when compared to mean histopathology scores of the group dosed with IgG1 isotype control (palivizumab). As a further control, four mice were sacrificed at 3 weeks of age, at the point of onset of the polyarthritic symptoms, and their ankle joint sections assessed for histopathological score. Each ankle joint of each animal was scored and the mean (±SEM) calculated. (C) Fixed section of Tg197 mouse ankle joint prepared after sacrifice at 10 weeks of age following twice weekly treatment with isotype control (palivizumab). The joint shows clear signs of severe onset of arthritic symptoms including erosion of bone (open triangle) and cartilage along with a high level of polymorphonuclear cell infiltration (down arrow). The synovial joint space is compressed (closed triangle) with some evidence of pannus formation at the left side of the joint. (D) Fixed section of Tg197 mouse ankle joint prepared after sacrifice at 10 weeks of age following twice-weekly treatment with 10 mg/kg CEP-37247. The joint has been protected from the onset of polyarthritic symptoms and maintains a healthy cartilage (open triangle) with no evidence of bone erosion. The synovial joint space is well defined (closed triangle) and there is some evidence of minor polymorphonuclear cell infiltration on the right side of the joint (down arrow).
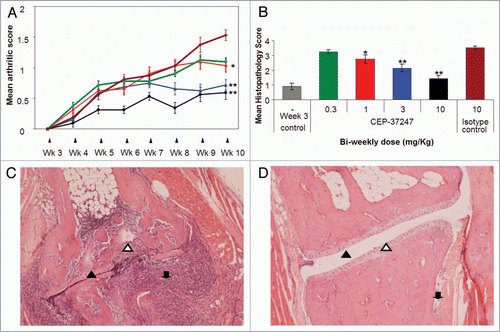
Figure 6 CEP-37247 is at least as potent as etanercept in the Tg197 transgenic mouse model. Mean arthritic scores for the treatment of 24 groups of 8 Tg197 mice across three separate studies were compared. Mice were treated with CEP-37247 (three groups each at doses of 0.3, 1, 3 and 10 mg/kg) and etanercept (three groups each at doses of 0.3, 1, 3 and 10 mg/kg). In each study, the mean arthritic score at week 10 for each treatment group was compared to the mean arthritic score for treatment with the IgG1 irrelevant isotype control (palivizumab, 10 mg/kg) and a percentage response score calculated. The percentage response scores for treatment with both CEP-37247 (red triangle) and etanercept (black square) are plotted.
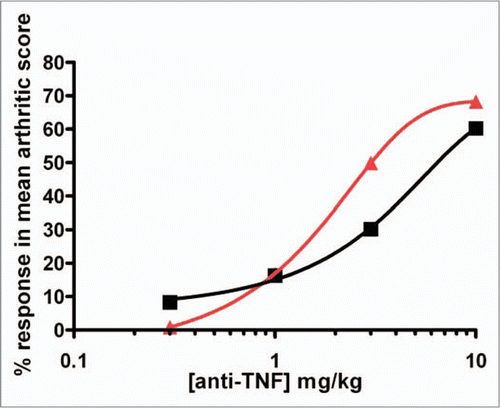
Figure 7 Pharmacokinetics of CEP-37247 in cynomolgus macaques. (A) Mean plasma levels (±SD) of CEP-37247 following the administration of a 50 mg/kg single bolus intravenous dose to three male (blue diamond) and three female (pink square) cynomolgus macaques. (B) Mean plasma levels (±SD) of CEP-37247 following the administration of three different single subcutaneous doses of CEP-37247 to groups of three male and three female cynomolgus macaques. Doses given were 0.5 mg/kg (males, blue diamond; females, pink square), 5.0 mg/kg (males, blue triangle; females, red square) and 50.0 mg/kg (males, purple star; females, dark red circle).
Table 1A Summary of mean (±SD) pharmacokinetic parameters determined for the administration of a single intravenous dose to a group of three male and three female cynomolgus macaques
Table 1B Summary of mean (±SD) pharmacokinetic parameters determined for the administration of single subcutaneous doses at three different dose levels to groups of three male and three female cynomolgus macaques
Acknowledgements
All authors are current or former employees of Cephalon Australia Pty Ltd. The authors would like to thank: Ben Woolven, Neil Brewis, Ian Tomlinson, Steve Grant and Jenny Lee from Domantis for their collaborative work isolating and affinity maturing the lead domain antibody, and the Australian Red Cross Blood Service for provision of human buffy coat samples.
References
- Ward ES, Gussow D, Griffiths AD, Jones PT, Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 1989; 341:544 - 546
- Holt LJ, Basran A, Jones K, Chorlton J, Jespers LS, Brewis ND, et al. Anti-serum albumin domain antibodies for extending the half-lives of short lived drugs. Protein Eng Des Sel 2008; 21:283 - 288
- Feldmann M. Maini RN, Anti-TNFalpha therapy of rheumatoid arthritis: what have we learned?. Annu Rev Immunol 2001; 19:163 - 196
- Hochberg MC, Lebwohl MG, Plevy SE, Hobbs KF, Yocum DE. The benefit/risk profile of TNF-blocking agents: findings of a consensus panel. Semin Arthritis Rheum 2005; 34:819 - 836
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000; 343:1586 - 1593
- Emery P, Genovese MC, van Vollenhoven R, Sharp JT, Patra K, Sasso EH. Less radiographic progression with adalimumab plus methotrexate versus methotrexate monotherapy across the spectrum of clinical response in early rheumatoid arthritis. J Rheumatol 2009; 36:1429 - 1441
- La Merie Business Intelligence. Top 20 biologics—2008. R&D Pipeline News 2009 1:1 - 25
- Tansey MG, Szymkowski DE. The TNF superfamily in 2009: new pathways, new indications and new drugs. Drug Discov Today 2009; 14:1082 - 1088
- Butler M. Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biot 2005; 68:283 - 291
- Langer ES. Trends in capacity utilization for therapeutic monoclonal antibody production. mAbs 2009; 1:151 - 156
- Chensue SW,, Remick DG, Shmyr-Forsch C, Beals TF, Kunkel SL. Immunohistochemical demonstration of cytoplasmic and membrane-associated tumor necrosis factor in murine macrophages. Am J Pathol 1988; 133:564 - 572
- Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell 1990; 63:251 - 258
- Kinkhabwala M, Sehajpal P, Skolnik E, Smith D, Sharma VK, Vlassara H, et al. A novel addition to the T cell repertory. Cell surface expression of tumor necrosis factor/cachectin by activated normal human T cells. J Exp Med 1990; 171:941 - 946
- Luettig B, Decker T, Lohmann-Matthes ML. Evidence for the existence of two forms of membrane tumor necrosis factor: an integral protein and a molecule attached to its receptor. J Immunol 1989; 143:4034 - 4038
- Dinarello CA. Differences between anti-tumor necrosis factor-alpha monoclonal antibodies and soluble TNF receptors in host defense impairment. J Rheumatol Suppl 2005; 74:40 - 47
- Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 1991; 10:4025 - 4031
- Rau R, Sander O, van Riel P, van de Putte L, Hasler F, Zaug M, et al. Intravenous human recombinant tumor necrosis factor receptor p55-Fc IgG1 fusion protein Ro 45-2081 (lenercept): a double blind, placebo controlled dose-finding study in rheumatoid arthritis. J Rheumatol 2003; 30:680 - 690
- Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther 2009; 11:1
- Finckh A, Simard JF, Gabay C, Guerne PA. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis 2006; 65:746 - 752
- Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, Bonfa E. Immunogenicity of Anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol 2010; 38:82 - 89
- Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007; 56:13 - 20
- Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods 2005; 36:3 - 10
- Harding FA, Stickler MM, Razo J, Dubridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. mAbs 2:256 - 265
- Tutuncu Z, Kavanaugh A, Zvaifler N, Corr M, Deutsch R, Boyle D. Fcgamma receptor type IIIA polymorphisms influence treatment outcomes in patients with inflammatory arthritis treated with tumor necrosis factor alpha-blocking agents. Arthritis Rheum 2005; 52:2693 - 2696
- Louis E, El Ghoul Z, Vermeire S, Dall'Ozzo S, Rutgeerts P, Paintaud G, et al. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn's disease. Aliment Pharmacol Ther 2004; 19:511 - 959
- Arora T, Padaki R, Liu L, Hamburger AE, Ellison AR, Stevens SR, et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine 2009; 45:124 - 131
- Redlich K, Hayer S, Maier A, Dunstan CR, Tohidast-Akrad M, Lang S, et al. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum 2002; 46:785 - 792
- Bessis N, Chiocchia G, Kollias G, Minty A, Fournier C, Fradelizi D, et al. Modulation of proinflammatory cytokine production in tumour necrosis factor-alpha (TNFalpha)-transgenic mice by treatment with cells engineered to secrete IL-4, IL-10 or IL-13. Clin Exp Immunol 1998; 111:391 - 696
- Schett G, Hayer S, Tohidast-Akrad M, Schmid BJ, Lang S, Turk B, et al. Adenovirus-based overexpression of tissue inhibitor of metalloproteinases 1 reduces tissue damage in the joints of tumor necrosis factor alpha transgenic mice. Arthritis Rheum 2001; 44:2888 - 2898
- Borth N, Strutzenberger K, Kunert R, Steinfellner W, Katinger H. Analysis of changes during subclone development and ageing of human antibody-producing heterohybridoma cells by northern blot and flow cytometry. J Biotechnol 1999; 67:57 - 66
- Schlatter S, Stansfield SH, Dinnis DM, Racher AJ, Birch JR, James DC. On the optimal ratio of heavy to light chain genes for efficient recombinant antibody production by CHO cells. Biotechnol Prog 2005; 21:122 - 133
- Jespers L, Schon O, James LC, Veprintsev D, Winter G. Crystal structure of HEL4, a soluble, refoldable human V(H) single domain with a germ-line scaffold. J Mol Biol 2004; 337:893 - 903
- Chang DM, Chang SY, Yeh MK, Lai JH. The pharmacokinetics of interleukin-1 receptor antagonist in Chinese subjects with rheumatoid arthritis. Pharmacol Res 2004; 50:371 - 376
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117:244 - 279
- Immunex Corporation. Nonclinical Pharmacology and Toxicology Review of BLA 98-0286 1998; www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm088697.pdf
- Abbott Laboratories. Pharmacology Review of BLA 125057/0 2002; www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm092772.pdf
- Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, et al. Efficacy, pharmacokinetic and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther 2003; 25:1700 - 1721
- Nestorov I. Clinical pharmacokinetics of TNF antagonists: how do they differ?. Semin Arthritis Rheum 2005; 34:12 - 18
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res 1993; 10:1093 - 1095
- Wang AM, Creasey AA, Ladner MB, Lin LS, Strickler J, Van Arsdell JN, et al. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science 1985; 228:149 - 154
- Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNFalpha and activates immune effector functions. Cytokine 1995; 7:251 - 259