Abstract
The use of antibodies in transplantation dates back to 1986 when muromonab CD3, a monoclonal antibody (mAb) targeting CD3, was first approved for prevention and treatment of renal allograft rejection. These agents have largely been used in a brief adjunctive manner to provide immunosuppression during the initial period after solid organ transplantation or during an episode of acute rejection. Recent advances in our understanding of transplant immunology have allowed emergence of numerous new mAbs, targeting co-stimulatory signals, cell surface receptors and novel protein constructs. During the next decade, transplant professionals will increasingly require knowledge of the mechanisms and pharmacologic characteristics of these novel therapeutic agents.
Introduction
In part I of this review, several antibody therapies currently in clinical use were discussed.Citation1 Here, experiences with use of newer antibodies and other agents targeting cytokines, co-stimulatory signals, certain surface molecules and various B-cell epitopes involved in the rejection process of allograft are explored ().
Co-Stimulatory Pathway
Activation of T cells requires both antigen-specific (signal 1) and co-stimulatory (signal 2) pathways. Naïve T cells contain the CD (cluster designation) 28 receptor to which ligands CD80 and CD86 may potentially bind to produce the co-stimulatory signal. Once T cells become active, the inhibitory molecule cytotoxic T-lymphocyte antigen 4 (CTLA4) is expressed to prevent further T-cell co-stimulation ().
Immunosuppression targeting the co-stimulatory pathway became available in 2005 when the CTLA4-immunoglobulin G fusion protein abatacept (Orencia®; Bristol-Myers Squibb, Princeton, NJ, USA) first underwent clinical trial to prevent acute rejection and prolong graft survival. After encouraging results in transplanted rodents, abatacept failed to sustain graft survival in non-human primates (NHP) due to weaker affinity for CD86 compared to CD80. Shortly thereafter, a CTLA4-immunoglobulin G fusion protein more specific for CD80, belatacept (Bristol-Myers Squibb, Princeton, NJ, USA), yielded better renal allograft survival in NHP.Citation2 Belatacept has also proven itself as a calcineurin inhibitor (CNI) replacement that does not compromise acute rejection rates. The goal of research on co-stimulatory blockades, however, is not only to keep acute rejection rates low, but also to improve long-term outcomes through the induction of tolerance.
In a Phase 3 trial (BENEFIT, Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial), belatacept was compared to cyclosporine as a component of maintenance immunosuppression in kidney transplant recipients. In this 666-patient trial, Vicenti et al. studied whether co-stimulatory pathway blockade could be used as maintenance immunosuppression instead of cyclosporine without adverse effects on survival, cardiovascular or renal profiles.Citation3 Although the BENEFIT showed a slightly higher incidence of early acute rejection in the belatacept group, these same patients had similar graft and patient survival rates at one year post-transplant compared to the cyclosporine group. Furthermore, the belatacept patients had superior renal, metabolic and cardiovascular function profiles. The study will continue to follow its patients for a total of three years in order to assess long-term outcomes.
Blockade of signal 2 by belatacept raises questions regarding its effects on regulatory T cell (Treg) function, as these cells depend on the CD28-CD80/86 interaction in addition to the interleukin-2 (IL-2)-mediated pathway. Because Tregs play a crucial role in immune tolerance, long-term belatacept usage could have a negative impact on chronic rejection rates. Bluestone et al. studied the concomitant effects of basiliximab, a monoclonal antibody (mAb) against IL-2 receptor, and belatacept in renal transplant recipients. The study showed that belatacept (CD28-CD80/86 blockade) in combination with basiliximab (IL-2 blockade) did not affect Tregs in the long-term compared to CNI regimens.Citation4
Anti-Adhesion Agents
Leukocyte function-associated antigen 1 (LFA-1) serves as an adhesion molecule in the communication between antigen presenting cells (APC) and T cells. Although primarily found on the surface of T cells, LFA-1 can also be found on B cells where it facilitates antigen presentation. LFA-1 has also been implicated in lymphocyte migration to target sites. Given these important roles in the immune response, LFA-1 has become a target of interest in transplant research.Citation5
Efalizumab (Raptiva; Genentech, San Francisco, CA, USA), a humanized LFA-1 IgG1 antibody, was initially approved for the treatment of psoriasis in 2003. Vincenti and colleagues studied efalizumab treatment in 38 kidney transplant recipients against a background of maintenance immunosuppression.Citation6 Four groups of patients were given high- or low-dose efalizumab biweekly injections with either full-dose cyclosporine, mycophenolate mofetil and corticosteroids or half-dose cyclosporine, sirolimus and corticosteroids. Although acute rejection rates remained low at six months in all groups, 8% of patients in the group receiving high-dose efalizumab and high-dose CNIs experienced early post-transplant lymphoproliferative disease (PTLD). The authors concluded that efalizumab's long-term safety and efficacy in renal transplant patients warrants further evaluation in larger prospective trials; however, efalizumab was voluntarily withdrawn by Genentech in 2009 due to an increased risk of progressive multifocal leukoencephalopathy (PML) observed in patients being treated for psoriasis.Citation7
Alefacept (Amevive®; Astellas, Tokyo, Japan), an LFA-3/IgG1 fusion protein, prevents the interaction of CD2 and LFA-3 binding. While this interaction has been deemed important in naïve T-cell activation, alefacept has also shown an ability to deplete peripheral blood memory T cells.Citation8 Weaver and colleagues studied the effects of alefacept combined with a co-stimulatory blockade by CTLA4-Ig and sirolimus and showed prolonged renal allograft survival in NHP. Alefacept's contribution to immunosuppression was attributed to its effect on T memory cells.Citation9 In combination with traditional immunosuppressive agents, alefacept is now being investigated in Phase 2 studies with renal allograft recipients.Citation10
Complement Pathway Inhibition
Rejection of a transplanted allograft may be mediated by either T cells (cellular rejection) or B cells (antibody-mediated rejection). The presence of antibodies specific for donor human leukocyte antigen (HLA) and ABO antigens increases the risk of antibody-mediated rejection (AMR). Still, detection of these antibodies and desensitization protocols make transplantation with blood-type and HLA incompatibilities possible. AMR is treated with intravenous immune globulin (IVIg), plasmapheresis and rituximab (Rituxan®; Genentech, San Francisco, CA, USA), an anti-CD-20 antibody. Pathologically, AMR in renal allograft is characterized by C4d+ staining of peritubular capillaries resulting from complement activation, although the exact role of complement in AMR is not entirely understood.Citation11,Citation12
Eculizumab (Soliris®; Alexion, Cheshire, CT, USA) is a humanized mAb that specifically binds complement 5 (C5), thereby inhibiting the formation of the membrane attack complex (MAC) and preventing cell lysis and death. Eculizumab is currently only approved in the United States for treatment of paroxysmal nocturnal hemoglobinuria,Citation13 but Locke and colleagues recently reported a case of severe AMR in a kidney transplant recipient that was successfully treated with eculizumab in conjunction with plasmapheresis, IVIg and rituximab. The authors concluded that eculizumab's benefit resulted from the prevention of antibody-induced damage while plasmapheresis and IVIg cleared donor-specific antibodies.Citation14
Ischemia and Reperfusion Injury Prevention
Ischemia and reperfusion injury (IRI) remains a problem in organ transplantation. IRI may delay allograft function, increase the incidence of acute and chronic rejection and increase morbidity and mortality.Citation15 During IRI, oxygen free radicals and inflammatory cytokines are generated, ultimately leading to apoptosis and cell death.
Selectins, cellular adhesion molecules expressed on endothelial cells, are responsible for the binding of leukocytes and platelets to sinusoidal endothelial cells in the initial phase of IRI. P-selectin specifically attracts platelets to the site of inflammation via P-selectin glycoprotein ligand 1 (PSLG-1), ultimately leading to leukocyte infiltration as well.Citation16 In animal models, P-selectin blockade has been shown to reduce IRI. By infusing P-selectin IgG mAb, Garcia-Criado and colleagues demonstrated a statistically-significant reduction in hepatic injury in rat models.Citation17 Tsuchihashi et al. confirmed this observation by studying the effect of P-selectin blockade during reperfusion injury in ex vivo rat livers. With infusion of PSLG-1 antibody prior to reperfusion, the study showed a reduction in hepatic injury and inflammation.Citation18 Although the specific pathophysiology of IRI has been elucidated, targeted P-selectin blockade has not made its way into clinical practice.
The complement cascade is another potential target in treating reperfusion injury. Cardiopulmonary bypass can cause a systemic inflammatory response in which the complement cascade appears to play a major role. TP-10, a recombinant soluble complement receptor type 1 (sCR1), has been studied in patients who are placed on cardiopulmonary bypass while undergoing cardiac surgery and lung transplantation. TP-10 blocks C3 and C5 convertases that are responsible for the formation of MACs.Citation19
Reperfusion of a transplanted allograft initiates a release of chemokines, which are proteins responsible for recruitment of leukocytes from blood into the reperfused tissue. CXC ligand 8 (CXCL8) binds to its receptors to promote neutrophil attraction. Accordingly, blockade of the CXCL8 receptors CXCR1 and CXCR2 results in decreased neutrophil infiltration and tissue damage.Citation20 Cugini et al. studied the effects of repertaxin, a noncompetitive inhibitor of CXCR1 and CXCR2, in a rat model following renal reperfusion. The study showed that repertaxin successfully blocked the signaling pathway via CXCR2, thus preventing granulocyte infiltration and subsequent kidney impairment.Citation21 Repertaxin also demonstrated a preventative effect against liver and intestine reperfusion injury in rat models.Citation20
Finally, small interfering RNAs (siRNAs) may also have a future role in the prevention of reperfusion injury. QPI-1002, a siRNA formerly known as AKI-5, selectively inhibits the expression of p53. This gene is expressed in times of cell stress and is responsible for the activation of cellular pathways that ultimately lead to cell death. If p53 is temporarily disabled, cell death will be delayed and repair mechanisms may have enough time to reverse cell injury. QPI-1002 showed promising results in rats and is undergoing Phase 1/2 clinical trials for the prevention of renal reperfusion injury in humans undergoing cardiopulmonary bypass.Citation22
Anti-B-Cell Therapies
Empirically, most anti-lymphocyte induction therapies have targeted the T cell; however, B cells may play an important role in antigen presentation, T-cell activation and alloantibody production that drives transplant rejection. A variety of new treatments, therefore, are currently being explored to deplete B-cell populations in the hopes of reducing acute rejection episodes and preserving long-term graft survival.
Although antibody-producing plasma cells do not display this antigenic marker, all mature B cells uniquely express CD20. The anti-CD20 rituximab has a long history of successful use in depleting CD20-expressing B cells in the setting of malignancies and PTLD. In kidney transplant patients, after CD20+ cells were shown to be present in large numbers in those with steroid-resistant rejection episodes, rituximab was proposed as an induction agent. In 2004, Becker et al. reported that single-dose rituximab reversed steroid-refractory kidney rejection episodes in 24 of 27 patients.Citation23 Shortly thereafter, Genberg et al. showed that single-dose rituximab significantly improved graft outcomes in ABO-incompatible patients and eliminated the need for co-surgical splenectomy (graft survival of 86.7% at 3 years mean follow up, equivalent to the graft survival in the ABO-compatible control group).Citation24 Attempting to broaden the spectrum of rituximab use, the same group recently conducted a prospective, double-blind, multicenter randomized trial comparing 68 kidney transplant patients treated with rituximab to 68 placebo-controlled patients. Rituximab or placebo was administered along with tacrolimus, mycophenolate mofetil and steroids. There were only eight biopsy-proven rejection episodes in the rituximab arm compared to 12 in the placebo group, although this result was not statistically significant (p = 0.317). They reported no statistically-significant difference in the overall incidence of cytomegalovirus, BK virus, bacterial or fungal infections between the two groups.Citation25
In 2009, Clatworthy et al. reported results of a study that compared single-dose rituximab with a well-established induction agent, daclizumab, in nonsensitized kidney transplant patients.Citation26 They attempted to enroll 120 patients in this study, but it was suspended after acute rejection rates were deemed too high in the rituximab arm. Five of the six rituximab patients experienced biopsy-proven acute rejection (BPAR) episodes within the first three months post-transplant, compared to only one of seven in the daclizumab group (83% versus 14%, respectively, p = 0.01).Citation26 The current data, therefore, suggests that rituximab may be useful as an induction agent in high-risk patients who are ABO-incompatible recipients or who experience steroid-resistant rejection episodes, but it may not be safe for more generalized organ transplant induction therapy.
A number of other case reports and studies have raised concerns regarding potential complications of rituximab use for AMR. Kamar et al. compared infectious complications among 77 rituximab-treated patients (2–8 h course for AMR episodes) with 902 retrospectively-reviewed control patients. Although the rate of infection was similarly high in both groups (45.5% rituximab-treated vs. 53.9% controls), the mortality rate was significantly higher in the rituximab group (9.1% vs. 1.6%).Citation27 Other reports have documented cases of late-onset Pneumocystis jiroveci pneumonia,Citation28 cryptogenic organizing pneumoniaCitation29 and JC virus associated PMLCitation30 in patients treated with rituximab in post-transplant period. These potential complications show that the particular indications for rituximab usage must be further elucidated through larger-scale randomized trials that compare rituximab with conventional therapies.
In a manner similar to rituximab, epratuzumab (Immunomedics, Morris Plains, NJ, USA) targets and depletes B-cell lines through antibody-dependent cell-mediated cytotoxicity. However, epratuzumab targets the surface antigen CD22, which, although not present on immature B or plasma cells, is increasingly expressed on certain differentiating B cells. Unlike rituximab, epratuzumab may preferentially deplete B cells committed to antibody production or memory functions rather than nonspecific lineage depletion.Citation31 Epratuzumab was originally developed for treatment of non-Hodgkin lymphoma, but has since been used to treat a variety of autoimmune inflammatory disorders, most notably systemic lupus erythematosus (SLE), as well. Its potential in organ transplant induction therapy is yet to be determined, although its indications may be similar to those of rituximab.
Belimumab (Human Genome Sciences, Rockville, MD, USA) is a human mAb that targets and inhibits B lymphocyte stimulator (BLyS), a vital B-cell surface protein survival factor. BLyS has two important signaling properties: (i) preventing apoptosis and (ii) stimulating differentiation to plasma cells.Citation32 By preventing this signaling, belimumab induces apoptosis and prevents immunoglobulin production. A marketing application for belimumab as a treatment for SLE was submitted to the United States Food and Drug Administration (US FDA) in June 2010 and the mAb is still in clinical studies for a variety of other autoimmune autoantibody disorders, but no trials are currently underway to evaluate belimumab's potential in post-transplant induction therapy.
Cytokine Targets
Cytokines play an extremely important role in the development and maintenance of the alloimmune response against a foreign graft. As such, a variety of these molecular pathways have been targeted as a means of immunosuppression and graft preservation, most successfully with IL-2 receptor (CD25) inhibitors such as daclizumab and basiliximab.Citation1 Other cytokine receptor-targeting mAbs that have been established in the treatment of autoimmune inflammatory disorders may soon be in the developmental pipeline for use in post-transplant induction therapy.
Interleukin 6 (IL-6) is a pleiotropic cytokine that supports a variety of processes, including the inflammatory response, cartilage and bone metabolism and hematopoiesis.Citation33 It exerts its immunomodulatory effects by (i) enhancing T-cell activation and differentiation by upregulating IL-2 receptors and IL-2 production, (ii) inducing thymocyte proliferation and thymic T-cell development, (iii) stimulating B-cell proliferation and (iv) activating acute-phase protein production.Citation34 Of note, IL-6 knockout mice show impaired abilities to localize lymphocytes to inflammatory foci and transition from a polymorphonuclear infiltrate to a mononuclear one, as occurs in chronic injury.Citation35,Citation36 The effects of IL-6 are mediated through the IL-6 receptor, which is expressed in all tissue types. Tocilizumab (Roche, San Francisco, CA, USA) is a humanized mAb that binds the IL-6 receptor (IL-6R) in both membrane-bound and soluble forms and consequently inhibits IL-6-mediated signaling. It is principally used in the treatment of rheumatoid arthritis, although it is being tested for use in other inflammatory autoimmune diseases. Because elevated levels of IL-6 have been observed in allograft recipients, tocilizumab may have a future role in transplant induction therapy.Citation37
Tumor necrosis factor (TNF), a pleiotropic cytokine important in the systemic immune response and induction of the acute phase proteins, has a paradoxical array of activating and pro-apoptotic influences. Its effects are mediated through TNF receptors, which are expressed on every somatic cell (with the exception of erythrocytes). TNF is primarily produced by macrophages in response to bacterial antigens such as lipopolysaccharide, although TNF is commonly elevated in a variety of other inflammatory scenarios. Infliximab (Remicade®; Centocor, Horsham, PA, USA) is a chimeric mAb that targets both soluble and membrane-bound forms of TNF. While the precise mechanism of action remains unclear, at least two things are known: (i) infliximab binds and neutralizes TNFCitation38 and (ii) it induces apoptosis in active T cells.Citation39,Citation40 Initially approved by the FDA for use in treating Crohn disease, infliximab has since been approved for use in ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis and plaque psoriasis. After serum TNF levels were shown to be significantly elevated during acute rejection episodes in kidney transplant patients relative to non-rejecting controls,Citation41 infliximab has been used as an off-label induction agent in several recent pilots. There is great interest in the forthcoming results of these studies, especially in light of contrary evidence from Karczerski et al. stating that TNF levels are not significantly elevated during acute rejection episodes in kidney allograft recipients.Citation41 This would suggest a TNF-independent rejection mechanism, one that infliximab would be unlikely to counteract.
Conclusion
The therapeutic use and potentials of available mAbs in transplant medicine have been explored here. Many of these agents have already delivered promising results by prolonging graft survival while minimizing complications. With better understanding of transplant biology and alloresponse, one sees an explosive growth of highly targeted therapies towards: signaling points, surface molecules and receptors, putative cytokines, complement products and novel protein constructs. mAbs raised against them will likely play a more prominent role in the prevention and treatment of allograft rejection and transplant management. Understanding the uses of such agents will be essential to improving the outcomes of solid organ transplant recipients.
Figures and Tables
Figure 1 Mechanisms of T-cell activation and proliferation targeted by drugs. New agents in transplantation target various mechanisms of T-cell activation and proliferation with the intent to minimize calcineurin inhibitor use and improve long-term outcomes. CD, cluster of differentiation; MHC, major histocompatibility complex; NK, natural killer; PSGL, P-selectin glycoprotein ligand; sCR, soluble complement receptor; TCR, T-cell receptor.
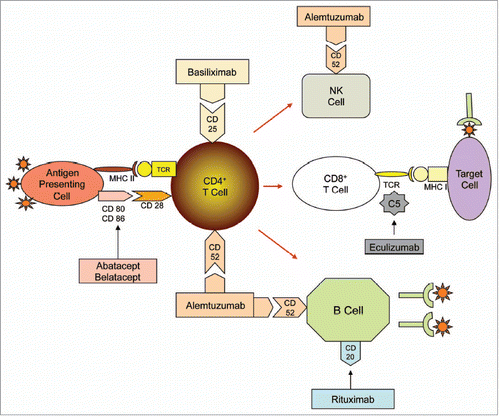
Table 1 Drugs approved or in clinical studies for allograft rejection
Note
Part I of this manuscript was previously published online at www.landesbioscience.com/journals/mabs/article/11159/
Mahumud N, Klipa D, Ahsan N. Antibody immunosuppressive therapy in solid-organ transplant: Part I mAbs 2010 2 148 156 http://dx.doi.org/10.4161/mabs.2.2.11159.
References
- Mahmud N, Klipa D, Ahsan N. Antibody immunosuppressive therapy in solid organ transplant—part I. mAbs 2010; 2:148 - 156
- Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high affinity variant of CTLA4Ig with potent immunosuppressive properties. Am J Transplant 2005; 5:443 - 453
- Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A pIII Study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010; 10:535 - 546
- Bluestone JA, Liu W, Yabu JM, Laszig ZG, Putnam A, Belingheri M, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant 2008; 10:2086 - 2096
- Nicolls MR, Gill RG. LFA-1 (CD11a) as a therapeutic target. Am J Transplant 2006; 6:27 - 36
- Vincenti F, Mendez R, Pescovitz M, Rajagopalan PR, Wilkinson AH, Butt K. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant 2007; 7:1770 - 1777
- United States Food and Drug Administration March 1 2010 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm133337.htm
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. NEJM 2001; 345:248 - 255
- Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV. Alefacept promotes co-stimulation blockade based allograft survival in primates. Nat Med 2009; 15:746 - 749
- Astellas Pharma Inc., study NCT00543569 September 12 2010 http://clinicaltrials.gov/ct2/show/NCT00543569?term=alefacept&rank=21
- Manthey HD, Woodruff TM, Taylor SM, Monk PN. Complement component 5a (C5a). Int J Biochem Cell Biol 2009; 41:2114 - 2117
- Stegall MD, Dean PG, Gloor J. Mechanism of alloantibody production in sensitized renal allograft recipients. Am J Transplant 2009; 9:998 - 1005
- Stegall MD, Gloor JM. Deciphering antibody-mediated rejection: new insights into mechanisms and treatment. Curr Opin Organ Transplant 2010; 15:8 - 10
- Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant 2009; 9:231 - 235
- Henderson JM. Liver transplantation and rejection: an overview. Hepatogastroenterology 1999; 46:1482 - 1484
- Morell CN, Sun H, Swain AM, Baldwin WM. Platelets an inflammatory force in transplantation. Am J Transplant 2007; 7:2447 - 2454
- Garcia-Criado FJ, Toledo-Pereyra LH, Lopez-Neblina F, Phillips ML, Paez-Rollys A, Misawa K. Role of P-selectin in total hepatic ischemia and reperfusion. J Am Coll Surg 1995; 181:324 - 334
- Tsuchihashi S, Fondevila C, Shaw DG, Lorenz M, Marquette K, Benard S. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J Immunol 2006; 176:616 - 624
- Li JS, Jaggers J, Anderson PA. The use of TP10, soluble complement receptor 1, in cardiopulmonary bypass. Expert Rev Cardiovasc Ther 2006; 4:649 - 654
- Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR + CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther 2006; 112:139 - 149
- Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int 2005; 67:1753 - 1761
- Quark Pharmaceuticals September 12 2010 http://quarkpharma.com/qbi-en/products/qpi-1002
- Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant 2004; 4:996 - 1001
- Genberg H, Kumlien G, Wennberg L, Berg U, Tydén G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation 2004; 85:996 - 1001
- Tydén G, Genberg H, Tollemar J, Ekberg H, Persson NH, Tufveson G. A randomized, doubleblind, placebo-controlled, study of single-dose rituximab as induction in renal transplantation. Transplantation 2009; 87:1325 - 1329
- Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med 2009; 360:2683 - 2685
- Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Mohamed AO, et al. Incidence and predictive factors for infectious diseases after rituximab therapy in kidney transplant patients. Am J Transplant 2010; 10:89 - 98
- Shelton E, Yong M, Cohney S. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology 2009; 14:696 - 699
- Bitzan M, Ouahed JD, Carpineta L, Bernard C, Bell LE. Cryptogenic organizing pneumomnia after rituximab therapy for presumed post-kidney transplant lymphoproliferative disease. Pediatr Nephrol 2010; 25:1163 - 1167
- Kamar N, Mengelle C, Rostaing L. Incidence of JC-virus replication after rituximab therapy in solid-organ transplant patients. Am J Transplant 2009; 9:244 - 245
- Vincenti F, Kirk AD. What's next in the pipeline. Am J Transplant 2008; 8:1972 - 1981
- Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 2000; 192:953 - 964
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008; 58:2968 - 2980
- Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 2006; 2:2
- Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997; 6:315 - 325
- Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001; 14:705 - 714
- Karczewski M, Karczewski J, Poniedzialek B, Wiktorowicz K, Smietanska M, Glyda M. Distinct cytokine patterns in different states of kidney allograft function. Transplant Proc 2009; 41:4147 - 4149
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002; 301:418 - 426
- Baert FJ, D'Haens GR, Peeters M, Hiele MI, Shaible TF, Shealy D, et al. Tumor necrosis factor-α antibody (infliximab) therapy profoundly downregulates the inflammation in Crohn's ileocolitis. Gastroenterology 1999; 116:22 - 28
- Di Sabatino A, Ciccocioppo R, Cinque B, Millimaggi D, Morera R, Ricevuti L, et al. Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn's disease. Gut 2004; 53:70 - 77
- Hribova P, Kotsch K, Brabcova I, Vitko S, Volk HD, Lacha J. Cytokines and chemokine gene expression in human kidney transplantation. Transplant Proc 2005; 37:760 - 763