Abstract
Brentuximab vedotin (SGN-35; Adcetris®) is an anti-CD30 antibody conjugated via a protease-cleavable linker to the potent anti-microtubule agent monomethyl auristatin E (MMAE). Following binding to CD30, brentuximab vedotin is rapidly internalized and transported to lysosomes where MMAE is released and binds to tubulin, leading to cell cycle arrest and apoptosis.
Several trials have shown durable antitumor activity with a manageable safety profile in patients with relapsed/refractory Hodgkin lymphoma, systemic anaplastic large cell lymphoma, or primary cutaneous CD30-positive lymphoproliferative disorders. Peripheral sensory neuropathy is a significant adverse event associated with brentuximab vedotin administration. Neuropathy symptoms are cumulative and dose-related. Multiple ongoing trials are currently evaluating brentuximab vedotin alone or in combination with other agents in relapsed/refractory patients, as well as patients with newly diagnosed disease.
Introduction
The successful application of immunotherapeutics in lymphoproliferative disorders, such as the anti-CD20 monoclonal antibodies (mAb) rituximab and ofatumumab, the anti-CD52 mAb alemtuzumab, and the radiolabelled antibodies 131I-tositumomab (Bexxar®) and 90Y-ibritumomab tiuxetan (Zevalin®) in non-Hodgkin lymphoma and chronic lymphocytic leukemia, has stimulated the development of mAbs for the treatment of other malignancies including Hodgkin lymphoma and systemic anaplastic large cell lymphoma (ALCL). Brentuximab vedotin (SGN-35; Adcetris®) is a promising antibody-drug conjugate directed against the CD30 antigen. It was approved in August 2011 by the United States Food and Drug Administration for patients with Hodgkin lymphoma after failure of autologous stem cell transplantation (auto-SCT) or after failure of at least two prior multi-agent chemotherapy regimens in auto-SCT-ineligible candidates, and for the treatment of systemic ALCL after failure of at least one multi-agent chemotherapy regimen.
Physiology of CD30
CD30 is a 120 kDa transmembrane protein that belongs to the tumor necrosis factor receptor (TNFR) superfamily. The protein encompasses six cysteine-rich pseudo-repeat motifs in its extracellular domain.Citation1 The cytoplasmic domain of CD30 contains several binding sequences for members of the TNFR-associated factor (TRAF) family, which are implicated in the activation of NF-κB, c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase pathways.Citation2-Citation4 CD30 is normally expressed in activated T cells, B cells, and NK cells. CD30 expression has also been detected in several malignancies including Hodgkin lymphoma, anaplastic large cell lymphoma (ALCL), certain subtypes of B cell derived non-Hodgkin lymphomas, mature T cell lymphomas, and germ-line malignancies. The restricted expression in these lymphocyte subsets renders it an attractive target for mAb therapy.
CD30 gene expression is regulated by several mechanisms, including Sp1 elements, microsatellite repressor elements, and histone deacetylases.Citation5,Citation6 In particular, in vitro administration of HDAC inhibitors induces downregulation of CD30 in Hodgkin lymphoma cell lines.Citation6 CD30 can also be proteolytically cleaved and released as a soluble form (sCD30). High levels of sCD30 are associated with poor prognosis in patients with anaplastic large cell lymphoma (ALCL) and Hodgkin lymphoma.Citation7,Citation8 The ligand of CD30 (CD30L) is a transmembrane protein that belongs to the tumor necrosis factor superfamily.Citation1 CD30L is expressed in activated T cells, B cells, NK cells, eosinophils, neutrophils, monocytes, and mast cells.
The function of CD30 and CD30L in human physiology remains unclear. No specific diseases or abnormalities have been linked to CD30 or CD30L mutations. The use of CD30-knockout animal models has produced inconclusive results with regard to the role of CD30 on removal of autoreactive T cells through apoptosis during development in the thymus (negative selection).Citation9,Citation10 However, CD30-mediated signaling is important for regulating the development of both effector and memory CD4-positive T cells.Citation11 Various studies suggest that CD30L/CD30 signaling is linked to both Th1- and Th2-responses and Th1- and Th2-associated diseases.Citation12-Citation15 Inhibition of this pathway could prove beneficial in the treatment of autoimmune diseases.Citation16,Citation17 In vitro studies show contradictory results with CD30/CD30L interaction either stimulating or inhibiting B cell proliferation and differentiation, but studies performed in mice suggest that CD30L/CD30 interactions promote secondary humoral immune responses.Citation18 In CD30-positive B cell and T cell lymphoma cell lines, the effect of CD30 signaling is cell type-dependent and varies from enhancement of proliferation to reduction of proliferation and induction of apoptosis.Citation19
CD30-positive hematologic malignancies
To date, brentuximab vedotin has mostly been evaluated in Hodgkin lymphoma, anaplastic large cell lymphoma, and the primary cutaneous CD30-positive lymphoproliferative disorders. We briefly describe these disorders here and discuss which patient groups could benefit most from new treatment strategies such as brentuximab vedotin.
Hodgkin lymphoma
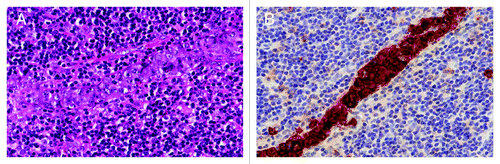
Classical Hodgkin lymphoma is a lymphoid neoplasm defined by the presence of CD30-positive Hodgkin/Reed-Sternberg cells in a background of inflammatory cells (). First line treatment of Hodgkin lymphoma consists of combination chemotherapy with or without additional radiotherapy. Combined modality therapy is given to patients with localized disease whereas chemotherapy alone is given to patients with advanced disease. During the last three decades, patients’ long-term survival has increased through improved multiagent chemotherapy, more accurate radiotherapy, tailored treatments to minimize late therapy-related complications, and better supportive care for myelosuppression, infections, and other complications.Citation20,Citation21 Despite this progress, survival remains substantially lower in elderly patients, likely due to patient characteristics such as lower performance status and comorbidities that strongly affect chemotherapy feasibility and tolerance. In addition, biologic differences between tumors may play a role.Citation20,Citation21 A recent study with elderly patients (median age: 67 y; range: 60–89 y) showed a 5-y progression-free survival (PFS) and 5-y overall survival (OS) of 44% and 58%, respectively.Citation22 Furthermore, current therapies also fail to cure a significant proportion of patients with advanced Hodgkin lymphoma with multiple risk factors such as stage IV disease, male sex, serum albumin < 4 g/dL, hemoglobin < 10.5 g/dL, leucocytes > 15 x 109/L, or lymphocytes < 0.6 x 109/L.Citation23 Patients without these risk factors have a 5-y OS of 89%, whereas patients with ≥ 5 of these risk factors have a 5-y OS of only 56%.Citation23
Figure 1. Classical Hodgkin lymphoma of the nodular sclerosis type. (A) Hematoxylin and eosin (H&E) staining. (B) The Reed-Sternberg cells positively stained for CD30.
In the case of refractory disease or relapse, ~50% of transplant-eligible patients can be cured by high-dose chemotherapy with autologous SCT,Citation24,Citation25 but survival following relapse after auto-SCT is very poor, with only 55% surviving at 2 y and 32% at 5 y.Citation26 Non-transplant eligible patients with relapsed or refractory disease also have a poor outcome. Altogether, these data suggest that new targeted treatment strategies in this disease are still warranted, especially in the elderly and in patients with poor risk disease.
Systemic anaplastic large cell lymphoma (ALCL)
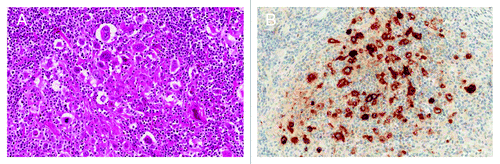
Systemic ALCL accounts for approximately 10% of all T cell non-Hodgkin lymphomas and is characterized by large anaplastic lymphoid cells with uniform and strong CD30 expression (). The chromosome translocation t(2;5)(p23;q35) leads to the formation of the nucleophosmin-anaplastic lymphoma kinase (NPM1-ALK) fusion gene. The fusion protein contains a constitutively activated ALK kinase, which results in activation of multiple downstream pathways, including PI3K/AKT, Ras/ERK, and JAK/Stat3, that leads to proliferation and protection against apoptosis. Other partner chromosomes have been identified that also result in ALK overexpression. Around 60% of systemic ALCL cases are ALK positive and these patients have significantly superior survival (5-y OS: 70%) when compared with ALK-negative patients (5-y OS: 49%).Citation27 Treatment consists of CHOP-like chemotherapy with radiotherapy added in patients with limited-stage disease. Consolidation with high-dose therapy and auto-SCT should be considered in ALK-negative transplant eligible patients. Given the better prognosis in ALK-positive cases, auto-SCT is only considered in case of relapse or refractory disease.Citation28 New treatment strategies are needed to improve cure rates of ALK-positive and especially ALK-negative ALCL patients.
Primary cutaneous CD30-positive lymphoproliferative disorders
The primary cutaneous CD30-positive lymphoproliferative disorders comprise approximately 25% of all cutaneous T cell lymphomas and include primary cutaneous ALCL (pcALCL) and lymphomatoid papulosis (LyP). Both diseases have a favorable prognosis, but differ with regard to their clinical presentation.Citation27,Citation29 LyP is characterized by recurrent papulonodular lesions that undergo spontaneous regression after weeks or months. In pcALCL, there are solitary or grouped, rapidly growing large tumors or thick plaques, but sometimes it presents with multifocal lesions. Generalized LyP can be treated with UV-light phototherapy, topical steroids or low-dose methotrexate, whereas localized pcALCL is treated with surgical excision or radiotherapy. Chemotherapy is often given for multifocal pcALCL. However, patients with these tumors tend to develop new lesions after therapy is stopped.
Brentuximab Vedotin
Antibody generation and mechanisms of action
Brentuximab vedotin is generated by conjugating the mouse-human chimeric IgG1 anti-CD30 mAb (cAC10; SGN-30), to the synthetic dolastatin 10 analog monomethylauristatin A (MMAE), via a protease-sensitive dipeptide linker. Each mAb molecule carries an average of 4 MMAE groups.Citation30 Brentuximab vedotin binds to the CD30 receptor and is internalized via endocytosis. Upon exposure to proteolytic lysosomal enzymes, MMAE molecules are released in the intracellular space.Citation31 Binding of MMAE to tubulin disrupts the microtubule network within the cell, leading to induction of G2/M-phase cell cycle arrest and apoptosis.Citation32 The advantage of the citrulline-valine linker is that it is highly stable in plasma, whereas the dipeptide linker is rapidly and efficiently cleaved by lysosomal enzymes after internalization in antigen-positive cells.Citation30 This results in lower in vivo toxicity and higher efficacy when compared with conjugates with lower stability in plasma.Citation30 Tumor cell killing by brentuximab vedotin may also be mediated by antibody-dependent cellular phagocytosis (ADCP) or via direct effects on tumor cell signaling, both of which are mechanisms of action of SGN-30. In addition, a small fraction of free MMAE diffuses out of the targeted CD30-positive cells, allowing it to kill surrounding cells in the tumor microenvironment which can support tumor growth by production of growth factors and via adhesion.Citation31
Preclinical evaluation of brentuximab vedotin
Brentuximab vedotin induced cell death of CD30-positive tumor cells in vitro including Hodgkin lymphoma cells, cutaneous T cell lymphoma, and anaplastic large cell lymphoma cells, with IC50 < 10ng/ml, but was more than 300-fold less active on CD30-negative cells.Citation32 In addition, brentuximab vedotin had antitumor activity in xenograft models of Hodgkin lymphoma and anaplastic large cell lymphoma.Citation30,Citation32 In the Hodgkin lymphoma xenograft model, improved antitumor activity was observed when brentuximab vedotin was administered with chemotherapeutic agents, including gemcitabine and the combination of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD).Citation33
Antibody pharmacokinetics
Increases in exposure to the antibody-drug conjugate and free MMAE are approximately proportional to dose when brentuximab vedotin is administered every 3 weeks.Citation34 Half-life estimates for the antibody-drug conjugate and for free MMAE are 4–6 d and 3–4 d, respectively.Citation34 When brentuximab vedotin was administered in a weekly schedule, increases in both brentuximab vedotin and free MMAE exposures were approximately dose-proportional.Citation35 Peak brentuximab vedotin blood concentrations were achieved at the end of the infusion in both dosing regimens, whereas peak free MMAE concentrations occurred within 1 to 3 d following each infusion.
Clinical development of brentuximab vedotin
1. Hodgkin lymphoma
Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma
Two unconjugated anti-CD30 antibodies, SGN-30 and MDX-060, had limited clinical activity in Hodgkin lymphoma.Citation36-Citation38 Brentuximab vedotin, however, showed high anti-tumor activity accompanied with limited toxicity in a recently published Phase 1 study (NCT00430846).Citation34 In this study, 42 (93%) out of the 45 patients who were treated had relapsed/refractory classical Hodgkin lymphoma, while 2 patients had systemic ALCL and 1 had CD30+-angioimmunoblastic T cell lymphoma (). They had received a median of 3 preceding lines of chemotherapy, including auto-SCT in 73% of the patients. Brentuximab vedotin was administered at doses of 0.1 to 3.6 mg/kg every 3 weeks; premedication was not required. Study treatment was stopped upon disease progression. A dose of 1.8 mg/kg administered every 3 weeks was considered the highest dose without unacceptable adverse effects. The most common adverse events, predominantly of grade 1 and 2, were fatigue (36%), pyrexia (33%), diarrhea (22%), nausea (22%), neutropenia (22%), and peripheral neuropathy (22%). Peripheral neuropathy typically presented with sensory findings such as numbness and tingling in hands or feet. The neuropathy was cumulative and dose-related. Neuropathy resolved after stopping treatment in the majority of patients (63%). Two patients developed anti-therapeutic antibodies during treatment. Complete response (CR) was achieved in 11 patients (25%) and partial response (PR) in 6 (14%) patients. In addition, 19 out of 44 evaluable patients (43%) had stable disease. Treatment was also accompanied with resolution of disease-related symptoms such as night sweats, weight loss, or fever. The response rate (CR+PR) was 50% in patients that received brentuximab vedotin at the MTD (1.8 mg/kg). Among the 41 evaluable patients with Hodgkin lymphoma, the CR and PR rate was 22% and 15%, respectively. Furthermore, 88% of the patients with objective response achieved it within 4 treatment cycles (2.8 mo). For the whole group, the median duration of response was at least 9.7 mo. Median PFS was 5.9 mo with a trend toward longer PFS in patients receiving brentuximab vedotin at doses ≥ 1.2 mg/kg. Interestingly, responses were accompanied with decreases in serum levels of cytokines TARC (CCL17), IL-6, and TNF-α.
Table 1. Results from clinical studies evaluating brentuximab vedototin in relapsed/refractory CD30-positive malignancies
Another study (NCT00051597) evaluated brentuximab vedotin in a more frequent (once weekly) dosing schedule in patients with relapsed/refractory CD30-positive malignanciesCitation35 (). This Phase 1 study enrolled 38 Hodgkin lymphoma patients, 5 patients with systemic ALCL, and 1 with a peripheral T cell lymphoma. Patients had received a median of 3 prior chemotherapy regimens and 68% had undergone prior auto-SCT. Brentuximab vedotin MTD was defined as 1.2 mg/kg. The most common side effects were again fatigue, nausea, diarrhea, arthralgia, and pyrexia. In addition, peripheral sensory neuropathy was observed in 66% of patients (predominantly grade 1 or 2). Only 6 patients (14%) experienced grade 3 peripheral sensory neuropathy. In addition, 4 patients (9%) experienced peripheral motor neuropathy, including 3 cases with grade 3. Eight patients discontinued treatment because of neuropathy. Grade 1/2 acute infusion reactions occurred in 14% of patients but routine pre-medications were not required. Both patients who developed anti-brentuximab antibodies experienced infusion reactions. At least PR was achieved in 59% of patients with a CR rate of 34%. Median time to objective response was 8.1 weeks. Stable disease was achieved in an additional 32% of patients. Six out of the 7 patients with B-symptoms had resolution of these symptoms. At least PR was achieved in 54% (CR in 29%) of the Hodgkin lymphoma patients and in 4 out of 5 patients with ALCL (all CR). The patient with peripheral T cell lymphoma (PTCL-NOS) achieved a PR. The remissions were durable with median duration of response not yet reached at a median follow-up of 45 weeks on study. Median PFS was 29 weeks and median OS was not reached. Treatment was accompanied with reductions of cytokine and chemokine concentrations.
Based on the encouraging results of these Phase 1 studies, a Phase 2 study (NCT00848926) was initiated in which patients with relapsed/refractory classical Hodgkin lymphoma were treated with brentuximab vedotin 1.8 mg/kg every 3 weeks for up to 16 cyclesCitation39 (). Preliminary data of 102 patients demonstrate an objective response rate (PR+CR) of 75%, with 34% CR. The median duration of CR was not yet reached. The most common treatment-related adverse events were sensory peripheral neuropathy, nausea, fatigue, neutropenia, and diarrhea.
Thus, the available data shows that single agent brentuximab vedotin induces responses in heavily pretreated patients with relapsed/refractory Hodgkin lymphoma, and has acceptable toxicity. More frequent administration with the once weekly schedule may be useful for short-term remission induction strategy in patients with bulky or symptomatic disease requiring rapid tumor debulking. However, the weekly dosing regimen was accompanied with a significantly higher rate of peripheral neuropathy compared with every 3-week dosing, indicating that, when a response is achieved, a less frequent dosing regimen can be initiated for consolidation and to minimize toxicity.
Brentuximab vedotin before or after auto-SCT
Several Phase 2 studies have been initiated to test single agent brentuximab vedotin as salvage therapy preceding auto-SCT in patients with relapsed/refractory Hodgkin lymphoma (NCT01393717; NCT01508312). Furthermore, a randomized, multicenter Phase 3 trial is currently enrolling patients with classical Hodgkin lymphoma who underwent auto-SCT for relapse or refractory disease (NCT01100502). This study will compare the efficacy and safety of brentuximab vedotin to placebo treatment in patients at high-risk of residual disease post-transplant.
Allogeneic transplant following brentuximab vedotin
Hodgkin lymphoma patients with relapse following auto-SCT have a very poor prognosis. Allo-SCT is a potentially curative procedure for these patients, but the lack of sufficient pre-transplant disease control limits the applicability of this approach. A retrospective analysisCitation40 and a subgroup analysis of patients participating in two Phase 2 studiesCitation41 examined the efficacy and toxicity of allo-SCT following induction of remission with brentuximab vedotin. This approach resulted in prolonged disease control, without delay in engraftment or increase in acute graft-vs.-host disease, chronic graft-vs.-host disease, or non-relapse mortality.
Brentuximab vedotin in newly diagnosed Hodgkin lymphoma
Based on the efficacy of single agent brentuximab vedotin in relapsed/refractory disease and in vitro data showing synergy between brentuximab vedotin and chemotherapy,Citation33 several studies are testing the efficacy and safety of combination treatments in newly diagnosed patients.
An ongoing, but not recruiting, Phase 1 study (NCT01060904) is evaluating the safety of brentuximab vedotin (0.6, 0.9, or 1.2 mg/kg; on days 1 and 15 of a 28-d cycle) in combination with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy or a modified standard (AVD; doxorubicin, vinblastine, and dacarbazine) in newly diagnosed patients with stage IIa or IIb-IV Hodgkin lymphoma (age, 18–60 y).Citation42 Of the first 31 patients treated in this study, 17 (55%) had stage IV disease. Combination therapy was generally well tolerated with no dose-limiting toxicities observed up to 1.2 mg/kg. The most common adverse events were nausea (77%), neutropenia (77%), peripheral sensory neuropathy (48%), and fatigue (45%). However, 40% of the patients treated with brentuximab vedotin and ABVD had pulmonary toxicity occurring during cycles 3–6, which is higher when compared with ABVD alone. Thus far, no patients in the AVD cohort experienced a pulmonary toxicity event indicating that brentuximab vedotin should not be combined with bleomycin (A. Younes, personal communication, March 2012). All 10 patients who had a response assessment available after completion of frontline therapy had achieved CR ().Citation42 Based on these data a Phase 3 trial is planned to assess frontline treatment with brentuximab vedotin plus AVD as compared with ABVD without brentuximab vedotin (A. Younes, personal communication, March 2012).
Table 2. Results from a clinical study evaluating brentuximab vedototin in newly diagnosed CD30-positive malignancies
The aim of another ongoing Phase 2 study is to compare the activity of brentuximab vedotin combined with AVD (doxorubicin, vinblastine, and dacarbazine) in older patients (> 60 y) with previously untreated stage II-IV classical Hodgkin lymphoma (NCT01476410).
2. Systemic anaplastic large cell lymphoma
Younes et al. treated 2 patients with relapsed/refractory systemic ALCL with brentuximab vedotin once every 3 weeks. Both patients achieved a CR with a duration of 17 and 5 mo.Citation34
In the Phase 1 study (NCT00051597) reported by Fanale et al.,Citation35 5 systemic ALCL patients with relapsed or refractory disease were treated with brentuximab vedotin. The weekly dosing schedule resulted in CR in 4 out of 5 patients, while the other patient who was treated at the lowest dose had stable disease. In contrast, unconjugated anti-CD30 antibodies had only modest clinical activity in systemic ALCL.Citation36-Citation38
Based on these promising results, a Phase 2 study (NCT00866047) was initiated in which brentuximab vedotin was administered at a dose of 1.8 mg/kg every 3 weeks to 58 patients with relapsed/refractory systemic ALCLCitation43 (). At least PR was achieved in 86% of patients, including CR in 57%. Patients with ALK-positive and ALK-negative disease had similar response rates. Notably, patients who never responded to previous therapy achieved a response to brentuximab vedotin (n = 13; ≥ PR: 77%; CR: 31%). The median duration of the response was 13 mo. Median PFS was 14.6 mo and median OS was not yet reached. Importantly, median PFS with brentuximab vedotin was significantly longer than the median PFS achieved with the most recent prior therapy. After discontinuing treatment, 16 patients received a transplant (allo-SCT in 8 and auto-SCT in 8). Peripheral sensory neuropathy was observed in 41% of patients, including 17% with a grade 3 neuropathy. Again, the majority (79%) of these patients experienced resolution or improvement of neuropathy.
Several reports indicate that retreatment with brentuximab vedotin is possible after previous response or stabilization of disease following brentuximab vedotin therapy.Citation44,Citation45 An ongoing Phase 2 retreatment study is enrolling patients who previously experienced an objective response to brentuximab vedotin (NCT00947856).
These data suggest that durable remissions can be achieved with brentuximab vedotin in patients with relapsed/refractory systemic ALCL. Based on these results, a Phase 1 study is now evaluating brentuximab vedotin administered in sequence and in combination with multiagent chemotherapy as first line treatment in systemic ALCL (NCT01309789).
3. Primary cutaneous CD30-positive lymphoproliferative disorders
A Phase 2 study (NCT00099255) evaluated the safety and efficacy of single agent SGN-30 in patients with pcALCL and LyP, or CD30-positive large cell transformation of mycosis fungoides who had failed to respond to local radiation therapy or systemic therapy.Citation46 The overall response rate in 23 evaluable patients was 70% with an acceptable toxicity profile. A Phase 2 study is currently enrolling patients with these skin lymphomas to test efficacy and toxicity profile of brentuximab vedotin (NCT01352520).
Future Perspectives
Although the majority of patients with Hodgkin lymphoma can be cured with frontline therapy, new agents are needed, particularly for elderly patients, relapsed/refractory patients ineligible for auto-SCT, patients with refractory disease after salvage chemotherapy, and patients who relapse after auto-SCT. Brentuximab vedotin is a promising novel cancer treatment and it has shown significant single-agent antitumor activity in heavily pretreated patients with acceptable toxicity. Various ongoing trials are currently evaluating brentuximab vedotin alone and in combination with other agents as frontline therapy or salvage therapy, including induction therapy preceding auto-SCT. In the front-line setting, the aim is to reduce toxicity in patients with very favorable disease and to improve outcomes in patients with high risk for relapse. In addition, the feasibility of using brentuximab vedotin as maintenance therapy after auto-SCT is under evaluation. These studies will provide important data with regard to the optimal regimens that incorporate brentuximab vedotin for the treatment of Hodgkin lymphoma. Several other promising novel agents that target the tumor cell or the microenvironment such as mTOR inhibitors, lenalidomide, and rituximab are currently under investigation in Hodgkin lymphoma as single agent or in combination with other therapies.Citation47-Citation49
Despite the relative efficacy of chemotherapy in systemic ALCL, novel agents are still needed for relapsed or refractory patients. Recent clinical trials in systemic ALCL showed clinical activity of brentuximab vedotin in heavily pretreated patients in both ALK-positive and –negative tumors. Ongoing studies are investigating the value of brentuximab vedotin in patients with newly diagnosed disease. Other promising novel agents for the treatment of systemic ALCL include inhibitors of the NPM1-ALK fusion protein in ALK-positive cases, histone deacetylase inhibitors, pralatrexate (a folate analog), and other mAbs such as the anti-CD25 daclizumab or anti-CD4 zanolimumab.Citation50-Citation54
Most studies focused on the treatment of Hodgkin lymphoma and ALCL. However, studies currently enrolling patients are also evaluating brentuximab vedotin in other tumors with variable CD30 expression such as mycosis fungoides, Sezary syndrome, acute lymphoblastic leukemia, acute myeloid leukemia, multiple myeloma, chronic lymphatic leukemia, and NK-cell neoplasms.
One of the clinically most important adverse events related to brentuximab vedotin treatment is the cumulative, dose-related peripheral neuropathy, which is predominantly sensory. The development of neuropathy is a consequence of the microtubule assembly inhibition by the cytotoxic component of brentuximab vedotin (MMAE). Dose adjustments, delays, or lengthening of dose interval should be considered to manage neuropathy and to enable continuation of treatment.Citation55 Grade 3 and 4 neutropenia must also be managed by dose delays or reductions. Support with growth factors can also be considered for subsequent cycles of therapy. Importantly, in January 2012 Seattle Genetics announced that progressive multifocal leukoencephalopathy may be associated with the use of brentuximab vedotin. In addition, a contraindication warning against concomitant use of bleomycin and brentuximab vedotin was given because of increased risk of pulmonary toxicity. Serious pulmonary toxicity was also observed when SGN-30 was combined with gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD).Citation56 This observation indicates that interactions between SGN-30 or brentuximab vedotin with certain agents such as gemcitabine and bleomycin, may potentially lead to development of pneumonitis.
An important challenge will be the identification of patient subsets that will benefit most from brentuximab vedotin-based therapeutics. Biomarker assessment in future studies will help to evaluate the risk/benefit profile and tailor individualized therapeutic approaches.
Also other CD30 targeting agents are currently in clinical testing either as single agent or in combination with other drugs in Hodgkin lymphoma and ALCL. These include the human IgG1 monoclonal antibody MDX-060Citation36 and its derivative MDX-1401, which has a nonfucosylated Fc region leading to higher affinity to Fcγ receptors and improved ADCCCitation57; the humanized monoclonal antibody XmAb2513,Citation58 which has an Fc region engineered to have increased binding affinity to Fcγ receptors leading to improved Fcγ receptor-dependent effector functions; and AFM13 which is a recombinant antibody construct targeting both CD30 and CD16a, which is present on the surface of NK cells, macrophages and neutrophils (NCT01221571).
In summary, brentuximab vedotin offers an important new therapeutic option for patients with heavily pretreated relapsed/refractory Hodgkin lymphoma, systemic ALCL, and primary cutaneous CD30-positive lymphoproliferative disorders. Future research will be directed at developing combinations with brentuximab vedotin for patients with relapsed or newly diagnosed disease.
Acknowledgments
The authors thank Dr. Roos Leguit (Department of Pathology, University Medical Center Utrecht, Utrecht, The Netherlands) for providing images for picture 1.
Financial Disclosures
NvdD: research funding/contracted research: Celgene
ED: none
References
- Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 1993; 73:1349 - 60; http://dx.doi.org/10.1016/0092-8674(93)90361-S; PMID: 8391931
- Duckett CS, Gedrich RW, Gilfillan MC, Thompson CB. Induction of nuclear factor kappaB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol 1997; 17:1535 - 42; PMID: 9032281
- Ansieau S, Scheffrahn I, Mosialos G, Brand H, Duyster J, Kaye K, et al. Tumor necrosis factor receptor-associated factor (TRAF)-1, TRAF-2, and TRAF-3 interact in vivo with the CD30 cytoplasmic domain; TRAF-2 mediates CD30-induced nuclear factor kappa B activation. Proc Natl Acad Sci U S A 1996; 93:14053 - 8; http://dx.doi.org/10.1073/pnas.93.24.14053; PMID: 8943059
- Harlin H, Podack E, Boothby M, Alegre ML. TCR-independent CD30 signaling selectively induces IL-13 production via a TNF receptor-associated factor/p38 mitogen-activated protein kinase-dependent mechanism. J Immunol 2002; 169:2451 - 9; PMID: 12193714
- Croager EJ, Gout AM, Abraham LJ. Involvement of Sp1 and microsatellite repressor sequences in the transcriptional control of the human CD30 gene. Am J Pathol 2000; 156:1723 - 31; http://dx.doi.org/10.1016/S0002-9440(10)65043-2; PMID: 10793083
- Buglio D, Mamidipudi V, Khaskhely NM, Brady H, Heise C, Besterman J, et al. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol 2010; 151:387 - 96; http://dx.doi.org/10.1111/j.1365-2141.2010.08342.x; PMID: 20880107
- Pizzolo G, Vinante F, Chilosi M, Dallenbach F, Josimovic-Alasevic O, Diamantstein T, et al. Serum levels of soluble CD30 molecule (Ki-1 antigen) in Hodgkin’s disease: relationship with disease activity and clinical stage. Br J Haematol 1990; 75:282 - 4; http://dx.doi.org/10.1111/j.1365-2141.1990.tb02664.x; PMID: 2164839
- Nadali G, Vinante F, Ambrosetti A, Todeschini G, Veneri D, Zanotti R, et al. Serum levels of soluble CD30 are elevated in the majority of untreated patients with Hodgkin’s disease and correlate with clinical features and prognosis. J Clin Oncol 1994; 12:793 - 7; PMID: 8151321
- Amakawa R, Hakem A, Kundig TM, Matsuyama T, Simard JJ, Timms E, et al. Impaired negative selection of T cells in Hodgkin’s disease antigen CD30-deficient mice. Cell 1996; 84:551 - 62; http://dx.doi.org/10.1016/S0092-8674(00)81031-4; PMID: 8598042
- DeYoung AL, Duramad O, Winoto A. The TNF receptor family member CD30 is not essential for negative selection. J Immunol 2000; 165:6170 - 3; PMID: 11086050
- Withers DR, Gaspal FM, Bekiaris V, McConnell FM, Kim M, Anderson G, et al. OX40 and CD30 signals in CD4(+) T-cell effector and memory function: a distinct role for lymphoid tissue inducer cells in maintaining CD4(+) T-cell memory but not effector function. Immunol Rev 2011; 244:134 - 48; http://dx.doi.org/10.1111/j.1600-065X.2011.01057.x; PMID: 22017436
- Tang C, Yamada H, Shibata K, Muta H, Wajjwalku W, Podack ER, et al. A novel role of CD30L/CD30 signaling by T-T cell interaction in Th1 response against mycobacterial infection. J Immunol 2008; 181:6316 - 27; PMID: 18941223
- Saraiva M, Smith P, Fallon PG, Alcami A. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J Exp Med 2002; 196:829 - 39; http://dx.doi.org/10.1084/jem.20020319; PMID: 12235215
- Romagnani S, Del Prete G, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leukoc Biol 1995; 57:726 - 30; PMID: 7759952
- Bengtsson A. The role of CD30 in atopic disease. Allergy 2001; 56:593 - 603; http://dx.doi.org/10.1034/j.1398-9995.2001.00137.x; PMID: 11421916
- Gaspal F, Withers D, Saini M, Bekiaris V, McConnell FM, White A, et al. Abrogation of CD30 and OX40 signals prevents autoimmune disease in FoxP3-deficient mice. J Exp Med 2011; 208:1579 - 84; http://dx.doi.org/10.1084/jem.20101484; PMID: 21788408
- Chakrabarty S, Nagata M, Yasuda H, Wen L, Nakayama M, Chowdhury SA, et al. Critical roles of CD30/CD30L interactions in murine autoimmune diabetes. Clin Exp Immunol 2003; 133:318 - 25; http://dx.doi.org/10.1046/j.1365-2249.2003.02223.x; PMID: 12930356
- Kennedy MK, Willis CR, Armitage RJ. Deciphering CD30 ligand biology and its role in humoral immunity. Immunology 2006; 118:143 - 52; http://dx.doi.org/10.1111/j.1365-2567.2006.02354.x; PMID: 16771849
- Gruss HJ, Boiani N, Williams DE, Armitage RJ, Smith CA, Goodwin RG. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood 1994; 83:2045 - 56; PMID: 8161776
- Sjöberg J, Halthur C, Kristinsson SY, Landgren O, Nygell UA, Dickman PW, et al. Progress in Hodgkin lymphoma: a population-based study on patients diagnosed in Sweden from 1973-2009. Blood 2012; 119:990 - 6; http://dx.doi.org/10.1182/blood-2010-08-302604; PMID: 22147892
- Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood 2008; 111:2977 - 83; http://dx.doi.org/10.1182/blood-2007-10-115493; PMID: 18096762
- Evens AM, Helenowski I, Ramsdale E, Nabhan C, Karmali R, Hanson B, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood 2012; 119:692 - 5; http://dx.doi.org/10.1182/blood-2011-09-378414; PMID: 22117038
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med 1998; 339:1506 - 14; http://dx.doi.org/10.1056/NEJM199811193392104; PMID: 9819449
- Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, et al, Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea Cooperative Group. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol 2005; 16:625 - 33; http://dx.doi.org/10.1093/annonc/mdi119; PMID: 15737986
- Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant 2006; 12:1065 - 72; http://dx.doi.org/10.1016/j.bbmt.2006.06.006; PMID: 17084370
- Martinez C, Canals C, Alessandrino E, Karakasis D, Leone G, Trneny M, et al. Relapse of Hodgkin's lymphoma (HL) after autologous stem cell transplantation (ASCT): Prognostic factors in 462 patients registered in the database of the EBMT. J Clin Oncol 2010; 28:8060
- Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al, International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008; 111:5496 - 504; http://dx.doi.org/10.1182/blood-2008-01-134270; PMID: 18385450
- Dearden CE, Johnson R, Pettengell R, Devereux S, Cwynarski K, Whittaker S, et al, British Committee for Standards in Haematology. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma). Br J Haematol 2011; 153:451 - 85; http://dx.doi.org/10.1111/j.1365-2141.2011.08651.x; PMID: 21480860
- Kempf W, Pfaltz K, Vermeer MH, Cozzio A, Ortiz-Romero PL, Bagot M, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood 2011; 118:4024 - 35; http://dx.doi.org/10.1182/blood-2011-05-351346; PMID: 21841159
- Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003; 21:778 - 84; http://dx.doi.org/10.1038/nbt832; PMID: 12778055
- Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res 2010; 16:888 - 97; http://dx.doi.org/10.1158/1078-0432.CCR-09-2069; PMID: 20086002
- Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 2003; 102:1458 - 65; http://dx.doi.org/10.1182/blood-2003-01-0039; PMID: 12714494
- Oflazoglu E, Kissler KM, Sievers EL, Grewal IS, Gerber HP. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol 2008; 142:69 - 73; http://dx.doi.org/10.1111/j.1365-2141.2008.07146.x; PMID: 18477046
- Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010; 363:1812 - 21; http://dx.doi.org/10.1056/NEJMoa1002965; PMID: 21047225
- Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res 2012; 18:248 - 55; http://dx.doi.org/10.1158/1078-0432.CCR-11-1425; PMID: 22080439
- Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol 2007; 25:2764 - 9; http://dx.doi.org/10.1200/JCO.2006.07.8972; PMID: 17515574
- Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol 2009; 146:171 - 9; http://dx.doi.org/10.1111/j.1365-2141.2009.07740.x; PMID: 19466965
- Bartlett NL, Younes A, Carabasi MH, Forero A, Rosenblatt JD, Leonard JP, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood 2008; 111:1848 - 54; http://dx.doi.org/10.1182/blood-2007-07-099317; PMID: 18079362
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results from a pivotal phase II study of brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma (HL). J Clin Oncol 2011; 29:8031
- Chen RW, Forman SJ, Palmer J, Tsai N-C, Popplewell L, Delioukina ML, et al. Brentuximab Vedotin (SGN-35) Enables Successful Reduced Intensity Allogeneic Hematopoietic Cell Transplantation in Relapsed/Refractory Hodgkin Lymphoma. ASH Annual Meeting Abstracts 2011; 118: 664.
- Illidge T, Bouabdallah R, Chen RW, Gopal AK, Moskowitz CH, Ramchandren R, et al. Allogeneic Transplant Following Brentuximab Vedotin Treatment in Patients with Relapsed or Refractory CD30+ Lymphomas. ASH Annual Meeting Abstracts 2011; 118: 3091.
- Younes A, Connors JM, Park SI, Hunder NNH, Ansell SM. Frontline Therapy with Brentuximab Vedotin Combined with ABVD or AVD in Patients with Newly Diagnosed Advanced Stage Hodgkin Lymphoma. ASH Annual Meeting Abstracts 2011; 118: 955.
- Advani RH, Shustov AR, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab Vedotin (SGN-35) in Patients with Relapsed or Refractory Systemic Anaplastic Large Cell Lymphoma: A Phase 2 Study Update. ASH Annual Meeting Abstracts 2011; 118: 443.
- Foyil KV, Kennedy DA, Grove LE, Bartlett NL, Cashen AF. Extended retreatment with brentuximab vedotin (SGN-35) maintains complete remission in patient with recurrent systemic anaplastic large-cell lymphoma. Leuk Lymphoma 2012; 53:506 - 7; http://dx.doi.org/10.3109/10428194.2011.614706; PMID: 21867368
- Bartlett NL, Grove LE, Kennedy D, Sievers EL, Forero A. Objective responses with brentuximab vedotin (SGN-35) retreatment in CD30-positive hematologic malignancies: A case series. J Clin Oncol 2010; 28:8062
- Duvic M, Reddy SA, Pinter-Brown L, Korman NJ, Zic J, Kennedy DA, et al. A phase II study of SGN-30 in cutaneous anaplastic large cell lymphoma and related lymphoproliferative disorders. Clin Cancer Res 2009; 15:6217 - 24; http://dx.doi.org/10.1158/1078-0432.CCR-09-0162; PMID: 19789316
- Johnston PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol 2010; 85:320 - 4; PMID: 20229590
- Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 2011; 118:5119 - 25; http://dx.doi.org/10.1182/blood-2011-07-362475; PMID: 21937701
- Blum KA. Upcoming diagnostic and therapeutic developments in classical Hodgkin’s lymphoma. Hematology Am Soc Hematol Educ Program 2010; 2010:93 - 100; http://dx.doi.org/10.1182/asheducation-2010.1.93; PMID: 21239777
- Merkel O, Hamacher F, Sifft E, Kenner L, Greil R, European Research Initiative on Anaplastic Large Cell Lymphoma. Novel therapeutic options in anaplastic large cell lymphoma: molecular targets and immunological tools. Mol Cancer Ther 2011; 10:1127 - 36; http://dx.doi.org/10.1158/1535-7163.MCT-11-0042; PMID: 21712478
- Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood 2011; 117:5827 - 34; http://dx.doi.org/10.1182/blood-2010-10-312603; PMID: 21355097
- d’Amore F, Radford J, Relander T, Jerkeman M, Tilly H, Osterborg A, et al. Phase II trial of zanolimumab (HuMax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma. Br J Haematol 2010; 150:565 - 73; http://dx.doi.org/10.1111/j.1365-2141.2010.08298.x; PMID: 20629661
- O’Connor OA, Horwitz S, Hamlin P, Portlock C, Moskowitz CH, Sarasohn D, et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol 2009; 27:4357 - 64; http://dx.doi.org/10.1200/JCO.2008.20.8470; PMID: 19652067
- Lindén O. Remission of a refractory, anaplastic large-cell lymphoma after treatment with daclizumab. N Engl J Med 2004; 351:1466 - 7; http://dx.doi.org/10.1056/NEJM200409303511424; PMID: 15459315
- Forero-Torres A, Berryman RB, Advani RH, Bartlett NL, Chen RW, Fanale MA, et al. Prolonged Treatment with Brentuximab Vedotin (SGN-35) in Patients with Relapsed or Refractory Hodgkin Lymphoma (HL) or Systemic Anaplastic Large Cell Lymphoma (sALCL). ASH Annual Meeting Abstracts 2011; 118: 3711.
- Blum KA, Jung SH, Johnson JL, Lin TS, Hsi ED, Lucas DM, et al, Cancer and Leukemia Group B. Serious pulmonary toxicity in patients with Hodgkin’s lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an FcγRIIIa-158 V/F polymorphism. Ann Oncol 2010; 21:2246 - 54; http://dx.doi.org/10.1093/annonc/mdq211; PMID: 20423913
- Cardarelli PM, Moldovan-Loomis MC, Preston B, Black A, Passmore D, Chen TH, et al. In vitro and in vivo characterization of MDX-1401 for therapy of malignant lymphoma. Clin Cancer Res 2009; 15:3376 - 83; http://dx.doi.org/10.1158/1078-0432.CCR-08-3222; PMID: 19401346
- Blum KA, Smith M, Fung H, Zalevsky J, Combs D, Ramies DA, et al. Phase I study of an anti-CD30 Fc engineered humanized monoclonal antibody in Hodgkin lymphoma (HL) or anaplastic large cell lymphoma (ALCL) patients: Safety, pharmacokinetics (PK), immunogenicity, and efficacy. J Clin Oncol 2009; 27:8531