Abstract
The role of Fc glycans on clearance of IgG molecule has been examined by various groups in experiments where specific glycans have been enriched or the entire spectrum of glycans was studied after administration in pre-clinical or clinical pharmacokinetic (PK) studies. The overall conclusions from these studies are inconsistent, which may result from differences in antibody structure or experimental design. In the present study a well-characterized recombinant monoclonal IgG1 molecule (mAb-1) was analyzed from serum samples obtained from a human PK study. mAb-1 was recovered from serum using its ligand cross-linked to Sepharose beads. The overall purity and recovery of all isoforms were carefully evaluated using a variety of methods. Glycans were then enzymatically cleaved, labeled using 2-aminobenzamide and analyzed by normal phase high performance liquid chromatography. The assays for recovering mAb-1 from serum and subsequent glycan analysis were rigorously qualified at a lower limit of quantitation of 15 μg/mL, thus permitting analysis to day 14 of the clinical PK study. Eight glycans were monitored and classified into two groups: (1) the oligomannose type structures (M5, M6 and M7) and (2) fucosylated biantennary oligosaccharides (FBO) structures (NGA2F, NA1F, NA2F, NA1F-GlcNAc and NGA2F-GlcNAc). We observed that the oligomannose species were cleared at a much faster rate (40%) than FBOs and conclude that high mannose species should be carefully monitored and controlled as they may affect PK of the therapeutic; they should thus be considered an important quality attribute. These observations were only possible through the application of rigorous analytical methods that we believe will need to be employed when comparing innovator and biosimilar molecules.
Abstract
Introduction
The role of Fc glycans on IgG molecules as well as novel IgG formats such as half-body molecules, dual variable domain molecules (DVDs) and Fc fusion proteins is much studied by the biotechnology industry. Interest in Fc glycans involves two major topics: (1) the well-established role of Fc glycans in antibody dependent cell-mediated cytotoxicity (ADCC)Citation1 and complement dependent cytotoxicity (CDC),Citation2 and (2) inference from studies demonstrating that glycoproteins in circulation are cleared much faster as a result of glycoreceptors such as the asialoglycoprotein receptor that binds terminal galactose residuesCitation3-Citation5 or mannose receptors that bind terminal mannose and N-acetylglucosamine residues.Citation6-Citation8
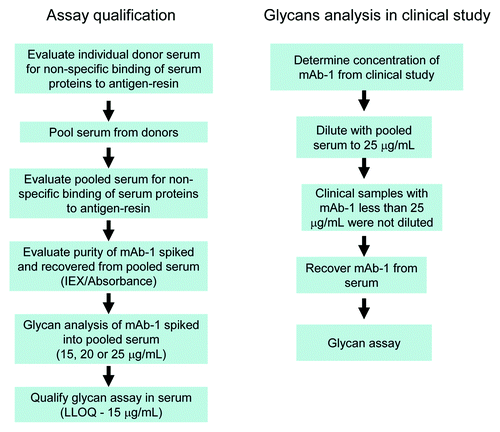
The N-linked Fc glycans present on IgG molecules are of the biantennary complex type composed of a core heptasaccharide structure with various sugars added to the core. The major species are classified into two groups (): the oligomannose type structures (M5, M6 and M7) and the fucosylated bianntenary oligosaccharide (FBO) type structures (NGA2F, NA1F, NA2F, NA1F-GlcNAc and NGA2F-GlcNAc). These glycans are partially buried in the CH2 domain, which may limit their exposure to serum glycoreceptors.Citation9 As summarized in , the results of many studies that evaluate the impact of Fc glycans on clearance are conflicting.Citation10-Citation19 The approaches are varied. For example, Fc glycan species may be enriched (enzymatically, genetically or via inhibitors added during cell culture) prior to administration. This approach has limitations because of the assumption that the only change in the molecule during enrichment is the glycan structure, and because the studies are limited to non-clinical settings. In a second approach, the total glycan pool is studied after administration. We used this approach in the present study because it permitted studies of a human IgG1 molecule (mAb-1) in a human pharmacokinetics (PK) study. The molecule was well-characterized at the primary, secondary and tertiary structure and the glycan profile was obtained using a qualified normal phase high performance liquid chromatography (HPLC) assay of 2-AB labeled glycans. To enhance the validity of the current study, we qualified the HPLC glycan assay at a limit of quantitation (LOQ) of 5 μg in a buffer matrix. We also qualified a ligand-based affinity method that was used to recover mAb-1 from serum and obtained the glycan profile at a lower LOQ (LLOQ) of 15 μg/mL. Both A280 readings and a weak ion exchange (IEX) chromatography method were used to ensure complete recovery of all species from serum; the IEX method provided a recognizable fingerprint of the molecule after recovery from serum.
Figure 1. Fc glycans observed on antibodies. Symbol nomenclature was adopted from the Consortium for Functional Glycomics. The nomenclature used for complex and oligomannose species are NA1F (Gal-1, G1), NA2F (Gal-2, G2), NGA2F (Gal-0, G0), Mann-5, Mann-6, Mann-7 (M5, M6, M7 or Oligomannose-5,6,7).
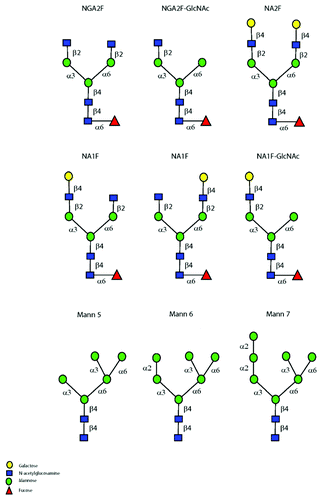
Table 1. Summary of studies to evaluate influence of the Fc glycan structure on serum clearance
Using qualified assays, we concluded that the oligomannose species were cleared at a faster rate than the FBOs. Serum α-1,2 mannosidases were found to have some role in the conversion of M6 and M7 species to M5 and, as observed in a previous report,Citation10 we found a slight elevation in M5 species at 6 h. However, the level of M5 then rapidly declined over 14 d; the rate of clearance was estimated to be 40% higher when compared with fucosylated biantennary species. Our studies parallel the observations of Goetze et al.Citation10 that high mannose species should be considered an important product quality attribute as they may affect PK of the therapeutic antibody. Recent guidance from the United States Food and Drug Administration for demonstrating biosimilarity to a reference product emphasize that totality of evidence includes not only analytical comparability, but also clinically relevant studies to assess PK and pharmacodynamics of the biosimilar.Citation20-Citation22 As described here, we applied rigorous analytical methods on a well-characterized IgG1 molecule to recover and analyze glycans from a clinical PK study. Such types of studies are recommended for other post-translational modifications when comparing reference and biosimilar molecules.
Results
Concentration of mAb-1 from clinical study
mAb-1, a human IgG1 molecule, was administered intravenously (IV) to 15 healthy subjects. Each volunteer was given a single dose of 700 mg IV and sufficient blood was then collected at various time points (days): pre-dose, 0.25, 0.5, 1, 1.5, 2, 3, 5, 7, 10, 14, 21, 28, 35, and 56 d. summarizes the PK results of mAb-1 concentration (μg/mL), obtained using an ECL based method, through week 4. The average serum concentration at day 10 is 34 μg/mL and by day 14 is about 23 μg/mL. The challenge in this study was to qualify assays that could (a) robustly recover mAb-1 from serum at day 14 without interfering species; and (b) enable reliable analysis of glycan species released from mAb-1.
Table 2. mAb-1 concentrations (µg/mL) measured at time intervals post-dose through week 4 in 15 healthy subjects (1–15)
Recovery from serum
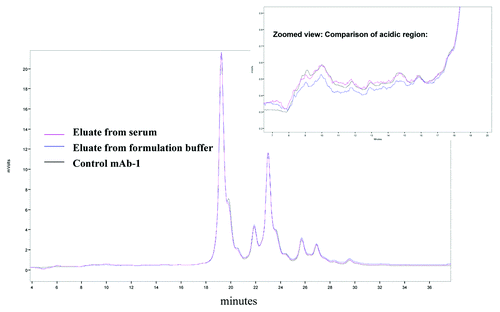
A weak cation exchange chromatography (WCX-10) method was used to evaluate antigen based affinity recovery of mAb-1 from serum. The method is highly resolving and provides a recognizable fingerprint of the molecule. The acidic region of mAb-1 is composed of poorly resolved species that elute in the first 10 min and are ~5% of the overall IEX profile. The relative area percentage of acidic, main isoform and basic regions from 10 to 30 μg are fairly consistent; however, the ability to integrate the acidic region is lost at around 5 μg (Table S1). The overall IEX profile of mAb-1 recovered from serum was comparable to that of mAb-1 recovered from formulation buffer and non-spiked mAb-1 (). An overlay of the acidic regions showed similar profiles and the relative area percentage of the acidic, basic and main isoform species were also found to be comparable (Table S1). From these results, we concluded that the antigen affinity resin consistently recovered all species observed on an IEX chromatogram.
Figure 3. Overlay of IEX profile. Weak cation exchange method was used to evaluate antigen-based affinity recovery of mAb-1 from serum. An overlay of eluted mAb-1 recovered from serum showed similar profile to mAb-1 recovered from formulation buffer. Zooming into acidic region also showed similar recovery of different species.
An absorbance reading at 280 nm (A280) of total protein eluted from the affinity resin was also evaluated. Both the IEX method and A280 measurement revealed consistent protein recovery from 5 separate studies (Table S2). While the A280 measurement measures total protein, the IEX method preserved the unique fingerprint of mAb-1, supporting consistent recovery of the majority of species.
Fragmentation in the hinge region of IgG molecules is typically observed during long-term storage or at elevated temperature. Loss of one of the Fab arms results in a fragment species (Fragment 2) that was purified by size exclusion chromatography from a stability sample. This species was spiked into serum and then incubated with affinity resin for 1 h. The resin was washed and eluted as described in the methods section and the eluate evaluated by IEX chromatography. Fragment 2 elutes in the early portion of the acidic region and its overall recovery from serum was estimated to be about 93% (data not shown). From these studies, we concluded that the antigen affinity resin effectively recovered mAb-1 with just one antigen binding arm.
Qualification of 2-Aminobenzamide NPHPLC oligosaccharide assay
Normal phase HPLC is a well-established method for separating oligosaccharides. The functional groups that aid in this separation have included diethylaminoethyl,Citation23-Citation25 imideCitation26 and amideCitation27,Citation28 groups. Guile et al.Citation29 in a landmark publication exploited the use of an acetonitrile:water gradient buffered with volatile salts to resolve different sugars on a silica based column with amide functional groups. Derivatization of sugars with the fluorescent label 2-aminobenzamide (2-AB) increased the sensitivity of the method. It is this combination of high resolution, improved sensitivity of detection, reproducibility, accurate prediction of glucose units and most importantly, quantitative responsiveness that resulted in many laboratories adopting this method for routine monitoring and for setting release specifications for oligosaccharides in quality control laboratories.
The first step, before labeling oligosaccharides with 2-AB, is enzymatic release of the sugars using the endoglycosidase, PNGase-F. When the quantity of available protein is not limiting, the standard method uses as much as 400 μg of antibody and 8 μL of enzyme (1:50 fold enzyme:protein ratio). In this clearance study, however, the method must quantify glycans after digesting low levels of protein; for example, by post-dose day 14 serum mAb-1 was ~20 μg/mL. We investigated glycan profiles obtained from digesting mAb-1 in the 1 to 10 μg range with and without use of a speed vacuum centrifugation step after the GlycoClean S step. While the speed vacuum step permitted analysis below 5 μg of mAb-1 (data not shown), we proceeded to qualify the oligosaccharide assay from 5 to 100 μgs of mAb-1 without the additional vacuum step; thereby minimizing sample handling. The antibody:enzyme ratio was also altered from the original protocol and details are provided in the Methods section. The relative percentage area of glycans that were enzymatically released from 5 to 100 μg of mAb-1 is consistent (Table S3). Three different evaluations found excellent repeatability of the total area of individual peaks when 5 μgs of mAb-1 was digested (Tables S4 and S5).
Pooling of serum from healthy human donors
mAb-1 was recovered from serum using the antibody’s ligand that was cross-linked to Sepharose beads. The isolation method developed to recover mAb-1 from serum was optimized using rat serum and the glycan profile of mAb-1 recovered from serum was comparable to mAb-1 analyzed in buffer (results not shown).
We then evaluated recovery of mAb-1 spiked into human serum. A serum lot (BRH235934) was obtained from pooling serum from 10 healthy donors. This pooled serum was evaluated for non-specific binding by adding affinity resin (60 μL) into 1 mL of serum and incubating for 1 h at room temperature. The resin was washed and eluted as described in the methods section. The eluate was then analyzed both by measuring absorbance at 280 nm and oligosaccharide profiling. A280 measurements from 3 separate analyses of pooled serum found low non-specific binding of serum proteins to the resin (Table S6).
Serum from ten donors was also obtained from Biological Specialty Corporation for creating a large “pooled” serum lot that was eventually used as a diluent for the clinical samples. Of the 10 donor serums, eight were evaluated for non-specific binding to the resin (55–21155A, 23–06358A, 55–20697A, 23–06438A, 55–20785A, 55–21019A, 23–07267A, and 23–06655A). The individual donor serums were first thawed at 4°C and then spun using the SS-34 rotor for 30 min at 16,000 rpm. Each of the serums was evaluated for non-specific binding by spiking the antigen affinity resin into 1 mL of serum. The resin was washed and eluted as described in the Methods section and the eluate was then evaluated by obtaining A280 readings (Table S7). Donor serum 23–06655A had high non-specific binding and was not used in the pooled serum. To create the final “pooled” serum lot that was used as a diluent for the clinical samples; we combined about 30 mL of each of the remainder donor serums.
Lower limit of quantitation for mAb-1 recovery and glycan analysis
The average serum concentration of mAb-1 at day 14 was ~20 μg/mL. The lower limit of quantitation (LLOQ) for recovering and analyzing glycans released from mAb-1 was obtained by spiking in triplicate 15, 20 or 25 μg/mL into the pooled serum lot. A handling control, where mAb-1 was just spiked into 1 mL of formulation buffer and concentrated using a 30,000 MWCO concentrator was evaluated in parallel to account for losses brought about by handling. Three blank serums were also included as controls.
The eluted samples were analyzed by A280 and 2-AB oligosaccharide analysis (). The blank pooled serum samples showed very little background contribution (data not shown). The percent results for each of the individual glycans from the spike and recovery experiment were consistent between the triplicate samples. The % CVs for all the major glycans NGA2F, M5, NA1F total and NA2F were ≤ 10% for all the 3 spikes. For the minor glycans, the % CVs were higher (≤ 36%) due to the low abundance of these glycans in mAb-1. With these results we proceeded to qualify the assay at an LOQ of 15 μg/mL - this permitted analysis of the clinical samples out to day 14 post-dose. The % CV for all major glycans was less than 11%.
Table 3. mAb-1 spike and recovery from serum. mAb-1 was spiked in triplicate into serum at 25, 20 and 15 μg/mL. mAb-1 was recovered from serum and the eluted samples were analyzed by 2-AB oligosaccharide analysis. The % CVs for all the major glycans NGA2F, M5, NA1F total and NA2F were ≤ 10% for all the 3 spikes. For the minor glycans, the % CVs were higher (≤ 36%) due to the low abundance of these glycans
Qualification of assay for recovery and glycan analysis at 15 μg/mL
The qualification of recovery of mAb-1 from serum and 2-AB oligosaccharide analysis was performed as described in . The glycans monitored in this study were NGA2F-GlcNAc, NGA2F, M5, NA1F-GlcNAc, NA1F, M6, NA2F, M7 and total oligomannose. The assay’s acceptance criterion was established using glycans NGA2F, total NA1F and M5 (consisting of greater than 80% of the total glycans of the mAb-1). summarizes the total standard deviation (SD) and % CV based on variance component analysis of these data. To have high confidence that limits would not be exceeded by analytical variance alone, limits were typically set at or beyond the grand mean ± 3x total SD. Because only nine results were available, Monte-Carlo simulations were also performed on the 3 most abundant glycans (NGA2F, NA1F total and M5) to predict the probability of exceeding the assays acceptance criteria. Monte Carlo simulations supported using the most abundant glycan (NGA2F) for deciding whether to reject an analysis. A range of ± 20% around the mean value of NGA2F (54.09%) would be exceeded only 0.1% of the time simply by chance alone and not due to an assay failure.
Table 4. Design of experiment for qualification of recovery of mAb-1 from serum and 2-AB oligosaccharide analysis
Table 5. Results from 3 separate experiments quantifying glycans recovered from pooled serum spiked with 15 ug/mL of mAb-1 (results are expressed as percentage of total peak area)
In vitro incubation of mAb-1
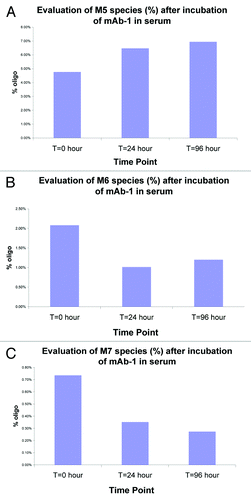
Chen et al.Citation11 revealed a role of serum mannosidases (mannosidase I) with specificity for α-1,2-mannose linkage observed in the high mannose species (M6-M9). We replicated these studies by spiking mAb-1 into pooled human serum and incubating for 96 h at 37°C. At various time points, we recovered mAb-1 and evaluated glycan levels. As shown in , we confirmed that the high mannose species (M6 and M7) were indeed cleaved in serum and that this cleavage could be blocked with the mannosidase I inhibitor, kifunensine (data not shown). In contrast, M5 levels were found to increase, presumably as a result of cleavage of the high mannose species (M6 and M7) to the M5 species. Decrease of the M6-M7 species and increase in M5 species was also confirmed by mass spectrometry analysis. Other low oligomannose species (M3-M4) were not detected (data not shown).
Figure 4. Evaluation of mannosidase activity following in vitro serum incubation studies. mAb-1 was spiked into pooled human serum and at various time points (24 and 96 hours) recovered and the glycans analyzed. As shown in and , the level of high mannose species (M6 and M7) was reduced within 24 hours of incubation in serum. In contrast, M5 levels increased over the same time period (); presumably as a result of enzymatic cleavage (mannosidases) of M6 and M7 to the M5 species.
Analysis of glycans from clinical study samples
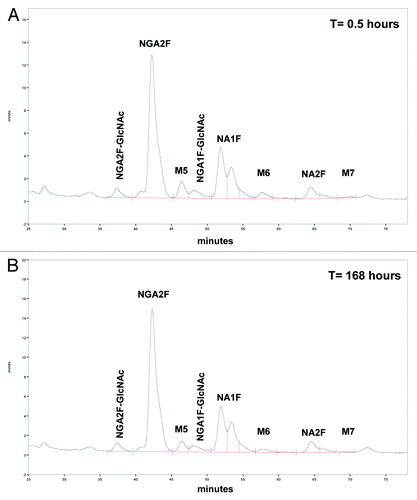
summarizes the mAb-1 concentrations results (μg/mL) from 15 min post-dose through 4 weeks thereafter from the clinical study. Based on the provided mAb-1 (μg/mL) concentrations, samples were diluted with pooled serum (see section on pooled donor serum) to 25 μg/mL prior to recovery by affinity chromatography. Samples with mAb-1 concentrations less than 25 μg/mL were purified neat. After affinity recovery, samples were analyzed by 2-AB oligosaccharide analysis. The glycan profile after separation by normal phase HPLC of volunteer-5 at t = 0.5 h (mAb-1 concentration in serum was 185.5 μg/mL) and t = 168 h (mAb-1 concentration in serum was 41.7 μg/mL) is shown in .
Figure 5. Glycan profile of mAb-1 separated by normal phase-HPLC after recovery from serum of healthy volunteer. Shown in is the glycan profile of volunteer #5 at t=0.5 hrs (mAb-1 concentration in serum was 185.5 μg/mL) and in at t=168 hrs (mAb-1 concentration in serum was 41.7 μg/mL). Samples were diluted with pooled serum to 25 μg/mL prior to recovery by affinity chromatography and analysis by 2-AB oligosaccharide assay.
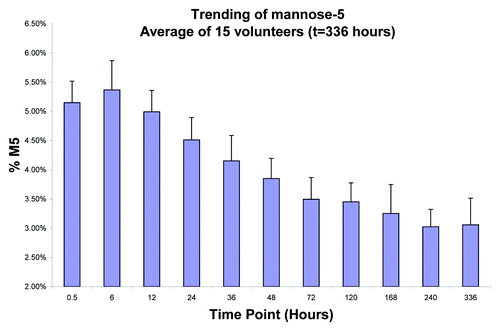
The profile of all glycans monitored from donor samples is shown in . To better illustrate trending, the average of the 15 donor samples of the major FBO (NGA2F) is shown in and the most abundant oligomannose (M5) is shown in . The results showed that levels of the FBOs (NGA2F, NA2F and NA1F) remained fairly consistent over 14 d. The levels of M5 were found to initially increase in the first 6 h, presumably as a result of mannosidase activity on the high mannose species (M6-M9). After 6 h, the levels of M5 were found to rapidly decrease to the observed 40% decrease in M5 by day 14 ().
Figure 6. Mean percentage of glycan species of mAb-1 in serum following single dose administration in healthy individuals.
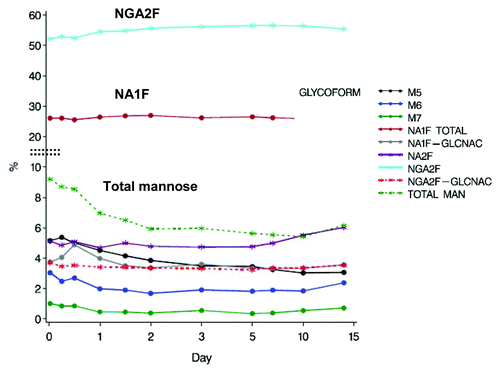
Figure 7. Average NGA2F fraction of mAb-1 in serum from 15 volunteers. The levels of NGA2F were found to remain fairly constant over 336 Hours (14 days).
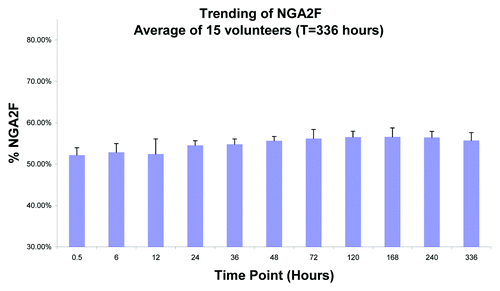
Figure 8. Average M5 fraction of mAb-1 in serum from 15 volunteers. The levels of M5 were found to initially increase in the first 6 hours, presumably as a result of mannosidase activity on the high mannose species (M6-M9). After 6 hours, the levels of M5 were found to rapidly decrease. There was about a 40% decrease in M5 by day 14.
Discussion
The Fc glycans found on recombinant antibodies expressed in Chinese hamster ovary (CHO) cells are predominantly biantennary fucosylated neutral glycans with either zero galactose (Gal-0 or G0F), one galactose (Gal-1 or G1F) or two galactose (Gal-2 or G2F) residues at the terminus. Varying levels of the immature high mannose species (M5-M9) are also found on the Fc site that we observed (unpublished data) can range from 1–2% to as high as 20% in some of our therapeutics in development. Interestingly, endogenous human IgG molecules are found to have trace levels (< 0.1%) of high mannose species.Citation30 Presence of Fc oligomannose species has attracted attention because mannose receptors are known to play a role in clearance of pathogens containing mannose on their surface as well and reports have linked increased clearance of glycoproteins to high mannose content.Citation6
A number of studies, summarized in , that address the impact of Fc oligomannose species on clearance are quite inconsistent. This lack of consistency stem in part from the varied technical approaches and models used to address the question.Citation31 Most of these studies, for example, are conducted in pre-clinical settings (frequently mice) with enriched species that are not rigorously characterized for other post-translational modifications that may have been introduced in the molecule. Further, the methods used for recovery and subsequent glycan characterization are not performed using qualified assays. A highly interesting study by Chen et al., in 2009Citation11 concluded that the larger (M6-M9) species are converted to M5 by serum mannosidases and that high mannose species do not affect serum clearance. In a subsequent study, using a mass spectrometry based approach; the authors conclude that high mannose species do affect serum clearance.Citation10 The discrepancy between the two studies was carefully evaluated and the conclusion was that the reverse phase method separating 2AB-labeled glycans did not adequately distinguish endogenous glycan species from the administered therapeutic.Citation10
In the study reported here we deliberately avoided potential problems by studying a very well-characterized human antibody in a human PK study and by using assays that were qualified to both recover the therapeutic and analyze glycans at an LOQ of 15 μg/mL. Using the rigorous methodology summarized in , we were able to make two very important conclusions: (1) oligomannose species are cleared at a faster rate that FBOs and (2) clearance of FBOs are unaffected over the course of the study. Route of administration is another important consideration as mAbs can be administered either via the IV or sub-cutaneous (SC) routes. While this manuscript evaluates only administration via the IV route, Goetze et al.Citation10 observed faster elimination half-lives of the M5 containing population of an IgG2 therapeutic via both SC and IV routes. Because high mannose species could alter PK of the therapeutic, they should be considered an important quality attribute to be carefully monitored not only during manufacturing changes, but also when comparing biosimilar to reference products.
Materials and Methods
Materials
Donor human serum (Bioreclamation, NY; Biological Specialty Corporation, PA), ammonium formate, 1 N hydrochloric acid, sodium acetate, sodium chloride, acetic acid, sodium-phosphate-dibasic-heptahydrate (JT Baker, NJ), drip columns, centrifuge columns (2 mL), Amicon Ultra-4 centrifugal filter and Ultra-15 centrifugal filter devices MWCO 30,000 (Millipore, MA), SignalTM 2-AB labeling kit, Enzyme, N-glycanase (PNGase F), GlycoCleanTM S Cartridge, guard column, GlycoSepTM N Guard, (Prozyme CA), CNBr Sepharose 4FF (GE Healthcare, PA), acetonitrile HPLC grade, Triton X-100 (Sigma-Aldrich), formic acid, (EMD, PA).
Cross-linking antigen to CNBr-Sepharose (Affinity-resin)
The method as described by the vendor was used. Briefly, coupling buffer was 0.1 M sodium bicarbonate, pH 8.3, containing 0.5 M sodium chloride. An aliquot (10 mg) of the antigen recognized by mAb-1 was buffer exchanged into the coupling buffer using a 30,000 MWCO concentrator (Amicon Ultra, Millipore). Then 0.5 g of the CNBr activated Sepharose 4 Fast Flow resin was washed on a table-top centrifuge at least 3 times with 30 mL of ice cold HCl between pH 2 and 3 (typically 1–4 mM of HCl was added until the right pH was obtained). After the last wash at low pH, the resin was quickly exchanged into coupling buffer. The activated resin was then quickly added to the antigen and a small aliquot taken to obtain an A280 reading. The coupling was allowed to proceed overnight (about 15 h) at 4°C and a small aliquot used to obtain an A280 reading. The percentage of cross-linked antigen was easily estimated (efficiency of ≥ 80% was acceptable). Excess ligand was washed away with coupling buffer and the reactive groups then blocked with 30 mL of 0.1 M TRIS-HCl, pH 8.0 for 3 h. The resin was finally washed alternatively with high pH (0.1 M Tris, 0.5 M NaCl, pH 8) and low pH (0.1 M acetate, 0.5 M NaCl, pH 3.8) at least 3 times using a drip column. After the final wash, the resin was suspended in 3 mL of 0.1 M Tris pH 8.0 and stored at 4°C.
Recovery of mAb-1 from serum using antigen affinity chromatography
The recovery of mAb-1 from serum was performed using antigen affinity chromatography and using drip columns. Serum was centrifuged on an Eppendorf 5418 table-top centrifuge at 15,000 rcf for 15 min prior to analysis. About 60 μL of the affinity-resin was added into 1 mL of serum and incubated on a rocking platform for 1 h. The wash conditions were 4x1 mL of wash buffer (20 mM sodium acetate, 0.25 M NaCl, pH 4.2) and elution was performed with two different buffers; elution buffer 1 (0.1 M acetic acid containing 0.15 M NaCl, pH 2.8) followed by elution buffer 2 (0.3 M acetic acid containing 0.15 M NaCl, pH 2.4). The eluates were immediately neutralized with 1 M Tris pH 7.5, then combined and finally exchanged into 50 mM sodium phosphate (pH 7.4) prior to analysis.
Evaluation of recovery by WCX-10
Recovery of the mAb-1 was also evaluated by weak cation exchange chromatography (WCX-10). The amount of mAb-1 was quantified from the area under the chromatogram (AUC). Injecting different amounts of mAb-1 onto the column provided a linear curve when AUC was plotted against the concentration.
Glycan analysis using 2-AB oligosaccharide analysis
mAb-1 (3 - 400 μg) was digested with N-glycanase at a ratio of 1:50 (enzyme:protein) for 18 h at 37°C. If there was less than 100 μg of antibody, then 2 μL of enzyme was added to the sample for digest for accuracy of pipetting. After digestion, the sample was heated at 95°C for 10 min to precipitate the protein. The sample was spun to pellet the precipitate and the supernatant, containing the glycans, was then transferred to a microcentrifuge tube.
The samples were labeled with 2-AB (2-aminobenzamide). After labeling at 65°C for 2–3 h, impurities such as excess dye and other labeling components were removed from the samples using a Glycoclean S cartridge, which contains a membrane that retains glycans in > 90% acetonitrile. Through a series of wash steps with multiple buffers, most hydrophobic non-glycan contaminants either pass through the membrane or are retained weakly and may be washed off. The glycans were then eluted with water. After elution, the samples were diluted 3.3X in acetonitrile to ensure binding to the normal phase column. 400 μL of each sample was injected onto a Glycosep™ N HPLC column using a Shimadzu HPLC system with fluorescent detection. Mobile phase A consisted of 100% acetonitrile and mobile phase B consisted of 50 mM ammonium formate pH 4.4. The glycans were separated using a gradient of 35% B at initial conditions to 40% B over 70 min. The gradient remained at 40% B for 20 min and then increased to 95%B over 5 min before going back to initial conditions. Flow rate was 0.4 mL/min and the column was heated at 30°C. Excitation was at 330 nm and emission was at 420 nm. Total run time was 120 min.
Measurement of total concentration of mAb-1 in serum
Analysis of mAb-1 concentrations in serum was performed using a validated bridging electrochemiluminescent (ECL) assay method at Intertek USA dba ALTA Analytical Laboratory, San Diego, CA. The lower limit of quantitation (LLOQ) for mAb-1 was established at 1.5 ng/mL using 1:5 dilution (7.5 ng/mL in undiluted serum).
Monte-Carlo simulations to predict the probability of exceeding the assays acceptance criteria
Future results for the most abundant glycans (NGA2F, NA1F total and M5) could vary due to between and within experiment sources of variation. To understand this variability and set run acceptance criteria for analysis, nine assay results (3 from each of 3 experiments) were available and were used to estimate the mean and the between and within experimental variance parameters using Gibbs sampling in WinBUGS. A posterior sample from Gibbs sampling (20,000 draws) was obtained for each parameter. Future results were obtained for each draw by Monte Carlo simulation. The percent of simulated future results that exceeded the +/− 20% limits was reported for each glycan.
| Abbreviations: | ||
| NA1F (Gal-1 or G1) | = | Asialo-monogalactosylated biantennary core-substituted fucose |
| NA2F (Gal-2 or G2) | = | Asialo-bigalactosylated biantennary core-substituted fucose |
| NGA2F (Gal-0 or G0) | = | Asialo-agalacto biantennary core-substituted fucose |
| GlcNAc | = | N, Acetylglucosamine |
| M5 | = | M6, M7, Mann-5, Mann-6, Mann-7 or Oligomannose-5, 6, 7 |
| PK | = | pharmacokinetics |
| FBO | = | fucosylated biantennary oligosaccharide |
| 2-AB | = | 2 aminobenzamide |
| IEX | = | ion exchange |
| A280 | = | absorbance at 280 nm |
| DVD | = | dual variable domains |
| ADCC | = | antibody dependent cell-mediated cytotoxicity |
| CDC | = | cell dependent cytotoxicity |
| LOQ | = | limit of quantitation |
| WCX | = | weak cation exchange chromatography |
| PNGase-F, Peptide | = | N-Glycosidase F |
Additional material
Download Zip (104.2 KB)Acknowledgments
We thank Susan Paulson, Matthew Hruska and Jeff Thomas (Project Manager at Intertek Chemicals and Pharmaceuticals) for the many discussions and contributions to this study. Gary Welch is gratefully acknowledged for his support of this project. George Avgerinos championed this study; his guidance and perseverance through various stages of the project is also gratefully acknowledged
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note
Supplemental materials can be found at: http://www.landesbioscience.com/journals/mabs/article/20450/
References
- Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov 2011; 10:101 - 11; http://dx.doi.org/10.1038/nrd3365; PMID: 21283105
- Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol Immunol 1995; 32:1311 - 8; http://dx.doi.org/10.1016/0161-5890(95)00118-2; PMID: 8643100
- Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem 1982; 51:531 - 54; http://dx.doi.org/10.1146/annurev.bi.51.070182.002531; PMID: 6287920
- Ashwell G, Morell AG. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol 1974; 41:99 - 128; PMID: 4609051
- Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev 1995; 75:591 - 609; PMID: 7624395
- Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol 2004; 24:179 - 92; http://dx.doi.org/10.1615/CritRevImmunol.v24.i3.20; PMID: 15482253
- Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 2002; 295:1898 - 901; http://dx.doi.org/10.1126/science.1069540; PMID: 11884756
- Stahl PD. The mannose receptor and other macrophage lectins. Curr Opin Immunol 1992; 4:49 - 52; http://dx.doi.org/10.1016/0952-7915(92)90123-V; PMID: 1317711
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 2011; 108:12669 - 74; http://dx.doi.org/10.1073/pnas.1108455108; PMID: 21768335
- Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011; 21:949 - 59; http://dx.doi.org/10.1093/glycob/cwr027; PMID: 21421994
- Chen X, Liu YD, Flynn GC. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 2009; 19:240 - 9; http://dx.doi.org/10.1093/glycob/cwn120; PMID: 18974198
- Jones AJ, Papac DI, Chin EH, Keck R, Baughman SA, Lin YS, et al. Selective clearance of glycoforms of a complex glycoprotein pharmaceutical caused by terminal N-acetylglucosamine is similar in humans and cynomolgus monkeys. Glycobiology 2007; 17:529 - 40; http://dx.doi.org/10.1093/glycob/cwm017; PMID: 17331977
- Keck R, Nayak N, Lerner L, Raju S, Ma S, Schreitmueller T, et al. Characterization of a complex glycoprotein whose variable metabolic clearance in humans is dependent on terminal N-acetylglucosamine content. Biologicals 2008; 36:49 - 60; http://dx.doi.org/10.1016/j.biologicals.2007.05.004; PMID: 17728143
- Wright A, Morrison SL. Effect of altered CH2-associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J Exp Med 1994; 180:1087 - 96; http://dx.doi.org/10.1084/jem.180.3.1087; PMID: 8064227
- Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology 2007; 17:104 - 18; http://dx.doi.org/10.1093/glycob/cwl057; PMID: 17012310
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, et al. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng 2008; 99:652 - 65; http://dx.doi.org/10.1002/bit.21598; PMID: 17680659
- Millward TA, Heitzmann M, Bill K, Längle U, Schumacher P, Forrer K. Effect of constant and variable domain glycosylation on pharmacokinetics of therapeutic antibodies in mice. Biologicals 2008; 36:41 - 7; http://dx.doi.org/10.1016/j.biologicals.2007.05.003; PMID: 17890101
- Newkirk MM, Novick J, Stevenson MM, Fournier MJ, Apostolakos P. Differential clearance of glycoforms of IgG in normal and autoimmune-prone mice. Clin Exp Immunol 1996; 106:259 - 64; http://dx.doi.org/10.1046/j.1365-2249.1996.d01-847.x; PMID: 8918571
- Wawrzynczak EJ, Cumber AJ, Parnell GD, Jones PT, Winter G. Blood clearance in the rat of a recombinant mouse monoclonal antibody lacking the N-linked oligosaccharide side chains of the CH2 domains. Mol Immunol 1992; 29:213 - 20; http://dx.doi.org/10.1016/0161-5890(92)90102-4; PMID: 1542298
- United States Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. Feb 2012.
- United States Food and Drug Administration. Quality considerations in demonstrating biosimilarity to a reference protein product. Feb 2012.
- United States Food and Drug Administration. Biosimilars: Questions and Answers regarding implementation of the biologics price competition and innovation act of 2009. Feb 2012.
- Green ED, Baenziger JU. Separation of anionic oligosaccharides by high-performance liquid chromatography. Anal Biochem 1986; 158:42 - 9; http://dx.doi.org/10.1016/0003-2697(86)90585-3; PMID: 3799968
- Mellis SJ, Baenziger JU. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem 1981; 114:276 - 80; http://dx.doi.org/10.1016/0003-2697(81)90480-2; PMID: 7304916
- Mellis SJ, Baenziger JU. Size fractionation of anionic oligosaccharides and glycopeptides by high-performance liquid chromatography. Anal Biochem 1983; 134:442 - 9; http://dx.doi.org/10.1016/0003-2697(83)90320-2; PMID: 6650829
- Alpert AJ, Shukla M, Shukla AK, Zieske LR, Yuen SW, Ferguson MA, et al. Hydrophilic-interaction chromatography of complex carbohydrates. J Chromatogr A 1994; 676:191 - 22; http://dx.doi.org/10.1016/0021-9673(94)00467-6; PMID: 7921176
- Alpert AJ. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr 1990; 499:177 - 96; http://dx.doi.org/10.1016/S0021-9673(00)96972-3; PMID: 2324207
- Tomiya N, Awaya J, Kurono M, Hanzawa H, Shimada I, Arata Y, et al. Structural elucidation of a variety of GalNAc-containing N-linked oligosaccharides from human urinary kallidinogenase. J Biol Chem 1993; 268:113 - 26; PMID: 8416919
- Guile GR, Rudd PM, Wing DR, Prime SB, Dwek RA. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem 1996; 240:210 - 26; http://dx.doi.org/10.1006/abio.1996.0351; PMID: 8811911
- Flynn GC, Chen X, Liu YD, Shah B, Zhang Z. Naturally occurring glycan forms of human immunoglobulins G1 and G2. Mol Immunol 2010; 47:2074 - 82; http://dx.doi.org/10.1016/j.molimm.2010.04.006; PMID: 20444501
- Correia IR. Stability of IgG isotypes in serum. MAbs 2010; 2:221 - 32; http://dx.doi.org/10.4161/mabs.2.3.11788; PMID: 20404539