Abstract
A major limitation to the application of therapeutic monoclonal antibodies (mAbs) is their reduced in vivo efficacy compared with the high efficacy measured in vitro. Effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC) are dramatically reduced in vivo by the presence of high amounts of endogenous IgG in the serum. Recent studies have shown that modification of the glycosylation moieties attached to the Fc part of the mAb can enhance binding affinity to FcγRIIIα receptors on natural killer cells and thus may counteract the reduced in vivo efficacy. In the present study, a humanized IgG1/κ monoclonal antibody recognizing the tumor-associated carbohydrate antigen Lewis Y was stably produced in a moss expression system that allows glyco-engineering. The glyco-modified mAb (designated MB314) showed a highly homogeneous N-glycosylation pattern lacking core-fucose. A side-by-side comparison to its parental counterpart produced in conventional mammalian cell-culture (MB311, formerly known as IGN311) by fluorescence-activated cell sorting analysis confirmed that the target specificity of MB314 is similar to that of MB311. In contrast, ADCC effector function of MB314 was increased up to 40-fold whereas complement dependent cytotoxicity activity was decreased 5-fold. Notably, a release of immunostimulatory cytokines, including interferon γ, monocyte chemotactic protein-1 (MCP-1), interleukin-6 and tumor necrosis factor (TNF) was particularly induced with the glyco-modified antibody. TNF release was associated with CD14+ cells, indicating activation of monocytes.
Introduction
Monoclonal antibodies (mAbs) such as rituximab (Rituxan®), trastuzumab (Herceptin®) or bevacizumab (Avastin®) have demonstrated their potential for anti-cancer therapy.Citation1-Citation4 Deficits of mAb therapy, however, are (1) the decreased effector function of therapeutic antibodies found in serumCitation5 and (2) infusion related toxicities.Citation6,Citation7
The reduction of antibody-related effector functions seems to be associated with the high levels of endogenous IgG present in human serum. Several investigators have shown that the presence of high amounts of endogenous serum IgG impairs the effector function of therapeutic antibodies such as the antibody-dependent cell-mediated cytotoxicity (ADCC). The competition for binding to Fcγ receptors, in particular FcγRIIIα expressed on natural killer (NK) cells, is believed to be the main reason.Citation5 Regarding infusion related toxicities, recent studies have shown that application of a chimeric anti-CD20 mAb can result in moderate to severe first-dose side effects, notably in patients with high numbers of circulating tumor cells. These side effects were found to correlate with activation of the complement system measured in serum of patients.Citation6
A clear correlation between therapeutic activity of humanized anti-CD20 mAb and the polymorphism in the FcγRIIIα gene has been demonstrated, with a significantly superior treatment benefit observed for patients carrying the homozygous high affinity type FcγRIIIα 158V/V compared with low affinity type FcγRIIIα 158F/F carriers.Citation8 Furthermore, effector cells of approximately 60% of the normal human population were found to express the low affinity FcγRIIIα on NK cells.Citation8,Citation9 Modification of the glycosylation moieties attached to the Fc part of the antibody, i.e., the reduction of the core-fucose content was shown to enhance the binding affinity to FcγRIIIα.Citation10-Citation14 In general, the effect of defucosylation on effector functions such as ADCC has extensively been demonstrated.Citation15,Citation16 Moreover, there are other sugar residues that affect effector functions of therapeutic antibodies such as terminal galactosylation, which correlates with enhanced CDC, and bisecting GlcNAc structures, which correlate with enhanced ADCC.Citation17-Citation19 In contrast, the effect of modified N-glycan structures on other effector functions, e.g., antibody-dependent cytokine release, that are an important part of the overall effector function profile of antibodies,Citation20-Citation25 has not been analyzed to date in detail.
In the study presented here, a unique plant expression host, based on a gene-engineered fucosyl-transferase and xylosyl-transferase deficient moss line, facilitated the completely animal component-free recombinant expression of therapeutic mAbs with tailor made N-linked glycosylation devoid of core fucose. Using this expression technology, increased ADCC activity of a transiently produced therapeutic antibody was shown previouslyCitation26 and was found to be independent of the FcγRIIIα genotype of the effector cells.Citation27 To characterize the effector function profile of such a glyco-engineered mAb in more detail, we generated a stably transformed moss line transgenic for the MB314 antibody. The resulting highly homogenous and defucosylated MB314 antibody was compared with its counterpart, the humanized, core-fucosylated parental mAb MB311 stably expressed by conventional mammalian cells. Both mAbs had the same binding specificity to the target structure, i.e., the tumor-associated Lewis Y carbohydrate that is broadly expressed on tumors of epithelial origin.Citation28-Citation32 In correlation with a complete lack of terminal galactose residues, CDC activity was decreased for the glyco-modified antibody compared with its parental counterpart. Most importantly, in parallel to the increased ADCC activity induced by MB314, a temporary release of stimulatory cytokines, including, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, monocyte chemotactic protein (MCP)-1 and interleukin (IL)-6 was observed. Moreover, TNF release was found to be associated with CD14+ cells indicating that beside NK cells mediating most of the ADCC activity, additional cells such as monocytes/macrophagesCitation33 may be involved in the enhanced cytotoxic activity of glyco-modified mAbs.
The data presented demonstrate that the effector function profile of therapeutic antibodies can be significantly changed by glyco-engineering, resulting in enhanced ADCC activity and the release of immune stimulatory cytokines.
Results
Expression and analytical characterization of MB314
The humanized Lewis Y-specific mAb MB 314 (IgG1/κ) was produced as described in Materials and Methods by greenovation’s Bryo-Master expression system using a double-knockout Physcomitrella patens moss cell line, resulting in an antibody lacking core fucose and xylose residues on the N-glycans. For transfection of moss protoplasts, genetic constructs containing open reading frames for light and heavy chains of the mAb MB 314 were used.Citation27 The yield was 25 mg MB314 / 364 g fresh weight.
Identity, integrity, molecular weight and purity of the purified glyco-modified MB314 were analyzed and compared with clinical grade MB311 material (produced in mammalian cells). Size exclusion chromatography of MB311 and MB314 showed for both mAbs a single peak (purity > 95%) with a retention time indicative for human IgG1 (data not shown). The stably expressed MB314 showed a homogeneous N-glycosylation pattern with quantitative removal of plant-specific xylose and fucose residues and characterized by the absence of core alpha1,6 fucose, and terminal galactosylation (G0). The parental mAb MB 311 displayed a N-glycan pattern typical for IgG molecules expressed in mammalian host cells with pronounced α1,6-fucosylation and substantial amounts of terminal galactose residues.Citation29 Stably expressed MB314 assembled correctly, showed the identical molecular weight of the heavy and light chains and revealed a homogenous glyco-optimized N-linked oligosaccharide pattern of the GnGn type without core fucosylation.
Binding to Lewis Y and FcγRIII
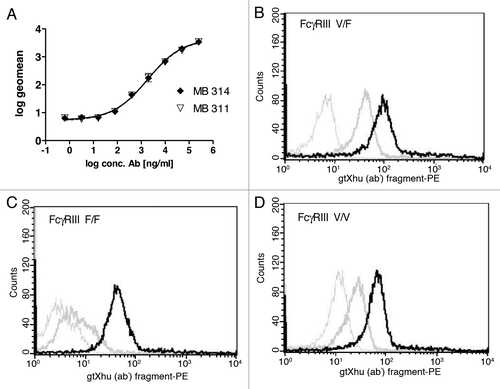
Binding of MB314 and MB311 to Lewis Y carbohydrate antigen presenting SKBR-3 cells was analyzed by a FACS. The logarithmized geo mean values of the obtained mean fluorescence intensities were set in relation of the antibody concentration. The data were fitted using a 4-PL algorithm with GraphPad Prism 2.0. There is no difference visible between MB311 and MB314, indicating that the moss-produced antibody retains its full Lewis Y binding capacity (). Next, binding to FcγRIII (CDC16) expressed on purified human NK cells was tested to confirm that the de-fucosylated MB314 antibody showed increased binding. Peripheral blood mononuclear cell (PBMCs) were prepared from the blood of healthy donors using Ficoll separation and NK cells were subsequently purified applying Miltenyi’s ‘negative purification’ approach. Binding to all three CD16 genetic variants V/V (homozygote, high affinity) V/F (‘heterozygote’) and F/F (homozygote, low affinity) was evaluated. The data demonstrate significantly increased binding of the de-fucosylated MB314 for all variants. In contrast, MB311 showed only low binding to NK cells from V/F donors and no binding to F/F low affinity donors (, , ). These results clearly demonstrate that de-fucosylated MB314 can bind to all naturally occurring FcγRIII variants expressed by human NK cells.
Figure 1. Binding activity MB314 and MB311. (A) Binding of MB311 and MB314 to the Lewis-Y positive tumor cell line SKBR-3 was determined by flow cytometry. Geometric Mean fluorescence intensity (MFI) was plotted vs. the logarithm of the antibody concentration and fitted using a sigmoidal four parameter fit using GraphPad Prism 4 software for calculation of EC50 values. Mean and SD of triplicates are shown. Binding of 100µg/ml MB311 (dark gray line) or MB314 (black line) to purified human NK-cells expressing the FcγRIII variant V/F (B), to the FcγRIII variant F/F (C) and the FcγRIII variant V/V (D). Results obtained with medium only are shown as light gray lines.
Effector functions: CDC and ADCC
CDC reactivity was tested using the Lewis Y positive target cell lines SKBR-3 and OVCAR-3 (). An approximate 5-fold lower CDC activity was found for the glyco-modified MB314 compared with the parental mAb MB311.
Figure 2. MB314 and MB311 mediated effector functions. (A, B) CDC reactivity against two Lewis Y positive tumor cell lines was tested using a FACS based approach. Tumor cells were incubated with different concentrations of antibodies in the presence of human complement for 1 h. As complement source frozen human serum was used. Cytotoxicity against SKBR-3 cells (A) or OVCAR-3 cells (B) was quantified by FACS™ analysis using a live/dead cell stain. Gated 7-AAD+ population (dead cells) as percentage of the whole population are plotted vs. the logarithm of the antibody concentration and fitted using a sigmoidal four parameter fit using GraphPad Prism 4 software. Mean and SD of triplicates are shown. (C, D) ADCC activity against two Lewis Y positive tumor cell lines was tested in a 51Cr release cell lysis assay. 51Cr labeled target cells (T) were incubated with effector cells (PBMCs from a healthy volunteer donor, E) at indicated E:T ratios for 16 h. Supernatants were counted for released 51Cr using a γ-counter. Specific lysis (%) was plotted against the logarithm of the antibody concentration (ng/ml) and fitted using a sigmoidal four parameter fit using GraphPad Prism 4 software.
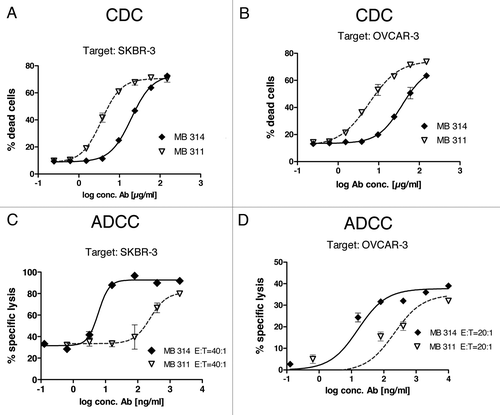
For ADCC measurements, Lewis Y positive tumor cells were used as target cells with PBMC from healthy donors as effector cells. Several parameters relevant for tumor therapy, such as different kinds of target cells and different effector to target ratios (E:T) were tested. First, MB314 and MB311 were tested against the Lewis Y positive tumor cell line SKBR-3. In contrast to the similar binding activity of the two mAbs determined by FACS (compare ), a significant, 30–40 fold higher cytotoxicity was found for the glyco-modified MB314 compared with the parental MB311 (). The increased ADCC activity of the glyco-modified mAb was evident at all E:T ratios tested, whereas the absolute amounts of killed cells showed a clear dependency on the E:T ratio (data not shown). Testing the ADCC activity against additional Lewis Y positive tumor cell lines such as OVCAR-3 () or KATOIII cells (data not shown) also resulted in significantly higher cytotoxicity mediated by MB314 compared with the parental MB311. The fold increase in ADCC observed with MB314 roughly correlated with the Lewis Y expression of the target cell lines as tested by FACS binding experiments (data not shown). These results are in accordance with increased binding of the glyco-modified MB314 to human FcγRIII (CD16) expressed on freshly isolated human NK cells (), confirming that increased binding to FcγRIII results in higher ADCC.
ADCC competition by excess IgG
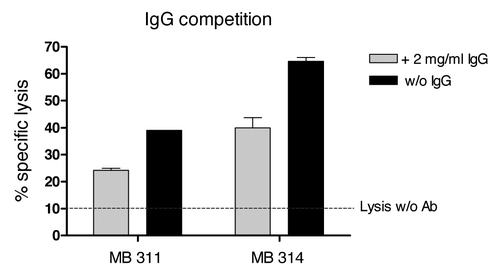
The inhibitory effect of the presence of excess of multi-specific immunoglobulin on the ADCC activity was tested. In this experiment, an E:T ratio of 30:1 was used and MB314 or MB311 were applied at a similar concentration of 400 ng/ml, respectively, to SKBR-3 cells. An excess of multi-specific IgG in the assay (addition of unspecific IgGs at a concentration of 2 mg/ml) resulted in a significant decrease in ADCC activity for both mAbs. However, the remaining ADCC activity of MB314 was significantly higher than that of MB 311, equal to at least that of MB311 without addition of unspecific IgG ().
Figure 3. Competition of the ADCC reaction with excess human IgG. 51Cr labeled SKBR-3 cells (T) were incubated with effector cells (E) at an E:T ratio of 30:1 for 16 h. Specific lysis (%) induced by 400 ng/ml of MB 314 or MB 311, respectively, in the presence or absence of excess of human IgG is shown. Horizontal line indicates lysis of target cells by effector cells without antibody. Mean and SD of triplicates are shown.
Antibody dependent cytokine release
To gain more insight into the differences observed in ADCC potency, an antibody dependent cytokine release (ADCR) assay was performed by using Multiplex measurement of a panel of cytokines (IL-1β, IL-2, IFNγ, IFNα2a, ΤΝFα, MCP-1, IL-6, IL-8, IL-12 and IL-13). These cytokines are known to be associated with cellular effector–target or effector–effector interactions during cellular interactions. The release of TNFα after 3 h incubation of PBMC (effector cells) with Lewis Y positive tumor cells (target cells) and various concentrations of MB314 or MB311 is shown in . For both mAbs a significant dose–dependent release of TNFα during ADCC was found. When comparing the dose dependency for the two mAbs in ADCR, the glyco-modified MB314 induced comparable cytokine levels at a 10-fold lower mAb concentration compared with the parental mAb MB311. This correlates well with the differences observed in ADCC activity (). Competition experiments with excess of multi-specific IgG (2 mg/ml) showed a significant decrease in cytokine release mediated by the two mAbs (). Similar results were found for MCP-1 (), IL-6 (), and IL-8 (data not shown). Most importantly, whereas no IFNγ was measured after incubation with MB311 (even at the highest concentrations), significant levels of IFNγ were measured after incubation with MB314 (). No measurable levels of IL-1β, IL-2, IL-12, IL-13 and IFNα2a were detectable for either of the two mAbs under investigation (data not shown).
Figure 4. Antibody-dependent cytokine release induced by MB314 or MB311. Antibody dependent cytokine release was measured in parallel to the antibody-dependent cell lysis assay. SKBR-3 target cells and effector cells (PBMCs from a healthy volunteer donor) at an E:T ratio of 25:1 were incubated with different concentrations of MB311 or MB314, respectively, for 3 h. Supernatants were analyzed for release of cytokines using xMAP multiplex technology. Release of TNFα (A, B), MCP-1 (C, D), IL-6 (E, F) and IFNγ (G, H) are shown. Mean and SD of triplicates are shown. Competition assay: Target and effector cells were incubated with the highest concentrations of MB314 or MB311 (400 ng/ml and 4 mg/ml, respectively) in the absence or presence of excess of human IgG.
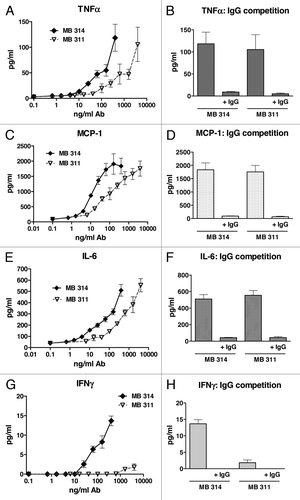
Figure 5. Intracellular Expression of tumor necrosis factor (TNF) by CD14+ cells, but not by CD3+ or CD56+ cells. SKBR-3 target cells and effector cells (PBMCs from a healthy volunteer donor) at an E:T ratio of 25:1 were incubated with 74 ng/ml of MB314 (A) or MB311 (B) or without antibody (C) for 5 h. Cells were harvested, stained with FITC-mouse anti-CD14 antibody or mouse anti-CD56 antibody and anti-mIgG Dylight 488, or Cy5-mouse anti-CD3 antibody, fixed, permeabilized, and subsequently stained with PE-mouse anti human TNFα antibody. Staining was measured by FACS analysis. The data reflect gating on PBMC effector cells (red) (and excluding target cells, green) based on forward and side scattered light signals (left panel). Intracellular staining of TNFα gated on CD14+ or CD56+ (middle panel) and CD3+ cells are shown (right panel). As positive control, PBMC effector cells were incubated with 1 µg/ml LPS (D). Effector cells (red) positive for intracellular TNF are encircled.
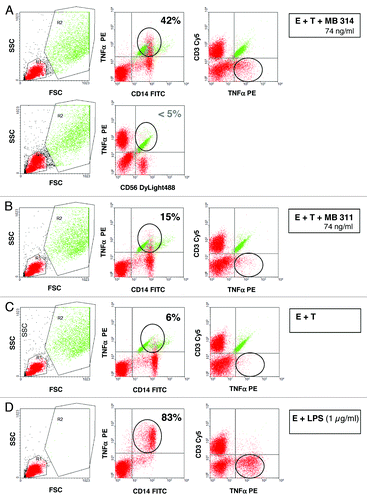
Cellular source of detected cytokines
To determine the source of the released cytokines, i.e., which effector or target cells are responsible for the cytokine release, a FACS based assay was set up for intracellular staining of selected cytokines of interest. The Lewis Y positive tumor cell line (SKBR-3) and PBMCs were used as target cells and effector cells, respectively, and incubated together with different concentrations of MB311 or MB314. Following 5 h incubation, the intracellular levels TNFα and IFNγ in the effector cells or target cells were measured by FACS analysis. As shown in significant amounts of TNFα staining CD14+ positive cells were detected, whereas no significant TNFα expression was found for any other cell population, including CD3+ and CD56+ cells, indicating that the vast majority of TNFα is released from mononuclear cells, such as macrophages. In contrast, intracellular staining for IFNγ was absent in CD14+ cells, but seemed to be associated with CD56+ cells, although no clear cut data regarding the cell subset responsible for its release could be obtained, probably due to the generally low amounts of IFNγ released (compare also ). In summary, the ADCR data provide evidence for a significant release of immunostimulatory cytokines during ADCC using both mAb glyco-types. In particular this holds true for those cytokines that are known to be associated with effector functions such as TNFα, IL-6, or cellular chemo-attraction such as IL-8 and MCP-1, which are, however, induced at lower concentrations of the glyco-modified MB 314. Interestingly, IFNγ, a cytokine involved in activation of effector cells such as macrophages and NK cells,Citation34 as well as regulation of MHC expression on target cells, was found only with the glyco-modified MB 314, and was absent for the parental MB311.
Discussion
A plant-based expression system employing a gene-engineered xylosyl- and fucosyl-transferase deficient moss cell line was used to produce a therapeutic mAb with tailor-made N-linked glycosylation. This glyco-engineered mAb, designated MB314, was compared with its parental mAb MB311, which was expressed in mammalian SP2.0 cells. MB311 binds to the tumor-associated carbohydrate antigen Lewis Y, which is expressed on 70–90% of epithelial cancers such as lung cancer, colon cancer, breast, ovarian cancer, cervical cancer, stomach and pancreas adenocarcinoma, but with only limited expression on normal tissues.Citation28 High levels of Lewis Y expression correlate with poor survival in patients with lung carcinomaCitation29 and breast cancer.Citation30-Citation32
For the parental mAb MB311, a dual mode of action leading to (1) signal transduction inhibition of Lewis Y glycosylated erbB receptors resulting in direct blocking of tumor cell growthCitation36,Citation37 and (2) destruction of Lewis Y expressing tumor cells by activation of Fc-mediated effector functions such as ADCC and CDC has previously been demonstrated.Citation35 MB311 has successfully completed an open-label dose escalation Phase I clinical trial in patients with Lewis Y positive epithelial cancers and has demonstrated good safety characteristics, a favorable pharmacokinetic profile, and significant ex vivo cytolytic activity.Citation35
Modification of the glycosylation moieties attached to the Fc-part of mAbs has recently become a promising approach to optimize effector functions.Citation11-Citation15,Citation38 In particular, core fucosylated oligosaccharides showed weaker binding to the FcγRIIIa receptor (CD16) expressed on effector cells and resulted in a decreased lytic potential. Accordingly, the expression of recombinant mAbs in glyco-optimized mammalian expression hosts with a reduced or complete lack of core fucosylation activity, have shown to generate mAbs with enhanced lytic potential, especially considering ADCC.Citation16-Citation20 However, a stable expression of a homogeneous glycosylation pattern has been a difficult challenge for most expression systems in use. Beside genetically-engineered mammalian expression systems, plant-based expression systems have also been used to modify glycosylation of mAbs.Citation18,Citation33 A murine Lewis Y-specific mAb, BR55–2, was expressed in transgenic tobacco plants with low alkaloid content. Because core fucosylation of that mAb was not affected in the transgenic tobacco plants, ADCC activity was comparable to the parental mAb BR55–2.Citation39
In the present study, a unique expression system based on a fucosyl- and xylosyl- transferase deficient, (Δxyl-t/Δfuc-t) double knockout Physcomitrella patens moss line was used to produce MB314, an α1,6-fucose free, humanized mAb with additional quantitative removal of plant-specific N-glycan alpha1,3-fucose and β1,2-xylose residues. MB314 stably expressed in Physcomitrella patens was demonstrated to assemble correctly, with intact heavy and light chains and showed a homogeneous N-linked oligo-saccharide pattern of a typical GnGn (G0) type, with no detectable core fucosylation (and plant-specific xylosylation).Citation27 The target specificity of the glyco-engineered MB314 was not affected compared with its parental mAb MB311 as confirmed by FACS. Binding data to CD16 expressed on the surface of NK cells isolated from healthy human donors proved the higher affinity of MB314. Most importantly, the ADCC effector function of the fucose-deficient MB314 was increased up to 40-fold (based on EC50 values) compared with MB311 (demonstrated here for different Lewis Y positive tumor cell lines). Most relevant from a clinical application perspective was the confirmation that the inhibitory effect of an excess of multi-specific immunoglobulin, simulating the situation in the serum of the patients, was largely compensated by the significantly higher ADCC activity of the glyco-engineered mAb. In contrast, ADCC activity mediated by the parental MB311 was almost completely abolished by addition of excess of unspecific IgG. These data indicate that a significantly higher ADCC activity of glyco-optimized therapeutic mAbs can be expected in a clinical setting whereas the ADCC activity of unmodified therapeutic mAb is largely abolished by the presence of high amounts of multi-specific endogenous IgG.Citation5,Citation26
A second critical finding of the present study, equal in interest to the increased ADCC activity induced by the glyco-modified mAb, was the significant increase in the dose-dependent temporary release of stimulatory cytokines. In particular, cytokines such as TNFα, IL-6, IFNγ or chemokines such as IL-8 and MCP-1, which have been described during antibody-dependent cytokine release,Citation22-Citation25 were found to be significantly enhanced for the glyco-modified antibody. Antibody-dependent cytokine release was dependent on the presence of both, target cells and effector cells. TNFα release was found to be associated with CD14+ cells, indicating that, besides the FcγRIIIα-carrying NK cells, other cells such as monocytes / macrophages are also involved in mediation of cytotoxic activity. This result is consistent with previous reports on unmodified mAb.Citation22-Citation25 The enhanced cytokine release found with the glyco-modified MB314 can be expected to lead also to enhanced chemo-attractance of inflammatory cells (IL-8, MCP-1), increased antigen expression on target cells (IFNγ) or increased effector functions of the effector cells (TNFα, IFNγ, IL-6). These effects are likely to result in higher therapeutic efficacy, but could theoretically also enhance the potential for toxic side effects.Citation40-Citation42 However, the moderate amounts measured in this study for most of the cytokines, together with the noteworthy finding that the cytokines were not systemically detectable in the presence of excess of human IgG, indicate that the stimulatory effects of the cytokines should manifest only in the vicinity of the releasing cells, i.e., in an autocrine/paracrine manner,Citation43 with the potential for side effects decreasing in serum with distance.
Finally, CDC reactivity against Lewis Y positive target cells was tested. In contrast to the significantly enhanced ADCC activity, a 4–5-fold lower CDC activity was found for the glyco-modified MB314. In contrast to the binding of the Fc-part of the mAb to effector cell expressed FcγRIII receptors, which is strongly enhanced by removal of the core fucosylation, the influence of absence/presence of core fucosylation on CDC activity has not been described. Regarding MB314, another effect of the utilized moss based expression system may play a role: in contrast to the parental MB311 expressed in mammalian cells that shows a substantial degree of terminal galactosylation, the glyco-engineered MB314 shows a very homogeneous ‘GnGn’ pattern with no N-terminal galactose. N-terminal galactose has been described to be associated with activation of complement system.Citation44,Citation45 Therefore, the lack of N-terminal galactose in the glyco-engineered MB314 might explain the lower CDC activity. However, although no terminal galactose is present on the glyco-modified MB314, moderate CDC activity remains. This might very well be a beneficial situation balancing the negative side effects and positive effector function. Noteworthy, complement activation seems not only to be part of the anti-tumor activity mediated by therapeutic mAbs, but has also been shown to be associated with infusion related toxicities. Recent reports have shown that application of therapeutic mAb can lead to severe infusion reactions in monkeys and human that were found to correlate with activation of the complement system.Citation6,Citation7 Application of a chimeric anti-CD20 mAb resulted in moderate to severe first-dose side effects, notably in patients with high numbers of circulating tumor cells.Citation6 Therefore, therapeutic mAbs with strongly enhanced ADCC activity combined with moderately reduced complement activation may have a broader therapeutic window regarding anti-tumor activity and toxic side effects.Citation21
The data presented demonstrate that the effector function profile of therapeutic mAbs can be significantly changed by glyco-engineering. The therapeutic window between different effector functions, i.e., ADCC, cytokine release and CDC, could be fine-tuned by adjusting the glycosylation of the particular therapeutic mAb.
Materials and Methods
Tumor cell lines
Tumor cell lines Ovcar-3, SKBR-3 and Kato-III were purchased from American Type Culture Collection (Manassas, CA, USA). The Lewis Y antigen density was determined by FACS analysis using the humanized, Lewis Y specific mAb MB311.
Generation of a stably transfected Δxyl-t/Δfuc-t double knockout moss line and production of humanized lewis Y specific mAb MB314
The humanized Lewis Y specific mAb MB314 (IgG1/κ) was stably produced by greenovation’s Bryo-Master expression system using a double-knockout Physcomitrella patens moss cell line.Citation27 Clones identified as positive by PCR for both heavy and light chain and by the presence of IgG in culture supernatant (48-well format) were propagated by further cultivation in liquid medium. Plant material was harvested from a photo bioreactor run and homogenized in ice cold TBSH buffer (20 mM Tris, 500 mM NaCl, pH 7.4 - 7.5) using high pressure homogenization (at 600 bar; Niro Soavi, Model Panda NS 1001L) and sedimented by centrifugation for 20 min, at 4°C at 9000 rpm. The resulting supernatant was filtered through 1 μm glass fiber filter (Pall) and 0.45 μm HV Durapore (Millipore) and purified twice using Protein A chromatography. Finally, MB314 was dialyzed against PBS using Slide-A-Lyzer® Dialysis Cassettes (Pierce). The yield was 25 mg MB314 / 364 g fresh weight.
Clinical grade material of the humanized Lewis Y-specific mAb MB311 was produced in SP 2.0 cells using a FCS containing hollow fiber production process and a classical down-stream process including a Protein A capture step.Citation35
Analytical characterization of MB314 and MB311
Concentration of MB311 and MB314 was determined by measuring the absorbance at 280 nm using an Ultraspec 3000 Pro Photometer (Amersham). Integrity, molecular weight, potential degradation products and the N-linked oligosaccharide profiles of purified expression products were analyzed by high-performance size exclusion chromatography, SDS-PAGE, Western Blot, and matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis exactly as described.Citation29 The endotoxin concentration was determined using the Endosafe®-PTS cartridge system (PTS cartridge 0.05, Charles River Laboratories) according to the manufacturer’s instructions. For both, the mammalian cell produced clinical grade material MB311 (stock concentration: 10 mg/ml) and for the plant expressed MB314 (stock concentration: 3 mg/ml) the endotoxin values were below 0.5 EU/ml, i.e., < 0.05 EU/mg and < 0.2 EU/mg, respectively. At the highest Ab concentrations used in the present study (i.e., 10 µg/ml) endotoxin levels were therefore below 0.0005 EU/ml and 0.002 EU/ml, respectively.
Antibody binding to lewis Y positive tumor cell lines
Binding of mAbs MB 311 and MB 314 to Lewis Y positive tumor cell lines was determined by FACS analysis. The mAbs were incubated at different concentrations with the cells for 30 min. on ice, washed and incubated with a FITC labeled anti-human IgG (Chemicon). After washing, the cells were incubated with 7-AAD (diluted 1:200) for 20 min on ice. The mean fluorescence intensity (MFI) was measured using a FACSCalibur instrument (Becton Dickinson). Geometric MFI were plotted against the logarithm of the mAb concentration (ng/ml) and fitted using a four parameter logistic fit using GraphPad Prism 4 software. EC50 values were calculated and used for quantification.
Antibody binding to freshly isolated human NK cells
NK cells were isolated from freshly donated whole blood. In a first step PBMCs were isolated via density gradient centrifugation with LymphoprepTM (Axis-Shield). In a second step, NK cells were isolated by negative depletion with MACS technology (NK cell isolation kit and MACS separation columns, Miltenyi Biotec). The purified NK were stained with CD16 and CD56 antibodies and analyzed by FACS technology as quality control. The purity was determined as > 80%. For the binding experiment, 1 × 105 NK cells were incubated with 10 µg/ml of MB311 or MB314 for 30 min on ice. Detection was done with PE labeled secondary antibody anti-human IgG goat F(ab’)2 fragment (H+L) (Beckton Coulter) and following FACS analysis (FACSCalibur).
Determination of complement dependent cytotoxicity activity
CDC activity was determined by FACS as described previously.Citation46 Two Lewis Y positive cell lines, the breast cancer cell line SKBR-3 and the ovarian cancer cell line OVCAR-3, were incubated with various concentrations of MB311 or MB314 in the presence of human complement for 1 h. As complement source, frozen human serum was used. For maximum lysis control, 50 µl Triton X-100 were added to the cells. Specific lysis (%) was calculated using the formula: specific lysis (%) = 100% * (sample lysis – spontaneous lysis) / (Max lysis – spontaneous lysis). After 1 h incubation with serial dilutions of MB311 or MB314 cytotoxicity was quantified by FACS analysis using a live/dead cell stain. Geometric MFI were plotted against the logarithm of the mAb concentration (ng/ml) and fitted using a four parameter logistic fit using GraphPad Prism 4 software. EC50 values were calculated and used for quantification.
Determination of antibody dependent cellular cytotoxicity activity
ADCC activity was determined as previously described.Citation47 ADCC was tested in triplicates in a 51Cr release assay using various Lewis Y positive cancer cell lines as target cells (SK-BR-3, Kato-III and Ovcar 3). Target cells were labeled by incubation for one hour with 100 μCi of Na251Cr2O7 solution in PBS, washed, and plated at a density of 25 x 103 cells per well into 96-well microplates. Effector cells (PBMCs from a healthy volunteer donor) were added to the target cells at indicated effector:target ratios (E:T) together with serial dilutions (100 pg-10 μg/ml) of MB311 or MB314. After incubation at 37°C for 16 h in a CO2-incubator, cell supernatants were collected and released 51Cr (Cs) was counted using a Cobra 5005 γ-counter (Canberra-Packard, Australia). Values for spontaneous release (Sr) and maximum release (Mr) were measured after incubation of representative samples with medium alone or with medium supplemented with detergent (25% SDS added 10 min before harvesting of cells), respectively. Cytotoxicity was calculated by the formula: specific cell lysis (%) = 100% * (Cs-Sr) / (Mr-Sr). The percentage cytotoxicity was plotted against the logarithm of the antibody concentration (ng/ml) and fitted using a sigmoidal four parameter fit using GraphPad Prism 4 software. EC50 values were calculated and used for quantification.
Determination of antibody dependent cytokine release
An ADCR assay was set up with Lewis Y positive tumor cell lines (SKBR-3 or OVCAR-3) as target cells and PBMC as effector cells together with different concentrations of MB311 or MB314, using xMAP multiplex technology. Following a 3 h incubation, ADCR, i.e., the levels of IL-1β, IL-2, IFNγ, IFNα2a, ΤΝFα, MCP-1, IL-6, IL-8, IL-12 and IL-13 in the supernatant was measured using Upstate (Millipore) multiplexing kits and a Bio-Plex Suspension Array system 100 (Bio-Rad) as described previously.Citation48
Intracellular staining of cytokine release
Analogously to the ADCR assay, an FACS based assay for intracellular staining of released cytokines was used. The Lewis Y positive tumor cell line (SKBR-3) and PBMCs were used as target cells and effector cells (E:t = 10:1), respectively, and incubated together with different concentrations of MB 311 or MB 314 for 5 h. Brefeldin A (5 mg/ml in DMSO, 1:500) at a final concentration of 10 µg/ml was added to block the release of the cytokine from the Golgi apparatus. Cells were harvested, washed with PBS containing 2% FCS and stained with a FITC-labeled mouse anti-human CD14 mAb (BD PharMingen) or a mouse anti-human CD56 mAb as primary antibody (Immunotech) and Dylight 488-labeled anti-mIgG as secondary antibody, or a Cy5-labeled mouse anti-human CD3 mAb (BD PharMingen), fixed with 2% PFA (for 20 min), permeabilized with 0.1% saponin, and subsequently stained with PE-labeled mouse anti-human TNFα antibody or PE-labeled mouse anti-human IFNγ antibody (BD PharMingen). Intracellular cytokine staining was measured with a FACSCalibur. Gating on PBMC effector cells (and excluding target cells) was based on forward and side scattered light signals. As positive control, PBMC effector cells were incubated 20 h with 1 µg/ml LPS.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dietmar Payrhuber for FcγRIII typing and Manual Mayer for technical assistance.
References
- Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs 2003; 63:803 - 43; http://dx.doi.org/10.2165/00003495-200363080-00005; PMID: 12662126
- Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol 2000; 18:3135 - 43; PMID: 10963642
- Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med 2005; 353:1734 - 6; http://dx.doi.org/10.1056/NEJMe058196; PMID: 16236745
- Collins TS, Hurwitz HI. Targeting vascular endothelial growth factor and angiogenesis for the treatment of colorectal cancer. Semin Oncol 2005; 32:61 - 8; http://dx.doi.org/10.1053/j.seminoncol.2004.09.026; PMID: 15726507
- Preithner S, Elm S, Lippold S, Locher M, Wolf A, da Silva AJ, et al. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol Immunol 2006; 43:1183 - 93; http://dx.doi.org/10.1016/j.molimm.2005.07.010; PMID: 16102830
- van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MHJ. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol 2001; 115:807 - 11; http://dx.doi.org/10.1046/j.1365-2141.2001.03166.x; PMID: 11843813
- Tawara T, Hasegawa K, Sugiura Y, Harada K, Miura T, Hayashi S, et al. Complement activation plays a key role in antibody-induced infusion toxicity in monkeys and rats. J Immunol 2008; 180:2294 - 8; PMID: 18250438
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754 - 8; http://dx.doi.org/10.1182/blood.V99.3.754; PMID: 11806974
- Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AEGK, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997; 90:1109 - 14; PMID: 9242542
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem 2001; 276:6591 - 604; http://dx.doi.org/10.1074/jbc.M009483200; PMID: 11096108
- Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol 2004; 336:1239 - 49; http://dx.doi.org/10.1016/j.jmb.2004.01.007; PMID: 15037082
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277:26733 - 40; http://dx.doi.org/10.1074/jbc.M202069200; PMID: 11986321
- Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res 2004; 10:6248 - 55; http://dx.doi.org/10.1158/1078-0432.CCR-04-0850; PMID: 15448014
- Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem 2006; 281:5032 - 6; http://dx.doi.org/10.1074/jbc.M510171200; PMID: 16330541
- Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther 2006; 6:1161 - 73; http://dx.doi.org/10.1517/14712598.6.11.1161; PMID: 17049014
- Jefferis R, Lund J, Pound JD. IgG-Fc-mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol Rev 1998; 163:59 - 76; http://dx.doi.org/10.1111/j.1600-065X.1998.tb01188.x; PMID: 9700502
- Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng 2001; 74:288 - 94; http://dx.doi.org/10.1002/bit.1119; PMID: 11410853
- Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 2006; 24:1591 - 7; http://dx.doi.org/10.1038/nbt1260; PMID: 17128273
- Schuster M, Umana P, Ferrara C, Brünker P, Gerdes C, Waxenecker G, et al. Improved effector functions of a therapeutic monoclonal Lewis Y-specific antibody by glycoform engineering. Cancer Res 2005; 65:7934 - 41; PMID: 16140965
- Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 2005; 21:1644 - 52; http://dx.doi.org/10.1021/bp050228w; PMID: 16321047
- Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood 2004; 104:2635 - 42; http://dx.doi.org/10.1182/blood-2004-03-1110; PMID: 15226177
- Dettke M, Loibner H. Different types of FCgamma-receptors are involved in anti-Lewis Y antibody induced effector functions in vitro. Br J Cancer 2000; 82:441 - 5; PMID: 10646902
- Marsh CB, Gadek JE, Kindt GC, Moore SA, Wewers MD. Monocyte Fc gamma receptor cross-linking induces IL-8 production. J Immunol 1995; 155:3161 - 7; PMID: 7673729
- Deo YM, Graziano RF, Repp R, van de Winkel JG. Clinical significance of IgG Fc receptors and Fc gamma R-directed immunotherapies. Immunol Today 1997; 18:127 - 35; http://dx.doi.org/10.1016/S0167-5699(97)01007-4; PMID: 9078685
- Debets JM, Van de Winkel JG, Ceuppens JL, Dieteren IE, Buurman WA. Cross-linking of both Fc gamma RI and Fc gamma RII induces secretion of tumor necrosis factor by human monocytes, requiring high affinity Fc-Fc gamma R interactions. Functional activation of Fc gamma RII by treatment with proteases or neuraminidase. J Immunol 1990; 144:1304 - 10; PMID: 2137489
- Nechansky A, Schuster M, Jost W, Siegl P, Wiederkum S, Gorr G, et al. Compensation of endogenous IgG mediated inhibition of antibody-dependent cellular cytotoxicity by glyco-engineering of therapeutic antibodies. Mol Immunol 2007; 44:1815 - 7; http://dx.doi.org/10.1016/j.molimm.2006.08.013; PMID: 17011625
- Schuster M, Jost W, Mudde GC, Wiederkum S, Schwager C, Janzek E, et al. In vivo glyco-engineered antibody with improved lytic potential produced by an innovative non-mammalian expression system. Biotechnol J 2007; 2:700 - 8; http://dx.doi.org/10.1002/biot.200600255; PMID: 17427997
- Kim YS, Yuan M, Itzkowitz SH, Sun QB, Kaizu T, Palekar A, et al. Expression of LeY and extended LeY blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res 1986; 46:5985 - 92; PMID: 2428490
- Miyake M, Taki T, Hitomi S, Hakomori S. Correlation of expression of H/Le(y)/Le(b) antigens with survival in patients with carcinoma of the lung. N Engl J Med 1992; 327:14 - 8; http://dx.doi.org/10.1056/NEJM199207023270103; PMID: 1317941
- Steplewska-Mazur K, Gabriel A, Zajecki W, Wylezoł M, Glück M. Breast cancer progression and expression of blood group-related tumor-associated antigens. Hybridoma 2000; 19:129 - 33; http://dx.doi.org/10.1089/02724570050031167; PMID: 10868792
- Inufusa H, Nakatani Y, Adachi T, Wakano T, Nakajima A, Nakamura M, et al. Correlation of prognosis of breast cancer patients and expression of Ley which acts as a cofactor of tumor procoagulant. Int J Oncol 1998; 13:481 - 7; PMID: 9683782
- Madjd Z, Parsons T, Watson NF, Spendlove I, Ellis I, Durrant LG. High expression of Lewis y/b antigens is associated with decreased survival in lymph node negative breast carcinomas. Breast Cancer Res 2005; 7:R780 - 7; http://dx.doi.org/10.1186/bcr1305; PMID: 16168124
- Ishida T, Ishii T, Inagaki A, Yano H, Kusumoto S, Ri M, et al. The CCR4 as a novel-specific molecular target for immunotherapy in Hodgkin lymphoma. Leukemia 2006; 20:2162 - 8; http://dx.doi.org/10.1038/sj.leu.2404415; PMID: 17039235
- Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007; 96:41 - 101; http://dx.doi.org/10.1016/S0065-2776(07)96002-2; PMID: 17981204
- Oruzio DV, Waxenecker G, Aulmann C, Märkl B, Wagner T, Mudde GC, et al.. Phase I dose escalation study with Le Y carbohydrate specific humanized mAb IGN311. J Cancer Therapy 2011; (2):760-71.
- Farhan H, Schuster C, Klinger M, Weisz E, Waxenecker G, Schuster M, et al. Inhibition of xenograft tumor growth and down-regulation of ErbB receptors by an antibody directed against Lewis Y antigen. J Pharmacol Exp Ther 2006; 319:1459 - 66; http://dx.doi.org/10.1124/jpet.106.107318; PMID: 16963623
- Klinger M, Farhan H, Just H, Drobny H, Himmler G, Loibner H, et al. Antibodies directed against Lewis-Y antigen inhibit signaling of Lewis-Y modified ErbB receptors. Cancer Res 2004; 64:1087 - 93; http://dx.doi.org/10.1158/0008-5472.CAN-03-2435; PMID: 14871842
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 2003; 278:3466 - 73; http://dx.doi.org/10.1074/jbc.M210665200; PMID: 12427744
- Brodzik R, Glogowska M, Bandurska K, Okulicz M, Deka D, Ko K, et al. Plant-derived anti-Lewis Y mAb exhibits biological activities for efficient immunotherapy against human cancer cells. Proc Natl Acad Sci U S A 2006; 103:8804 - 9; http://dx.doi.org/10.1073/pnas.0603043103; PMID: 16720700
- Wing MG, Moreau T, Greenwood J, Smith RM, Hale G, Isaacs J, et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest 1996; 98:2819 - 26; http://dx.doi.org/10.1172/JCI119110; PMID: 8981930
- Tax WJ, Tamboer WP, Jacobs CW, Frenken LA, Koene RA. Role of polymorphic Fc receptor Fc gammaRIIa in cytokine release and adverse effects of murine IgG1 anti-CD3/T cell receptor antibody (WT31). Transplantation 1997; 63:106 - 12; http://dx.doi.org/10.1097/00007890-199701150-00020; PMID: 9000670
- Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999; 94:2217 - 24; PMID: 10498591
- Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol 1995; 13:399 - 415; http://dx.doi.org/10.1146/annurev.iy.13.040195.002151; PMID: 7612229
- Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol Immunol 1995; 32:1311 - 8; http://dx.doi.org/10.1016/0161-5890(95)00118-2; PMID: 8643100
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol 2008; 20:471 - 8; http://dx.doi.org/10.1016/j.coi.2008.06.007; PMID: 18606225
- Nechansky A, Szolar OH, Siegl P, Zinoecker I, Halanek N, Wiederkum S, et al. Complement dependent cytotoxicity (CDC) activity of a humanized anti Lewis-Y antibody: FACS-based assay versus the ‘classical’ radioactive method -- qualification, comparison and application of the FACS-based approach. J Pharm Biomed Anal 2009; 49:1014 - 20; http://dx.doi.org/10.1016/j.jpba.2009.01.029; PMID: 19250790
- Co MS, Baker J, Bednarik K, Janzek E, Neruda W, Mayer P, et al. Humanized anti-Lewis Y antibodies: in vitro properties and pharmacokinetics in rhesus monkeys. Cancer Res 1996; 56:1118 - 25; PMID: 8640770
- Kircheis R, Siegl P, Grunt S, Halanek N, Loibner H, Mudde GC, et al. Immunization of Rhesus monkeys with a SialylTn-mAb17-1A conjugate vaccine co-formulated with QS-21 induces a temporary systemic cytokine release and NK cytotoxicity against tumor cells. Cancer Immunol Immunother 2007; 56:863 - 73; http://dx.doi.org/10.1007/s00262-006-0231-x; PMID: 17009044