Abstract
The effector functions of therapeutic antibodies are strongly affected by the specific glycans added to the Fc domain during post-translational processing. Antibodies bearing high levels of N-linked mannose-5 glycan (Man5) have been reported to exhibit enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) compared with antibodies with fucosylated complex or hybrid glycans. To better understand the relationship between antibodies with high levels of Man5 and their biological activity in vivo, we developed an approach to generate substantially homogeneous antibodies bearing the Man5 glycoform. A mannosidase inhibitor, kifunensine, was first incorporated in the cell culture process to generate antibodies with a distribution of high mannose glycoforms. Antibodies were then purified and treated with a mannosidase for trimming to Man5 in vitro. This 2-step approach can consistently generate antibodies with > 99% Man5 glycan. Antibodies bearing varying levels of Man5 were studied to compare ADCC and Fcγ receptor binding, and they showed enhanced ADCC activity and increased binding affinity to the FcγRIIIA. In addition, the clearance rate of antibodies bearing Man8/9 and Man5 glycans was determined in a pharmacokinetics study in mice. When compared with historical data, the antibodies bearing the high mannose glycoform exhibited faster clearance rate compared with antibodies bearing the fucosylated complex glycoform, while the pharmacokinetic properties of antibodies with Man8/9 and Man5 glycoforms appeared similar. In addition, we identified the presence of a mannosidase in mouse serum that converted most Man8/9 to Man6 after 24 h.
Introduction
The study of antibody glycosylation is growing in importance because of the essential role carbohydrates play in modulating effector functions, which affect the safety and efficacy of therapeutic antibodies.Citation1,Citation2 Induction of antibody-dependent cell-mediated cytotoxicity (ADCC) requires binding of the Fc region of the antibody to Fcγ receptors that are present on the surface of effector cells. The antibody simultaneously binds to its respective antigen (usually present on the surface of tumor cells) via the Fab region. The activation of effector cells (e.g., natural killer cells) through Fcγ receptor binding then initiates a signal cascade that results in the destruction of the tumor cells.Citation1 The level of ADCC activity is modulated by the binding affinity between the Fc region of the antibody and the Fcγ receptor on the surface of the effector cell. The Fc region of antibodies carries an N-linked glycan at the asparagine 297 residue that has been shown to affect Fcγ receptor binding and ADCC activity of the antibodies.Citation3,Citation4 Crystal structures of the Fcγ receptor complexed with fucosylated and afucosyl Fc that help explain the observed differences in affinity were recently reported.Citation5 It should be noted that this discussion pertains primarily to the IgG1 isotype, which is the isotype of the antibodies used in the present study.
Modulation of the glycosylation pattern on therapeutic antibodies to improve ADCC activity has been widely studied.Citation3,Citation6-Citation11 A major focus has been on the study of afucosylated antibodies whose Fc N-linked glycans lack core fucose, which results in an up to 50-fold increase in ADCC activity.Citation8,Citation11 Different methods to generate the complex type afucosylated (AF) glycoform have been investigated, including cell line engineering, knockout/knockdown of the α-1,6-fucosyltransferase that is responsible for adding fucose to the glycan,Citation12 and glycoengineering of a yeast expression system to generate complex human glycoproteins.Citation13,Citation14 Some groups have focused on the addition of small molecule inhibitors of specific reactions along the glycosylation pathway to produce the desired glycoform.Citation7,Citation9 Finally, many proof of concept studies were conducted with a series of CHO cell lines derived by Stanley using lectin selection, which generated a variety of glycosylation phenotypes.Citation8,Citation10,Citation15
Several reports have compared the biological activity and pharmacokinetics (PK) properties of antibodies bearing various glycoforms.Citation7,Citation10 In particular, Kanda et al. generated a panel of different glycoforms with an anti-CD20 antibody, including complex with and without core fucose, hybrid with and without core fucose, and two types of high mannose glycan (Man5 and Man8/9).Citation7 The different glycoforms were generated using methods that included cell lines generated by Stanley and incorporation of small molecule inhibitors during cell culture to manipulate the glycosylation pattern of the antibodies. Mass spectrometry was used to confirm the glycan structure of these antibodies, and further characterization of the properties of these antibodies was reported. One interesting observation is the increased ADCC activity associated with both Man8/9 and Man5 high mannose glycoforms compared with the typical complex-fucosylated glycoform. This result was confirmed in a study by Zhou et al. in which kifunensine was added to cell culture media to generate antibodies bearing mainly Man8/9 glycoforms.Citation9 These findings suggested that therapeutic antibodies bearing high mannose glycans may have enhanced potency and efficacy.
Although the high mannose glycoform has potentially advantageous biological activities, studies have shown that proteins having high mannose glycoforms may have more rapid clearance. Binding of high mannose glycoproteins to receptors such as mannose receptors and mannose binding proteins may lead to differential clearance of the molecule.Citation16,Citation17 Wright and Morrison reported rapid clearance associated with antibodies generated using the Lec1 cell lineCitation10,Citation18,Citation19 that were found to consist primarily of high mannose glycoforms.Citation20 However, the clearance profile published by Wright and Morrison appeared to be bi-phasic. They observed that 80% of all high mannose antibodies were cleared in the α phase, while the clearance rate in β phase appeared similar to other antibody glycoforms.Citation10 Kanda et al. reported a slightly different clearance pattern of the Man5 glycoform antibodies. They observed accelerated clearance of Man5 glycoform antibodies, but the clearance in the α phase seemed comparable to antibodies with other glycoforms. A divergence in the PK profile from antibodies bearing complex glycans was not apparent until ~5 d after the start of the study.Citation7 The comparable clearance profile observed in the first 5 d by Kanda et al. was consistent with a study by Zhou et al. They found no difference in clearance profile between antibodies with high mannose and complex glycans in a PK study with mice for up to 7 d.Citation9 Marian and Harris also reported no difference in clearance in mice between omalizumab (Xolair®) glycoforms having either Man5 or complex glycans (The impact of Fc glycans on the clearance of Xolair, WCBP Congress, 2002).
In addition, Chen et al. reported a mannosidase activity in human serum when investigating the clearance of different glycoforms of a therapeutic antibody in a human clinical study. They concluded that the change of clearance rate of high mannose glycoforms was a result of glycan cleavage, and not due to differential clearance of specific types of high mannose glycoform;Citation21 however, a later study using more refined analytical methods and an extended study length concluded that high mannose glycans were cleared faster.Citation22
Because the clearance profile of different types of high mannose glycoforms remains unclear, we focused on the production of antibodies bearing nearly homogeneous mannose-5 (Man5) glycans to enable better characterization of their biological activity and PK properties. The goal was to determine whether antibodies bearing only Man5 glycans show enhanced effector function, as well as a similar clearance rate, compared with the typical complex glycoform. To date most groups that have generated antibodies with Man5 glycans utilized the Lec1 cell line;Citation7,Citation20 however, the high mannose glycan distribution of antibodies from the Lec1 cell line appears inconsistent, with Kanda et al. reporting 100% Man5 and Wright et al. reporting various levels of Man5, Man6, Man7, Man8, and Man9. In this study, a 2-step approach was successfully developed to consistently generate antibodies with a substantially homogeneous Man5 glycoform. The first step involved adapting a method using kifunensine during cell cultureCitation9 to generate antibodies bearing more highly mannosylated glycans. After purification, the antibodies were processed in vitro to the Man5 glycoform using an α-1,2-mannosidase from Aspergillus saitoi.Citation20,Citation23,Citation24 The antibody preparations with different levels of Man5 and Man8/9 were then characterized for their biological activity including ADCC activity, CDC activity, and binding affinity to Fcγ receptors. Finally, antibodies with > 99% Man5 and > 99% Man8/9 glycans were studied in a PK study in mice. The serum samples collected from the PK study were isolated and further characterized to investigate the metabolism of the glycan in mouse serum.
Two different cell lines expressing humanized IgGs targeting different antigens were used in this study. The first model cell line expresses mAb1, which targets a soluble human growth factor, and produces unusually high levels of Man5 compared with other in-house cell lines (cell line A).Citation25 This cell line was used to optimize the conditions for generating the Man5 glycoform and its product, mAb1, was used for the PK study. To characterize the effector function of antibodies with different glycoforms, a second antibody (mAb2) targeted to a cell surface protein found on human B cells was expressed using a similar cell line.
Results
Cell Culture Performance in the Presence of Kifunensine
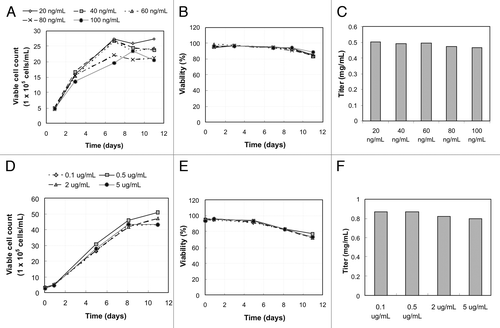
To confirm the minimal effect of kifunensine on cell growth and viability described by Zhou et al.,Citation9 kifunensine at different concentrations was first tested with the two model cell lines. For mAb1, kifunensine at 20, 40, 60, 80, and 100 ng/mL in basal media was tested, while 0.1, 0.5, 2, and 5 μg/mL of kifunensine were tested for mAb2. illustrates the growth characterization and the final titer for mAb1 (a-c) and mAb2 (d-f) after 11 d of culture. The viable cell count profile shown in and the viability profile in appeared very similar between the different concentrations of kifunensine, suggesting that the amount of kifunensine added has no effect on cell growth. show that final titers were also comparable for the different cases, confirming that kifunensine indeed has no effect on either growth or productivity for the cell lines selected for this study.
Figure 1. The growth profile, viability profile during course of culture, and the final titer on day 11. (A-C) are data for mAb1 when adding 20 (diamond), 40 (square), 60 (triangle), 80 (cross), and 100 ng/mL (circle) of kifunensine into basal media; (D-F) are data from mAb2 when adding 0.1 (diamond), 0.5 (square), 2 (triangle), 5 μg/mL (circle) of kifunensine into basal media. (A) and (D) are viable cell density at 1 × 105 cells/ mL measured on selected days using Vi-Cell XR (Beckman Coulter, Fullerton, CA). (B) and (E) are viability measured in percent. (C) and (F) are titer of antibody on day 11 after harvest.
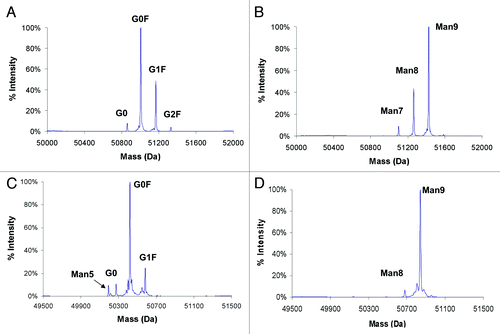
As reported in Zhou et al.Citation9 the overall percentage of high mannose glycoform increased as higher concentrations of kifunensine were used during cell culture. Our results were similar; as the kifunensine concentration increased from 20 to 100 ng/mL for mAb1, the percentage of high mannose glycoform increased while the percentage of complex glycoform decreased (data not shown). summarizes the glycan distribution profile of mAb1 and mAb2 with and without kifunensine at the corresponding highest concentration during cell culture. In the control case with no kifunensine, G0F, G1F and G2F glycoforms were the most prominent forms (). Kifunesine at 100 ng/mL for mAb1 production generated a mixture of Man7, Man8 and Man9, and kifunensine at 5 μg/mL resulted in mostly the Man9 glycoform for mAb2 with a small amount of Man8 ().
Figure 2. Mass spectra of the heavy chain of antibodies with different glycans measured by rpHPLC ESI-MS. Antibodies were expressed with or without kifunensine treatment during cell culture. (A) mAb1 without kifunensine; (B) mAb1 with 100 ng/mL kifunensine; (C) mAb2 without kifunensine; (D) mAb2 with 5 μg/mL kifunensine.
In Vitro Trimming Reaction Using α-1,2-mannosidase from Aspergillus Saitoi to Generate the Man5 Glycoform
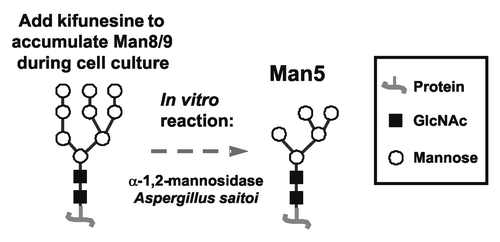
The addition of kifunensine during cell culture served as an excellent first step to the 2-step approach to generate Man5-enriched antibodies (). High mannose glycoforms were generated very efficiently with no hybrid or complex glycoforms found. The next step was to assess the ability of a mannosidase to trim the mannose in vitro from Man8/9 to Man5. The α-1,2-mannosidase from Aspergillus saitoi was selected for this purpose, and optimized reaction conditions were found that generated nearly homogeneous Man5 glycoform.
Preliminary experiments with commercially available mannosidase revealed that the addition of calcium and the extension of reaction time appeared to enhance the trimming reaction, which yielded higher Man5 content. These parameters were further optimized, and a CaCl2 concentration of 0.5 mM and a reaction time of 72 h were selected for subsequent mannosidase reactions (data not shown).
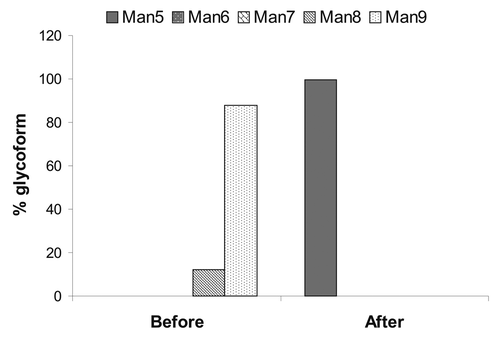
The Aspergillus saitoi α-1,2-mannosidase was later produced in-house and results from optimized conditions are shown in . The reaction mixture contained 0.5 mM CaCl2, 10 mg/mL of mAb2, and 0.2 mg/mL of α-1,2-mannosidase (enzyme specific activity was not determined) in 100 mM sodium acetate at pH 5.0. The reaction was kept at 37°C for 3 d, and then the samples were analyzed by reverse phase high performance liquid chromatography (rpHPLC) electrospray ionization mass spectrometry (ESI-MS) (). It appeared that the enzyme was very efficient at trimming all Man8/9 to Man5, indicated by the > 99% of Man5 obtained after in vitro trimming. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry assay also confirmed that the same sample contained > 99% Man5 (data not shown).
Antibody-dependent Cell-mediated Cytotoxicity Activity
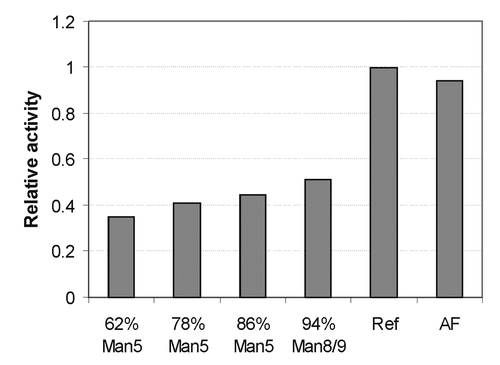
To assess whether antibodies with a high level of high mannose glycoforms have altered effector functions, evaluation in an ADCC assay was performed for mAb2. Four samples with different levels of high mannose (62% Man5, 78% Man5, 86% Man5, and 94% Man8/9) were tested against the complex-fucosylated glycoform as a reference. For comparison, mAb2 bearing the afucosyl (AF) glycoform (complex glycan lacking core fucose), which exhibits substantially higher ADCC activity,Citation7,Citation8 was also included in this study. Of note, the reference mAb2 was produced from regular CHO cells whereas the AF glycoform mAb2 was produced from a CHO cell line deficient in α-1,6-fucosyltransferase. Except for the difference in content of core fucose, the glycosylation profiles of the reference and the AF material are very similar. Both showed predominantly G0F and G1F glycoforms with a small amount of G2F, G0-GlcNAc, and Man5 glycoforms.
The ADCC data () show that all 4 different levels of high mannose glycoforms exhibit enhanced ADCC activity compared with the complex-fucosylated glycoform. A representative set of dose-response ADCC curves is shown in , and the effective concentration that reached 50% of its maximal activity (EC50) is presented in . Comparing the EC50 values of the different test antibodies, it appeared that all high mannose glycoforms exhibited a five- to seven-fold increase in ADCC (), similar to the AF glycoform, which has an eight-fold increase in this particular data set. This observation confirms that the high mannose glycoform can also enhance effector functions and increase activity of therapeutic antibodies similar to the AF complex glycoform. The increase in ADCC for the AF glycoform is modest in comparison with other reports in the literature where substantially higher increases in ADCC activity due to fucose depletion were reported.Citation7 However, the quantification of relative ADCC activity in these reports was largely based on estimated values extrapolated from incomplete dose response curves. In contrast, our data was based on EC50 values derived from complete dose response curves fitted with a 4-parameter model. Additionally, compared with the ADCC dose response curve of the reference molecule, those for the AF and the high mannose glycoforms both showed leftward shifts, indicating reduced EC50, and a higher upper asymptote indicating increased maximal ADCC level. Since our calculation of relative ADCC activity is based solely on the EC50 value, it is possible that we might have under-quantified the effect of afucosylation and high mannose by not accounting for changes in the upper asymptote of the dose response curves.
Figure 5. ADCC activity of mAb1 using peripheral blood mononuclear cells (PBMCs) from healthy donors as effector cells. (A) Percentage of ADCC measured at different concentrations of mAb1 for different levels of high mannose, 100% complex-afucosyl (AF), and complex-fucosylated (Ref) glycoforms. The data are shown as an average from duplicate experiments. (B) EC50 of the different glycoforms extracted from (A), and then normalized to reference.
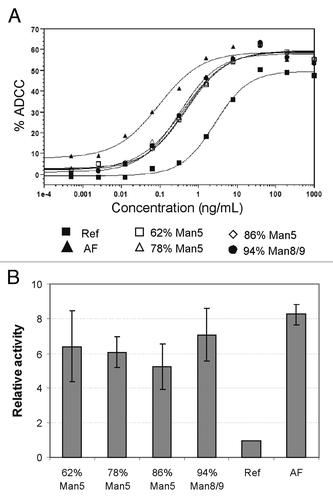
One additional observation from the ADCC data set is that the ADCC activity of the high mannose glycoforms does not appear to be affected by the ratio of Man5/Man9. Since all samples were generated via the kifunensine method, all 4 samples contained nearly all high mannose glycans regardless of the relative proportion of Man5, suggesting that any form of Man5-Man9 glycoform can result in enhanced ADCC activity.
Fcγ Receptor Binding Activity
Fcγ receptor binding activity of high mannose glycoform mAb2 was assessed in ELISA-based binding assays and compared against mAb2 with complex fucosylated and AF glycoforms. The EC50 values were determined from dose response binding curves and then normalized to the reference, and the relative binding affinities are portrayed in . FcγRIIIA binding is the most relevant with respect to ADCC mediated by NK cells since it is the only Fcγ receptor expressed on NK cells and binding between the antibodies and FcγRIIIA is required to induce ADCC activity. As shown in , all high mannose glycoforms have a similar level of increased binding affinity of 6 to 8-fold to FcγRIIIA [F158] and 4 to 6-fold to FcγRIIIA [V158] compared with reference glycoform. Of note, the FcγRIIIA binding activities of high mannose glycoforms were still lower compared with the AF glycoform. These data are consistent with the ADCC results that all high mannose glycoforms have a similar degree of increased activity compared with the reference but still lower than that of the AF glycoform regardless of the absolute level of Man5. However, given that the core fucose is absent in the high mannose glycoforms, it remains unclear if the observed increase in FcγRIIIA binding was due to the presence of multiple mannose moieties or the absence of core fucose.
Figure 6. Binding affinity of mAb1 with different glycoforms toward Fcγ receptors. All data are EC50 values, normalized to reference. (A) Binding affinity toward FcγRIA; (B) binding affinity toward FcγRIIA and FcγRIIB with two allotypes (H131 and R131); (C) binding affinity toward FcγRIIIA with two allotypes (F158 and V158).
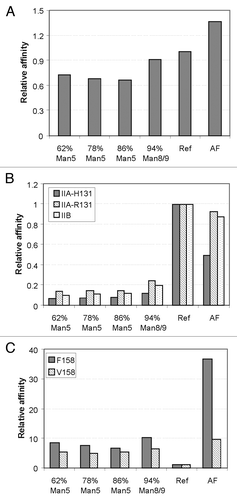
Binding affinities toward other FcγRs were also investigated. For FcγRIA, a high affinity receptor, the relative binding affinities of the different glycoforms appeared to be comparable (). Binding to FcγRIIA and IIB are illustrated in . Unlike FcγRIA binding, the data indicates that all high mannose glycoforms have five- to ten-fold lower binding affinities toward both IIA (both H131 and R131) and IIB compared with both the reference and the AF glycoforms. This result may have biological significance as FcγRIIB has been reported to exert an inhibitory effect toward ADCC activity.Citation4 It is conceivable that a combination of increased binding to FcγRIIIA and decreased binding to FcγRIIB by high mannose glycoforms may potentially provide a synergistic effect for effector functions in vivo.Citation26 Additional studies will be required to assess in vivo ADCC activity, which incorporates all of the interactions between the antibodies bearing different glycoforms with all of the various Fcγ receptors.
Complement-dependent Cytotoxicity (CDC) Activity
CDC activity can also be modulated by the glycosylation of the antibody,Citation2 therefore it was also tested for mAb2 and the result is shown in . Interestingly, all high mannose glycoforms exhibited an approximately 50% decrease in CDC activity compared with the complex-fucosylated glycoform, while complex-fucosylated and AF glycoforms had similar activity. This observation is consistent with other published data.Citation7 CDC activity may be linked to an undesirable side effect in some patients, and it has been suggested that decreasing CDC activity could potentially diminish this side effect.Citation27,Citation28 For this reason, we speculate that in some instances antibodies bearing the high mannose glycoform may have a therapeutic advantage.
Pharmacokinetic Study in Mice
All characterization assays including ADCC, CDC, and FcγR binding activity demonstrated that antibodies with high mannose glycoforms have similar activity. However, the question of potential differences in clearance rate between the different forms of high mannose, namely Man5 vs. Man8/9 glycoforms, remains. Wright and Morrison previously reported a significantly faster clearance rate observed with an antibody bearing the Man5 glycoform, in which the antibody was expressed using a Lec1 cell line having a deficient N-acetylglucosamine transferase I.Citation10,Citation18 N-acetylglucosamine transferase I is required in the conversion of high mannose glycans to complex glycans. The PK clearance curve reported by Wright and Morrison appears to be biphasic, with later phase clearance comparable to antibodies bearing complex glycans. It is unclear whether a distribution of Man5, 6, 7, 8 and 9 high mannose glycoformsCitation20 could result in an atypical biphasic clearance profile due to more rapid α phase clearance behavior from different high mannose glycoforms. Another study by Zhou et al. demonstrated comparable clearance between high mannose antibodies (primarily with Man 7, 8 and 9) and those bearing complex glycans,Citation9 which is inconsistent with the result from Wright and Morrison. In a more recent study, a serum mannosidase activity in humans that trims Man6–9 glycoforms to Man5 for an IgG2 antibody has been reported.Citation21 Due to the contradictory data reported by different groups,Citation29 we conducted a PK study in mice to directly compare mAb1 antibodies with > 99% Man5 and > 99% Man8/9 glycans to determine the clearance properties of different form of high mannose glycoform. Glycan analysis was also conducted on the collected PK samples to investigate the presence of serum mannosidase activity in mice, which could potentially act on the antibodies with Man8/9 glycans.
The serum concentration-time profiles of mAb1 with > 99% Man5 and > 99% Man8/9 are shown in . It appears that antibodies with Man5 and Man8/9 glycoforms have similar profiles (), as well as PK parameters (), suggesting no difference between antibodies with Man5 vs. Man8/9 glycoforms. Historical data for the complex-fucosylated glycoform of mAb1 at the same dose of 10 mg/kg was overlaid on the graph. The data illustrates that there is a noticeable difference in the concentration-time profiles between the high mannose and complex-fucosylated glycoforms with approximately 3-fold slower total clearance (CL) for the complex-fucosylated glycoform (). The faster CL of the high mannose glycoforms was characterized by an extended distribution phase leading to larger volumes of distribution (). This could potentially be due to more rapid clearance rather than distribution. The high mannose glycoforms also had shorter terminal (β) half-lives and lower exposure as measured by the area-under-the curve (AUC) compared with the complex-fucosylated glycoform (). These data are consistent with more tissue uptake of the high mannose glycoforms compared with the complex-fucosylated glycoform, which may be mediated by receptors such as mannose-binding proteins, and are also consistent with the data reported by Kanda et al.Citation7 This result suggested that antibodies bearing the Man5 glycoform have a faster clearance rate and the different forms of high mannose did not appear to differ in overall clearance profile. The clearance rate of the high mannose glycoform determined in the current study and in Kanda’s study differ from that reported by Wright and Morrison since the rapid drop in serum antibody concentration in α phase was not observed.Citation10,Citation18 Differences in our study and those of Wright and Morrison include dose, route of administration, as well as the method to monitor serum antibody concentration, and these differences may account for the different results. Goetze et al. pointed out that Man5 glycans are cleared relatively faster at lower doses, which may be due to saturation of the mannose receptor at higher doses.Citation22
Figure 8. Pharmacokinetic profiles of mAb variants in Harlan athymic nude mice. The two groups conducted in this study were mAb2 with > 99% Man5 (solid black circle) and mAb2 with > 99% Man8/9 (solid gray triangle). The complex-fucosylated profile (open squares) was from a separate study conducted in a similar fashion to the current study. Mice were injected with single I.V. dose of 10 mg/kg mAbs with specific glycoform, and serum samples were collected in the indicated time points up to 28 d. Triplicate sample set was collected for each time point. Average values are reported and the error bars represent standard deviation. The data was fit to a 2 compartment model for the calculation of pharmacokinetic parameters illustrated in .
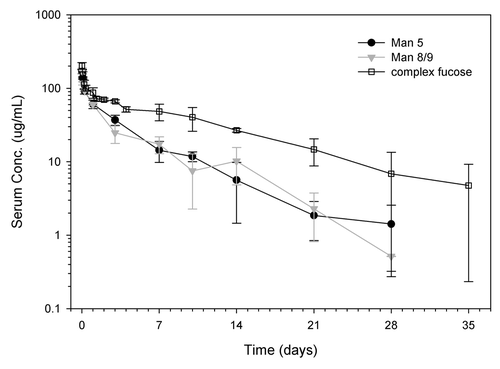
Table 1. Pharmacokinetics parameters calculated from pharmacokinetic profile shown in . Parameters are estimated from mean data calculated from 3 mice at each time point per group. CV stands for coefficient of variation. AUC – area under curve; CL – clearance rate; T ½ α – α half life; T ½ β – β half life; V1 – central compartment volume of distribution; V2 – tissue compartment volume of distribution
Interestingly, antibodies bearing afucosyl complex glycans have also been reported to have more rapid clearance than antibodies bearing fucosylated complex glycans.Citation30
Mannosidase Activity In Vivo
Another aspect of the clearance properties of antibodies bearing high mannose glycans is the report of a mannosidase activity in human serum that could potentially trim Man8/9 antibodies to Man5.Citation21 To determine whether mannosidase activity is present in mice, the serum samples collected at different time points were purified and then analyzed using rpHPLC ESI-MS to generate a glycan profile of mAb1 at different times post injection. The data are summarized in . For the study group that was dosed with > 99% Man5 antibodies, the results show that Man5 antibodies were very stable up to 10 d post injection (). The antibody concentration of samples beyond day 10 was too low for sufficient antibody recovery. In contrast, the study group that was dosed with > 99% Man8/9 antibodies clearly showed a shift from Man9 to Man6 after 24 h post-injection (), suggesting the presence of serum mannosidase activity. Somewhat in contrast to the work by Chen et al., generation of the Man5 glycoform from Man8/9 was not significant in this study. This may due to the fact that different mannosidases from a range of organisms or even from the same organism have a wide variety of substrate specificities.Citation31,Citation32 The first evidence of mannosidase activity in human serum also reported trimming to Man6 only.Citation33 It appears that the mouse serum mannosidase cannot efficiently trim Man6 to Man5.
Figure 9. Glycan analysis showing high mannose glycoform distribution for samples collected at the different time points of the PK study. The antibodies were first purified from serum, and then subsequently analyzed using rpHPLC ESI-MS method. (A) Glycan profile of mAb2 for mice injected with 100% Man5 glycoform; (B) glycan profile for mAb2 for mice injected with 100% Man8/9 glycoform.
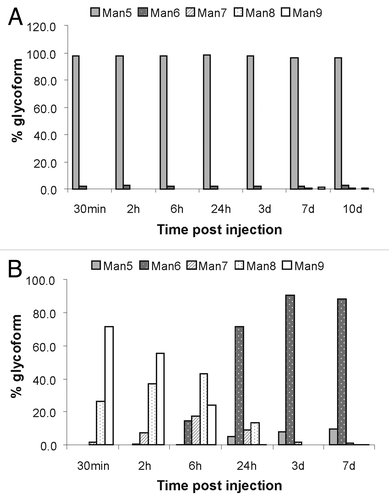
The discovery of mannosidase activity in mouse serum is consistent with work published by Chen et al.,Citation21 confounding the determination of the clearance properties of antibodies bearing Man8/9 glycans. Because the detection ELISA assay does not distinguish between different glycoforms and mAb1 measured post 24 h in both study groups (shown in ) was essentially all Man5/6, PK data post 24 h for the two glycoforms cannot be compared directly. To more accurately ascertain the PK profile of antibodies with primarily Man8/9 glycans, sampling more time points post-injection before significant conversion takes place would be required, as well as a means to knockout or inhibit the mannosidase activity.
Mannosidase Activity in Mouse Serum In Vitro
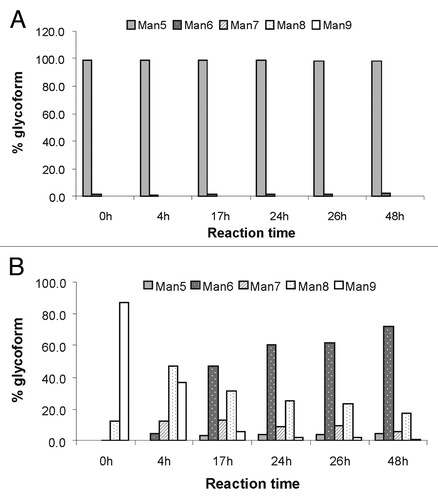
To further confirm the presence of mannosidase activity in mouse serum, the trimming reaction was confirmed in vitro using commercially purchased mouse serum. Purified mAb1 with > 99% Man5 or > 99% Man8/9 were spiked into the mouse serum, and incubated at 37°C for 2 d. Samples were taken and analyzed at multiple time points and the results are illustrated in . Similar to the glycan trimming observed in vivo, the majority of the Man9 glycoform was trimmed to Man6 after 24 h (), and the Man5 glycoform remained stable up to 2 d for this study (). This in vitro data confirms the finding of mannosidase activity in mouse serum in vivo, similar to the results of Chen et al. in human serum.Citation21
Figure 10. Glycan profile of mAb2 after incubation for indicated time period with mouse serum in vitro. The antibodies were spiked into mouse serum, and then incubated at 37°C to investigate the presence of mannosidase activity in commercially available mouse serum. The antibodies were then purified from serum and analyzed using rpHPLC-ESI MS. (A) Mouse serum spiked with mAb2 with 100% Man5; (B) mouse serum spiked with mAb2 with 100% Man8/9.
Discussion
This study focuses on the effector function and PK properties of antibodies bearing Man5 glycans. Whereas high mannose glycoforms have been previously studied, antibodies with demonstrated homogeneous Man5 glycans have not been characterized due to a lack of a facile method to generate these antibodies consistently. We developed a method to generate antibodies with > 99% Man5 by producing the antibodies in cell culture in the presence of kifunensine to generate antibodies bearing primarily Man 8/9 glycans, which were subsequently trimmed to Man5 using a mannosidase in vitro. The in vitro biological activity of antibodies with various levels of Man5 and Man8/9 were further characterized using ADCC, CDC and FcγR binding assays. All high mannose glycoforms appeared to have increased ADCC activity, decreased CDC activity, increased binding affinity to FcγRIIIA, and decreased binding affinity to FcγRIIA and IIB. Since all high mannose glycoforms lack core fucose, it is likely a combination of the Man5 pentasaccharide moiety and the lack of core fucose that are responsible for these properties relative to fucosylated and afucosylated complex glycoforms. The fact that Fc glycan structure can affect CDC activity has been demonstrated for two antibodies for which removal of galactose reduced CDC activity.Citation38,Citation39 These changes in effector functions of high mannose glycoform antibodies may benefit their therapeutic potential in certain applications but further testing is required.
We also studied antibodies with homogeneous Man5 or Man8/9 glycans in a PK study in mice. No major differences were observed in clearance profiles between the different high mannose glycoforms. However, we found a 3-fold faster clearance rate with both Man5 and Man8/9 glycoforms compared with the complex-fucosylated glycoform. Furthermore, this interpretation is complicated by the in vivo conversion of Man8/9 glycans to Man6 glycans by a serum mannosidase. As a result, the clearance rate observed for antibodies bearing the Man8/9 glycoform in this study is confounded by conversion to the Man6 glycoform as quickly as 24 h post injection.
Together with the PK analysis, this study has shown that both the Man5 and Man6 glycoforms exhibited more rapid clearance rate than the complex-fucosylated glycoform. To mitigate this, it is possible that introducing mutations to the FcRn binding site to prolong serum half-life could counteract this. The therapeutic impact of antibodies bearing high mannose glycoforms, having a combination of enhanced ADCC and decreased affinity for the inhibitory receptor FcγRIIB, will need to be evaluated in vivo.
Materials and Methods
Materials
For this study, two in-house Chinese hamster ovary (CHO) cell lines were used to generate recombinant human IgG1 monoclonal antibodies (mAb1 and mAb2). Afucosyl mAb2 was also expressed in an in-house cell line that lacked FUT6 activity. Alpha-1,2-mannosidase was first obtained from Prozyme and later made in-house by expressing the gene of interest in a CHO DP12 cell line. Freestyle MAX was purchased from Invitrogen, and kifunensine was purchased from Cayman Chemical. Sodium acetate, calcium chloride, and mouse serum were obtained from Sigma. TCEP HCl, formic acid, trifluoroacetic acid, and Ultralink Biosupport were purchased from Thermo Fisher Scientific. Goat anti-human IgG (Fcγ fragment specific) was obtained from Jackson ImmunoResearch.
Cell Culture Process to Generate Antibodies With High Mannose Glycoform
Two CHO cell lines expressing human IgG1 monoclonal antibodies of interest were used for this study. Kifunensine, a small molecule inhibitor of the enzyme α-mannosidase I, was added directly into the cell culture media. Zhou et al. have demonstrated that the accumulation of antibodies with the high mannose glycoform can be modulated by the amount of kifunensine added.Citation9 To begin the production process, antibody-expressing CHO cells were seeded at 3 × 105 cells/mL in shake flasks. All media used for cell line maintenance and production were formulated in-house using proprietary recipes. Different concentrations of kifunensine were added into the basal media to assess the effects on cell growth, viability, and antibody production (titer) for the generation of mAb1 and mAb2. Initial experiments also determined the appropriate concentration of kifunensine needed to generate antibodies with desired levels of the high mannose glycoform. Based on these results, kifunensine was added at a concentration of 0.1 μg/mL for material generation in the initial characterization assays, and at 5 μg/mL for the PK studies. The culture was shaken in a CO2-humidified incubator at 37°C for 3–4 d, and then the temperature was shifted to 33°C for the remainder of the process (temperature shift optional). The culture was harvested at the end of 11 d by centrifugation. Cell counts and viability were monitored throughout the course of the culture. The harvested cell culture fluid (HCCF) was collected at day 11 and the antibody titer was determined. Subsequently, antibodies were purified from HCCF via Protein A affinity chromatography. The Protein A eluate, containing the antibodies with the high mannose glycoform, was then dialyzed and concentrated into the mannosidase trimming reaction buffer (100 mM sodium acetate, pH 5.0).
In Vitro Trimming Reaction by α-1,2-mannosidase of Aspergillus Saitoi
The antibodies with higher mannosylated glycans obtained from the cell culture process with kifunensine addition were subjected to an additional processing step to generate mainly the Man5 glycoform. The general scheme of this process is depicted in . In short, the step of mannosidase trimming from mannose-9 (Man9) to mannose-5 (Man5) glycoform in the endogenous glycosylation pathway is replaced by an in vitro enzymatic trimming reaction. A commercially available α-mannosidase I, α-1,2-mannosidase from Aspergillus saitoi (Prozyme), was tested for its ability to carry out the in vitro trimming reaction from highly-enriched Man8/9 glycoform to Man5. Preliminary experiments were performed at various enzyme concentrations (0.4 to 20 mU/mL). In the reaction mixture, 10 to 16 mg/mL of protein-A purified, Man9-enriched antibodies were incubated at 37°C for 24 h in 100 mM sodium acetate at pH 5.0 and then analyzed using mass spectrometry. Optimization experiments were also conducted to test different reaction factors including temperature, time and the addition of metal ion co-factor. Because calcium is reported to be a co-factor for other mannosidases,Citation31,Citation34 we tested whether it could enhance the activity of the α-1,2-mannosidase from Aspergillus saitoi. For all optimization reaction conditions, 20 mU/mL of mannosidase and 10 mg/mL of protein A-purified, Man9-enriched antibodies were used. Elevated temperature (55°C), the addition of calcium chloride at 0.25, 0.5, and 1 mM, and reaction times of 24, 48 and 72 h were tested.
The use of higher enzyme concentrations in the trimming reaction was enabled by cloning, expressing, and purifying in-house the α-1,2-mannosidase from Aspergillus saitoi to allow production of larger quantities of enzyme. The gene was synthesized by BioPioneer using the published sequence,Citation23 with a 6 × HIS-tag added for simplified purification. The gene was cloned into an in-house vector for expression using a CHO transient system. CHO DP12 cells were transfected with the vector using FreeStyle™ MAX reagent (Invitrogen). The culture was grown for 7 d before harvest and the HCCF was collected for purification. A HisTrap FF column (GE Healthcare Bio-Sciences AB) was selected to purify the histidine-tagged α-1,2-mannosidase according to the manufacturer’s protocol. The purified mannosidase was dialyzed into reaction buffer (100 mM sodium acetate, pH 5.0) and concentrated before use for enzymatic trimming of Man9-enriched antibodies.
Analysis of Antibodies With Different Glycoforms Using Mass Spectrometry
Antibodies with different glycoforms were analyzed using mass spectrometry. All samples were characterized using a method developed in-house with rpHPLC coupled with electrospray ionization mass spectrometry (ESI-MS). Selected samples were confirmed using a well-established matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometric glycan characterization assay.Citation35,Citation36
In the rpHPLC ESI-MS method, the distribution of glycans attached to the antibody was deduced from the overall mass of the heavy chain with its associated N-glycan. This method does not require release of glycan using N-glycosidase F (PNGaseF),Citation35,Citation36 thereby allowing rapid characterization of the antibody glycoform. To prepare the samples for analysis, 0.1 to 0.5 mg of antibody was mixed with a 1:1 (w/w) ratio of reducing agent tris(2-carboxyethyl)phosphine hydrochloride (TCEP) and solvent (10% acetonitrile/0.1% formic acid/HPLC-grade water) to reach a final antibody concentration of 0.5–1 mg/mL. The mixture was incubated at 60°C for 10 to 20 min to reduce the antibody, and then subsequently centrifuged at 10,000 × g for 5 min to remove any precipitate. The supernatant was collected and analyzed using the rpHPLC ESI-MS system. To begin the analysis, 25 μL of the prepared sample was injected onto a PLRP-S column (Varian) on an Agilent 1200 series HPLC system (Agilent Technologies). The light chain and heavy chain were eluted with a gradient of 25% to 40% Buffer A to Buffer B at a flow rate of 0.5 mL/min (Buffer A: HPLC-grade water with 0.025% trifluoroacetic acid and 0.1% formic acid; Buffer B: Acetonitrile with 0.025% trifluoroacetic acid and 0.1% formic acid). A MDS Sciex/ QStar Elite mass spectrometer (Applied Biosystems) is installed in-line with the HPLC, which allowed the eluted protein to be directly injected into the ESI-MS unit after separation. The masses reconstructed from the heavy chain were measured and analyzed to determine the type of glycan attached to the heavy chain. The percentages of different glycoforms were then calculated based on the expected mass of the different glycans added to the heavy chain of the specific antibody.
Antibody-dependent Cell-mediated Cytotoxicity (ADCC) Assay
The ADCC assay was adapted from Shields et al.Citation3 ADCC assays were performed using peripheral blood mononuclear cells (PBMCs) from healthy donors as effector cells, and a human B-lymphoma cell line, WIL2-S, as target cells. To reduce inter-donor variations due to FcγRIIIa polymorphism, donors were selected for those carrying the heterozygous FcγRIIIA V/F-158 genotype.
Serial dilutions of test antibodies were added to the plates containing the target cells, followed by incubation at 37°C with 5% CO2 for 30 min to allow opsonization. The final concentrations of antibodies ranged from 1000 to 0.0038 ng/mL following serial four-fold dilutions. After the incubation, PBMC effector cells were added to each well to give a ratio of 25:1 effector:target cells and the plates were incubated for an additional 4 h. The plates were centrifuged at the end of incubation and the supernatants were assayed for lactate dehydrogenase (LDH) activity using a Cytotoxicity Detection Kit (Roche Diagnostics Corporation, Indianapolis, IN). Cell lysis was quantified through absorbance at 490 nm using a microplate reader. The absorbance of wells containing only the target cells served as the control for background (Low Control), whereas wells containing target cells lysed with Triton-X100 provided maximum signal available (High Control). Antibody-independent cellular cytotoxicity (AICC) was measured in wells containing target and effector cells without the addition of antibody. The extent of specific ADCC was calculated as followed:
% Cytotoxicity (ADCC) = 100 × ![]()
The mean ADCC values from duplicates of sample dilutions were plotted against the antibody concentration, and the EC50 values (half maximal effective concentration) and the maximum extent of ADCC (%) were generated by fitting the data to a four-parameter equation with SoftMax Pro (Molecular Devices).
For comparison, the EC50 value of the reference material was set at 1 and the relative activity of each sample was calculated as follows:
Relative Activity =
Complement-dependent Cytotoxicity (CDC) Assay
The CDC assays were performed using WIL2-S cells as target cells and complement derived from human serum, as previously described.Citation37 Briefly, the antibody samples were serially diluted in assay medium (RPMI 1640 medium supplemented with 1% FBS), and distributed into a 96-well opaque-walled microtiter plate (Costar Corning Inc.). Following the addition of WIL2-S cells (5 × 104 cells/well) and human serum complement (Quidel Corporation), the plate was incubated with 5% CO2 for 2 h at 37°C. After the incubation, the CellTiter-Glo reagent (Promega Corp.), which assays for ATP in metabolically active cells, was added and the plate was incubated at room temperature for 10 min with constant shaking. The extent of cell lysis was quantified by measuring intensity of luminescence with a plate reader (SpectraMax M5, Molecular Devices).
Fcγ Receptor Binding ELISA
The binding affinities of test antibodies for various human Fcγ receptors were assessed with ELISA-based ligand binding assays.Citation3 The human Fcγ receptors were expressed as fusion proteins containing the extracellular domain of the receptors linked to a Gly-6 × His-glutathione S-transferase (GST) polypeptide tag at the C-terminus. For the low-affinity receptors (FcγRIIA [H131 and R131 allotypes], FcγRIIB, and FcγRIIIA [F158 and V158 allotypes], the antibodies were tested as multimers, cross-linked withF(ab’)2 fragments of goat anti-human κ chain (MP Biomedicals) at an approximate molar ratio of 1:3 antibody:F(ab’)2. Antibody affinities for the high-affinity receptor (FcγRIA) were assayed in monomeric form (without cross-linking). Sample and reagent dilutions were prepared in an assay buffer containing phosphate-buffered saline (PBS), 0.5% bovine serum albumin (BSA), 0.1% casein and 0.05% Tween-20. Plates were washed with PBS containing 0.05% Tween-20 using an ELx405™ plate washer (Biotek Instruments) after each incubation step.
Briefly, plates were coated with a monoclonal mouse anti-GST antibody (Genentech) in a 0.05 M sodium carbonate buffer (pH 9.6) overnight at 4°C. After blocking with the assay buffer, the plates were incubated with Fcγ receptors at room temperature for 1 h. Serial dilutions of test antibodies were added either as monomers (for binding with FcγRIA) or multimeric complexes (for binding with FcγRIIA, IIB, and IIIA), and the plates were incubated at room temperature for 2 h. Antibodies bound to the Fcγ receptors were detected with horseradish peroxidase (HRP) conjugated goat anti-human F(ab’)2 (Jackson ImmunoResearch Laboratories) followed by addition of the substrate 3,3′,5,5′-tetramethylbenzidine (Kirkegaard and Perry Laboratories). The plates were incubated at room temperature for 5−20 min to allow color development, depending on the Fcγ receptors tested. The reaction was terminated with 1 N phosphoric acid, and absorbance was measured at 450 nm (the background measured at 650 nm was subtracted for each well) using a microplate reader SpectraMax® 190 (Molecular Devices).
Dose response binding curves were generated by plotting the mean absorbance values from duplicates of sample dilutions against the sample concentrations. The data points were fitted with a four-parameter model and the EC50 value was calculated using SoftMax Pro (Molecular Devices). For comparison, the EC50 value of the reference molecule was set at 1 and the relative affinity of each sample was calculated as follows:
Relative Affinity = 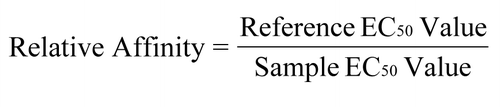
Pharmacokinetic Study in Mice
Sixty female athymic nude mice were obtained from Harlan Laboratories. The mice were 6−8 weeks old and weighed 19.0 − 23.8 g at the start of the study. Only animals that appeared to be healthy and were free of obvious abnormalities were used for the study. Sixty animals (n = 3/timepoint/group) were randomized into four dosing groups and received a single intravenous (IV) bolus of antibodies with 100% Man5 or 100% Man8/9 glycoforms at 10 mg/kg. For the PK study, samples were taken from 3 mice per group on 0.5, 2, 6, 24 h and 3, 7, 10, 14, 21, 28 d. Approximately 0.7 mL of whole blood was collected via cardiac puncture into serum separator tubes. Samples were allowed to clot at room temperature (15–30°C) for 30–60 min, centrifuged at 11,000 rpm for 5 min and decanted into sample collection tubes. The PK samples were stored at -70°C until determination of serum concentrations by ELISA or glycan analysis.
For data analysis, serum concentrations determined by ELISA were used to construct semi-logarithmic serum concentration vs. time curves. PK parameters were determined using a 2-compartment method (Model 8) using WinNonlin, version 5.2.1 (Pharsight Corp.).
ELISA Assay to Analyze PK Samples
An ELISA assay was developed to investigate the PK profiles of mAb1 with various levels of high mannose glycoforms. Nunc-Immuno Maxisorp 96-well plates (Thermo Fisher Scientific) were coated with 100 μL/well of 1 μg/mL antigen-Flag-His protein in 0.05 M sodium carbonate buffer at pH 9.6, and stored overnight at 4°C. The next day, plates were washed three times with wash buffer (PBS pH 7.4, 0.05% Polysorbate 20), followed by blocking with 150 μL/well of blocking buffer (PBS pH 7.4, 0.5% BSA, 15ppm Proclin) for 1 h at room temperature (RT) with gentle agitation. Plates were then washed three times with wash buffer before the addition of 100 μL/well of mAb2 standards or serum samples. The plates were incubated for 1 h at RT with gentle agitation. After incubation, plates were subsequently washed six times. Next, antibodies of interest were then detected by adding F(ab')2 donkey anti-human IgG, Fcγ conjugated to HRP (Jackson ImmunoResearch) at 12.5 ng/mL (100 μL/well). The plates were again incubated for 1 h at RT with gentle agitation. Plates were washed six times before the final addition of 100 μL/well of tetramethylbenzidine (TMB) developing solution (Moss). After sufficient color development, 100 μL of 1 M phosphoric acid was added to each well to stop the reaction. Plates were then read on a microplate reader (Biotek EL311 or equivalent) at 450/620 nm.
Purification of PK Samples for Glycan Analysis
For each mouse serum sample collected from the PK study, a 100 μL aliquot was purified for further glycan analysis. Ultralink Biosupport Beads from Thermo Fisher Scientific were covalently linked to goat anti-human IgG (Fcγ fragment specific) from Jackson ImmunoResearch based on the manufacturer’s protocol and used for capture of the humanized antibody. A coupling efficiency of > 95% was achieved consistently. The resins were kept as a 50% slurry with phosphate-buffered saline (PBS) at 4°C until use (no longer than 7 d).
Small scale purification using a 96-well deep well filter plate and collection plate was used to purify the injected humanized antibody from mouse serum. For every 100 μL of mouse serum sample, 50 μL of resin at 50% slurry was used to reach a 1:4 (solid: liquid) phase ratio. The 50% slurry resin was first added to each well, followed by removal of liquid using centrifugation at 180 × g for 3 min. The liquid filtered into the collection plate was discarded. The resin was then washed 2 times with 100 μL PBS by mixing on a shaker platform for 10 min, followed by centrifugation for the removal of liquid. After washing, 100 μL of selected PK mouse serum samples were loaded into corresponding wells, and the 96-well plate was shaken on the shaker platform for 1 h to allow binding to occur. It was important to make sure that the resin was well-suspended into the serum to guarantee optimal binding and recovery. After binding for 1 h, the filter/collection plate was again centrifuged at 180 × g for 3 min to remove unbound materials along with the serum. The resin samples were then washed with PBS 3 times, followed by elution in 3 stages. For each well, 100 μL of elution buffer (100 mM phosphoric acid, pH 1.74) was added and mixed for 10 min on shaker platform. The eluate was collected by centrifugation into a new collection plate at 180 × g for 3 min. The elution process was repeated 2 additional times for a total of three elution stages. The pH of all eluates was adjusted to ~pH 5.0 using 1.5 M Tris base.
Reverse phase HPLC coupled with ESI-MS was used to analyze the antibodies purified from mouse serum. Sample preparation was slightly different due to the small amount of antibodies available. Purified antibodies from the eluates were reduced with ~1 mg/mL TCEP, and then heated at 60°C for 20 min. Instead of injecting 25 μL into the LC as described above, 100 μL of reduced sample was injected for each sample to maximize sensitivity.
In Vitro Trimming of Man9-enriched Antibodies Using Mouse Serum
Man9-enriched antibodies were spiked into commercially available mouse serum from Sigma to test serum mannosidase activity in vitro. The reaction was performed in test tubes with 100 μg of Man9-enriched antibody spiked into ~600 μL mouse serum in the presence of 30 mM calcium chloride. The reaction was incubated at 37°C for 2 d, while 100 μL samples were collected at different time points (0, 4, 17, 24, 26, 48 h). The collected samples were frozen at -80°C prior to purification and glycan analysis using the same methods as the ones developed for the analysis of the PK samples.
| Abbreviations: | ||
| mAb | = | monoclonal antibody |
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| HCCF | = | harvested cell culture fluid |
| CHO | = | Chinese hamster ovary |
| GlcNAc | = | N-acetylglucosamine |
| Man9 | = | mannose-9 glycan |
| Man8 | = | mannose-8 glycan |
| Man7 | = | mannose-7 glycan |
| Man6 | = | mannose-6 glycan |
| Man5 | = | mannose-5 glycan |
| rpHPLC | = | reverse phase high performance liquid chromatography |
| ESI-MS | = | electrospray ionization mass spectrometry |
| MALDI-TOF | = | matrix-assisted laser desorption/ionization time-of-flight |
| TCEP | = | tris(2-carboxyethyl)phosphine hydrochloride |
| PNGaseF | = | N-glycosidase F |
| ADCC | = | Antibody-dependent cell-mediated cytotoxicity |
| PBMC | = | peripheral blood mononuclear cells |
| EC50 | = | half maximal effective concentration |
| CDC | = | Complement-dependent cytotoxicity |
| ELISA | = | enzyme-linked immunosorbent assay |
| PK | = | pharmacokinetic |
| PBS | = | phosphate-buffered saline |
| AF | = | complex glycan lacking core fucose, afucosylated complex glycan |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Feng Li, Efren Pacis, Amy Shen, and Domingos Ng for initial efforts to generate the Man5 glycoform. We thank Mike Laird and Clarissa Chui for cloning the Aspergillus saitoi a-1,2-mannosidase gene into our in-house vector, and Dr. Roland Contreras for providing the a-1,2-mannosidase from Trichoderma reesei. We also thank Rod Keck and Tomasz Baginski for support with the MALDI-TOF assay. Finally, we thank everyone mentioned above and Reed Harris, Steve Meier, Peter Thiel, and Ashraf Amanullah for valuable discussions, review of this manuscript, and support for this work.
Reference
- Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther 2007; 7:1401 - 13; http://dx.doi.org/10.1517/14712598.7.9.1401; PMID: 17727329
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 2009; 8:226 - 34; http://dx.doi.org/10.1038/nrd2804; PMID: 19247305
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs JB, et al. High resolution mapping of the binding site on human IgG1 for Fc γ RI, Fc γ RII, Fc γ RIII, and FcRn and design of IgG1 variants with improved binding to the Fc γ R. J Biol Chem 2001; 276:6591 - 604; http://dx.doi.org/10.1074/jbc.M009483200; PMID: 11096108
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8:34 - 47; http://dx.doi.org/10.1038/nri2206; PMID: 18064051
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 2011; 108:12669 - 74; http://dx.doi.org/10.1073/pnas.1108455108; PMID: 21768335
- Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 1999; 17:176 - 80; http://dx.doi.org/10.1038/6179; PMID: 10052355
- Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology 2007; 17:104 - 18; http://dx.doi.org/10.1093/glycob/cwl057; PMID: 17012310
- Shields RL, Lai J, Keck RG, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277:26733 - 40; http://dx.doi.org/10.1074/jbc.M202069200; PMID: 11986321
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, et al. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng 2008; 99:652 - 65; http://dx.doi.org/10.1002/bit.21598; PMID: 17680659
- Wright A, Morrison SL. Effect of C2-associated carbohydrate structure on Ig effector function: studies with chimeric mouse-human IgG1 antibodies in glycosylation mutants of Chinese hamster ovary cells. J Immunol 1998; 160:3393 - 402; PMID: 9531299
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 2003; 278:3466 - 73; http://dx.doi.org/10.1074/jbc.M210665200; PMID: 12427744
- Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, et al. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng 2004; 88:901 - 8; http://dx.doi.org/10.1002/bit.20326; PMID: 15515168
- Li H, Sethuraman N, Stadheim TA, Zha D, Prinz B, Ballew N, et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris.. Nat Biotechnol 2006; 24:210 - 5; http://dx.doi.org/10.1038/nbt1178; PMID: 16429149
- Hamilton SR, Bobrowicz P, Bobrowicz B, Davidson RC, Li H, Mitchell T, et al. Production of complex human glycoproteins in yeast. Science 2003; 301:1244 - 6; http://dx.doi.org/10.1126/science.1088166; PMID: 12947202
- Stanley P. Lectin-resistant CHO cells: selection of new mutant phenotypes. Somatic Cell Genet 1983; 9:593 - 608; http://dx.doi.org/10.1007/BF01574260; PMID: 6623313
- Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci U S A 1986; 83:2501 - 5; http://dx.doi.org/10.1073/pnas.83.8.2501; PMID: 3458213
- Schlesinger PH, Doebber TW, Mandell BF, White R, DeSchryver C, Rodman JS, et al. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J 1978; 176:103 - 9; PMID: 728098
- Wright A, Morrison SL. Effect of altered CH2-associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J Exp Med 1994; 180:1087 - 96; http://dx.doi.org/10.1084/jem.180.3.1087; PMID: 8064227
- Chen W, Stanley P. Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology 2003; 13:43 - 50; http://dx.doi.org/10.1093/glycob/cwg003; PMID: 12634323
- Wright A, Sato Y, Okada T, Chang KH, Endo T, Morrison SL. In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology 2000; 10:1347 - 55; http://dx.doi.org/10.1093/glycob/10.12.1347; PMID: 11159927
- Chen X, Liu YD, Flynn GC. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 2009; 19:240 - 9; http://dx.doi.org/10.1093/glycob/cwn120; PMID: 18974198
- Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011; 21:949 - 59; http://dx.doi.org/10.1093/glycob/cwr027; PMID: 21421994
- Inoue T, Yoshida T, Ichishima E. Molecular cloning and nucleotide sequence of the 1,2-α-D-mannosidase gene, msdS, from Aspergillus saitoi and expression of the gene in yeast cells. Biochim Biophys Acta 1995; 1253:141 - 5; http://dx.doi.org/10.1016/0167-4838(95)00195-6; PMID: 8519794
- Ichishima E, Taya N, Ikeguchi M, Chiba Y, Nakamura M, Kawabata C, et al. Molecular and enzymic properties of recombinant 1, 2-α-mannosidase from Aspergillus saitoi overexpressed in Aspergillus oryzae cells. Biochem J 1999; 339:589 - 97; http://dx.doi.org/10.1042/0264-6021:3390589; PMID: 10215597
- Pacis E, Yu M, Autsen J, Bayer R, Li F. Effects of cell culture conditions on antibody N-linked glycosylation-what affects high mannose 5 glycoform. Biotechnol Bioeng 2011; 108:2348 - 58; http://dx.doi.org/10.1002/bit.23200; PMID: 21557201
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443 - 6; http://dx.doi.org/10.1038/74704; PMID: 10742152
- van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MHJ. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol 2001; 115:807 - 11; http://dx.doi.org/10.1046/j.1365-2141.2001.03166.x; PMID: 11843813
- Clark EA, Ledbetter JA. How does B cell depletion therapy work, and how can it be improved?. Ann Rheum Dis 2005; 64:Suppl 4 iv77 - 80; http://dx.doi.org/10.1136/ard.2005.042507; PMID: 16239394
- Correia IR. Stability of IgG isotypes in serum. MAbs 2010; 2:1 - 12; http://dx.doi.org/10.4161/mabs.2.3.11788; PMID: 20065628
- Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res 2010; 70:4481 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-09-3704; PMID: 20484044
- Lal A, Pang P, Kalelkar S, Romero PA, Herscovics A, Moremen KW. Substrate specificities of recombinant murine Golgi α1, 2-mannosidases IA and IB and comparison with endoplasmic reticulum and Golgi processing α1,2-mannosidases. Glycobiology 1998; 8:981 - 95; http://dx.doi.org/10.1093/glycob/8.10.981; PMID: 9719679
- Tremblay LO, Herscovics A. Characterization of a cDNA encoding a novel human Golgi α 1, 2-mannosidase (IC) involved in N-glycan biosynthesis. J Biol Chem 2000; 275:31655 - 60; http://dx.doi.org/10.1074/jbc.M004935200; PMID: 10915796
- Porwoll S, Fuchs H, Tauber R. Characterization of a soluble class I α-mannosidase in human serum. FEBS Lett 1999; 449:175 - 8; http://dx.doi.org/10.1016/S0014-5793(99)00422-6; PMID: 10338126
- Gonzalez DS, Karaveg K, Vandersall-Nairn AS, Lal A, Moremen KW. Identification, expression, and characterization of a cDNA encoding human endoplasmic reticulum mannosidase I, the enzyme that catalyzes the first mannose trimming step in mammalian Asn-linked oligosaccharide biosynthesis. J Biol Chem 1999; 274:21375 - 86; http://dx.doi.org/10.1074/jbc.274.30.21375; PMID: 10409699
- Papac DI, Briggs JB, Chin ET, Jones AJS. A high-throughput microscale method to release N-linked oligosaccharides from glycoproteins for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Glycobiology 1998; 8:445 - 54; http://dx.doi.org/10.1093/glycob/8.5.445; PMID: 9597542
- Keck RG, Briggs JB, Jones AJS. Oligosaccharide release and MALDI-TOF MS analysis of N-linked carbohydrate structures from glycoproteins. Methods Mol Biol 2005; 308:381 - 96; PMID: 16082050
- Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol 2000; 164:4178 - 84; PMID: 10754313
- Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 2005; 21:1644 - 52; http://dx.doi.org/10.1021/bp050228w; PMID: 16321047
- Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol Immunol 1995; 32:1311 - 8; http://dx.doi.org/10.1016/0161-5890(95)00118-2; PMID: 8643100