Abstract
TAM-163, an agonist monoclonal antibody targeting tyrosine receptor kinase-B (TrkB), is currently being investigated as a potential body weight modulatory agent in humans. To support the selection of the dose range for the first-in-human (FIH) trial of TAM-163, we conducted a mechanistic analysis of the pharmacokinetic (PK) and pharmacodynamic (PD) data (e.g., body weight gain) obtained in lean cynomolgus and obese rhesus monkeys following single doses ranging from 0.3 to 60 mg/kg. A target-mediated drug disposition (TMDD) model was used to describe the observed nonlinear PK and Emax approach was used to describe the observed dose-dependent PD effect. The TMDD model development was supported by the experimental determination of the binding affinity constant (9.4 nM) and internalization rate of the drug-target complex (2.08 h−1). These mechanistic analyses enabled linking of exposure, target (TrkB) coverage, and pharmacological activity (e.g., PD) in monkeys, and indicated that ≥ 38% target coverage (time-average) was required to achieve significant body weight gain in monkeys. Based on the scaling of the TMDD model from monkeys to humans and assuming similar relationship between the target coverage and pharmacological activity between monkey and humans, subcutaneous (SC) doses of 1 and 15 mg/kg in humans were projected to be the minimally and the fully pharmacologically active doses, respectively. Based on the minimal anticipated biological effect level (MABEL) approach for starting dose selection, the dose of 0.05 mg/kg (3 mg for a 60 kg human) SC was recommended as the starting dose for FIH trials, because at this dose level < 10% target coverage was projected at Cmax (and all other time points). This study illustrates a rational mechanistic approach for the selection of FIH dose range for a therapeutic protein with a complex model of action.
Introduction
TAM-163, a recombinant humanized IgG1κ monoclonal antibody (mAb) engineered to act as an agonist of tyrosine kinase receptor B (TrkB), is being investigated for the treatment of disorders associated with weight loss, such as anorexia and cancer cachexia. TrkB and its ligands are expressed in three major appetite-regulatory centers located in the hypothalamus (HT), the dorsal vagal complex (DVC) of the brain stem and the enteric nervous system of the gut.Citation1,Citation2 Strong genetic evidence links TrkB and its primary endogenous ligand, brain derived neurotrophic factor (BDNF), to the regulation of body weight in rodents and in humans. In addition, pharmacological administration of either BDNF or the second endogenous ligand of TrkB, neurotrophin-4 (NT-4), profoundly affects food intake and body weight in rodents. Interestingly, while peripheral administration of NT-4 inhibits food intake and body weight gain in rodents, peripheral administration of the same agent increases food intake and body weight in non-human primates (NHPs).Citation3,Citation4 It has been suggested that TrkB ligands can act to modulate food intake differently at sites outside and behind the blood brain barrier (BBB), and that the peripheral site may be different between rodents and NHPs.
The important role of TrkB and its ligands on appetite regulation and body weight in multiple species provides a rationale for investigating the utility of TrkB agonists in weight associated disorders in humans. TAM-163 modulates body weight and food intake in rodents and non-human primates in a manner similar to NT-4 (Perreault et al., PLOS One, in press), and might be expected to increase body weight in humans similar to its effects on NHPs. Because of its complex pharmacology, agonistic mechanism of action, and potential species differences, efficient dose selection strategy is critical to the successful clinical development of TAM-163. The selection of doses that are too high may lead to significant safety concerns, as exemplified by the widely publicized case of TGN1412, an agonistic antibody against a T cell target.Citation5 On the other hand, a starting dose that is too low can lead to the need for clinical testing of a wide dose range, which is associated with high cost and time burdens. To ensure that the selected human doses adequately test the pharmacology while avoiding any potential safety issues resulting from over-activation or exaggerated pharmacology, it is essential to develop a quantitative pharmacology-driven modeling approach to advance TAM-163 from preclinical to clinical evaluation. Additionally, although we expect TAM-163 to be pharmacologically active upon single-dose administration in human, testing the effect of TAM-163 on body weight may require larger cohorts than typically utilized in first-in-human (FIH) studies, in which safety and pharmacokinetics (PK) are the primary endpoints.
Various empirical approaches for the selection of the clinical doses for initial human testing of therapeutic proteins have been used in the past.Citation6-Citation10 However, there is an increased emphasis on mechanistic approaches that incorporate the effect of physiological variables or disease status on PK and PD parameters.Citation11,Citation12 In the case of antibodies exhibiting target-mediated drug disposition (TMDD), the nonlinearity in the PK data has been analyzed by well-established mathematical models that take into account the receptor expression and turnover kinetics.Citation13-Citation16 A mechanism-based pharmacokinetic/pharmacodynamic (PK/PD) approach is particularly useful for therapeutic proteins because quantitative models can be used to predict target coverage at the clinical doses as a function of biological systems parameters (also referred to as biomeasures).Citation11,Citation12,Citation17
In this study, we applied mechanistic modeling to analyze preclinical PK and PD (e.g., body weight) data following administration of TAM-163 to lean and obese NHPs. Our main objective was to guide the dose selection for a FIH study of TAM-163. The quantitative approach for PK data analysis included an initial non-compartmental analysis followed by detailed mechanistic modeling to predict the target coverage achieved at various doses of TAM-163 in NHPs, whereas the Emax approach was used to describe the dose-dependent PD effect. To ensure a robust model development, in vitro experiments were conducted to estimate the critical TrkB/TAM-163 biomeasures. The TMDD model together with the Emax analyses were applied to link exposure, TrkB coverage and pharmacological activity in monkeys. The TMDD model parameters and target occupancy/PD link were subsequently scaled to humans and utilized to project a starting dose, as well as a dose range with desired degree of pharmacological activity for a FIH study.
Results
Non-compartmental analyses (NCA) of PK data and Emax analyses of PD data
In all monkey studies, serum concentrations of TAM-163 ( and ) and body weights (Fig. S2) were monitored over time and used as PK and PD (using body weight as the percentage of baseline) endpoints, respectively. Specifically, PK and PD profiles of the TrkB agonist antibody TAM-163 were assessed after a single intravenous (IV) dose over the 0.3–60 mg/kg dose range in lean cynomolgus monkeys and over the 1–10 mg/kg dose range in obese rhesus monkeys (study designs are summarized in ). Additionally, a group of lean cynomolgus monkeys was dosed subcutaneously (SC) with 5 mg/kg of TAM-163 to evaluate the bioavailability and absorption kinetics.
Table 1. Monkey Study Design Summary
Initial PK calculations were performed using non-compartmental analyses (NCA). Following a single IV dose of TAM-163 to lean cynomolgus monkey, nonlinear PK was observed at all doses lower than 20 mg/kg IV, such that both the total body clearance (CL) was faster and elimination half-life (t1/2) was shorter at the lower dose levels ( and ). Specifically, the mean total body CL at the lowest dose of 0.3 mg/kg was 0.737 mL/hr/kg, while that at the 20 mg/kg dose was 0.312 mL/hr/kg. PK parameters of TAM-163 in lean cynomolgus monkeys appeared similar to those in obese rhesus monkeys when similar doses were used. For example, following a single 1 mg/kg IV dose, the mean CL was 0.460 and 0.468 mL/hr/kg in lean cynomolgus and obese rhesus monkeys, respectively ().
Figure 1. Individual and mean (± SD) total body clearance (CL) of TAM-163 in lean cynomolgus and obese rhesus monkeys after a single IV dose determined by non-compartmental analysis (NCA). Monkeys were administered a single IV bolus dose of TAM-163 at indicated dose levels using study designs summarized in . TAM-163 concentrations in serum samples and total body CL were determined by a specific immunoassay and NCA, respectively, as described in the text. Cyno = cynomolgus monkeys.
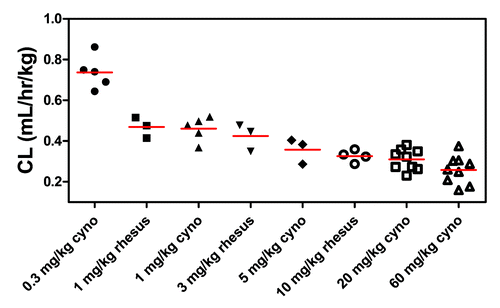
Table 2. Mean (± SD) PK parameters of TAM-163 in lean cynomolgus and obese rhesus monkeys after a single IV or SC dose determined by non-compartmental analysis (NCA)
Following a single SC administration of 5 mg/kg of the TAM-163 to lean cynomolgus monkeys, the bioavailability of TAM-163 was approximately 66% and the mean Tmax was 88 h, similar to the corresponding parameters for other IgG1 antibodies.Citation18
Significant body weight gain after a single dose of TAM-163 antibody was observed in both lean cynomolgus and obese rhesus monkeys at doses of greater or equal to 1 mg/kg (Fig. S2) in a dose-dependent manner. The mean body weight change-time profile for each dose group was analyzed using an Emax approach to determine both the magnitude and time of maximum effect (defined as Emax and TmaxE, respectively, in ). This analysis indicated that single administration of TAM-163 resulted in statistically significant dose-dependent body weight gain in both lean cynomolgus and obese rhesus monkeys at doses of greater or equal to 1 mg/kg, with Emax of ~5–22% at doses examined. Based on the Emax analysis, the doses of 1 and 20 mg/kg were defined as minimally and fully pharmacologically active doses, respectively. The PD effect appeared to be driven by the AUC rather than the Cmax, as TmaxE ranged from 17 to28 d. The results from the Emax-based PD analysis are consistent with a population modeling analysis of body weight-time course that established PK/PD relationships for body weight effect based on the individual cynomolgus monkey data (see Supplemental Data).
Table 3. Maximal observed mean body weight gain relative to baseline (Emax) and time to reach Emax (TmaxE) after a single dose of TAM-163 to lean cynomolgus and obese rhesus monkeys
TAM-163 binding affinity and internalization rate
In vitro experiments were conducted to measure the critical parameters determining the target coverage upon antibody dosing: antibody affinity to the TrkB receptor, and the internalization rate of antibody-receptor complex. The binding affinity constant (Kd) of TAM163 antibody against TrkB receptor was estimated using surface-plasma resonance (SPR)-based BiAcore instrument. The SPR experiments allowed the estimation of association (Kon = 1.54 nM*hr−1) and dissociation (Koff = 14.5hr−1) constants for antibody-receptor binding. Based on these values, the binding affinity constant (i.e., Kd) was calculated to be 9.4nM. The internalization rate of the endogenous receptor upon antibody binding was measured in a human neuroblastoma cell line (SH-SY5Y). The half-life of receptor internalization rate was estimated to be ~20 min, which corresponds to a rate constant (i.e., Kint) of 2.08 h–1.
Mechanistic analysis of monkey data using TMDD model
PK data from cynomolgus and rhesus monkeys were combined and fitted using a TMDD model () with a single set of parameters for both species except for the central volume of distribution (V1). In addition to the antibody-target binding kinetics, the TMDD model incorporated the in vivo target, drug, and target-drug complex turnover rates, such as elimination rate of free antibody (Kel), synthesis and degradation of TrkB (Ksyn and Kdeg) and internalization rate of antibody-target complex (Kint). In general, the model fitted the observed data reasonably well for both the lean cynomolgus and obese rhesus monkeys for most dose groups with the exception of the 10 mg/kg IV dose group in rhesus, where the data collected after 1000 h were under-predicted ( and ). The reason for the bias is currently unknown but is deemed to have minimal impact on the overall conclusions of the analyses presented in this report. All the estimated parameters in the mechanistic model were determined with high precision and relative standard error (RSE) well below 50%. A complete list of model estimated parameters are provided in . The absorption rate constant (Ka) through SC route was determined to be 0.019 (1/hr), similar to the value of 0.011 (1/hr) for a typical human IgG.Citation18 The bioavailability value (F) through SC route was estimated to be 0.644 or 64.4%. The effective in vivo concentration of TrkB receptor was estimated to be 171 pM with RSE of 4%; this is a model-based estimation that warrants experimental verification. At present, the total number of cells in the body expressing TrkB receptor and its expression level in primary cell lines are not known.
Figure 2. Schematic representation of the TMDD model. Ka and F denote the SC absorption rate and bio-availability, respectively. The Kel, K12 and K21 denote the first order rate constants corresponding to serum elimination, serum to peripheral, and peripheral to serum distribution, respectively. TrkB receptor internalization rate (Kint), baseline levels (R0), and turnover rate (Kdeg) are incorporated into the model along with the association (Kon) and dissociation (Koff) rate constants of TAM-163 binding to TrkB receptor. Ac and Ap denote antibody amount in central and peripheral compartment, respectively.
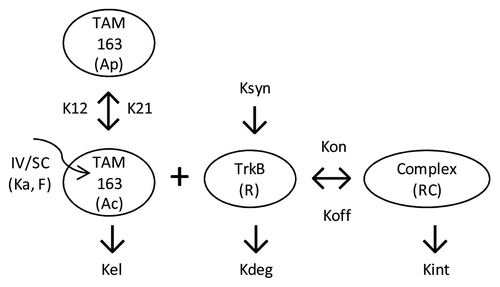
Figure 3. TAM-163 serum concentrations in lean cynomolgus monkeys fitted with the TMDD model. The observed data are represented by open circles, whereas model fit is denoted by a solid line. Monkeys were administered a single IV bolus or SC dose of TAM-163 at indicated dose levels using study designs summarized in . TAM-163 concentrations in serum samples were determined by a specific immunoassay as described in the text. Cyno, cynomolgus monkeys; Conc., concentration.
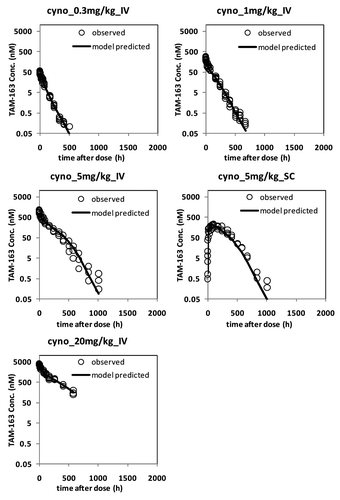
Figure 4. TAM-163 serum concentrations in obese rhesus monkeys fitted with the TMDD model. The observed data are represented by open circles, whereas model fit is denoted by a solid line. Monkeys were administered a single IV bolus dose of TAM-163 at indicated dose levels using study designs summarized in . TAM-163 concentrations in serum samples were determined by a specific immunoassay as described in the text.
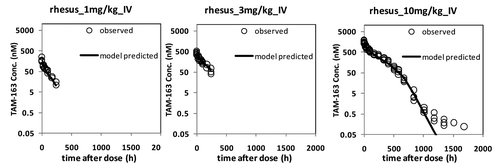
Table 4. The TMDD model parameters for monkeys and humans
Target coverage required for pharmacological activity in monkeys
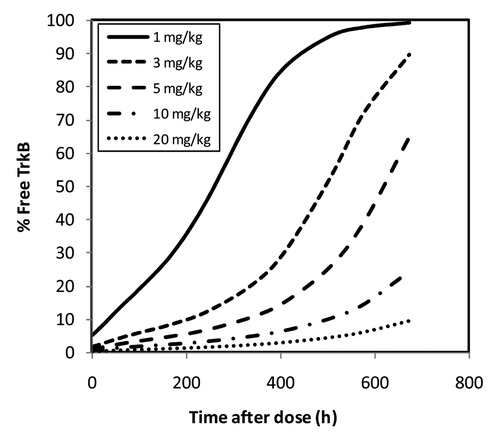
The TMDD model with the parameters estimated using monkey data was subsequently utilized to calculate % target coverage (defined as 100 - % free target levels), which was then linked to the pharmacological activity (e.g., Emax for the body weight change in ) observed after single IV doses in cynomolgus and rhesus monkeys. presents the time course of % free target levels at the doses of 1, 3, 5, 10 and 20 mg/kg. Based on the predicted profiles, the % free target level for 1 and 20 mg/kg IV doses averaged over a 28 d period was 62% and 3%, respectively. Conversely, the % target coverage (averaged over the 28-d period) was estimated to be 38% and 97% (defined as 100 - % free target levels) for 1 and 20 mg/kg doses, respectively. Note that the 1 mg/kg IV dose in lean cynomolgus monkeys resulted in a statistically significant increase in body weight relative to the baseline, with Emax of ~5% (p < 0.05) (). Thus, a relative low level of target coverage (38%) at 1 mg/kg IV dose resulted in a statistically significant pharmacological activity. At 20 mg/kg dose, the Emax for body weight gain was ~18% and appeared to be approximately the maximal Emax value observed for the lean cynomolgus monkeys, which is in agreement with close to maximal target coverage (97%) achieved at this dose.
Figure 5. Projection of free target levels, as percentage of total target levels, after a single IV dose of TAM-163 in cynomolgus and rhesus monkeys using the TMDD model. Monkeys were administered a single IV bolus dose of TAM-163 at indicated dose levels using study designs summarized in . The observed serum concentration and biomesure data were used to estimate % free target at each time point for each dose group, as described in the text. The 28-d (672 h) average free target percentages were calculated to be 62% and 3% for 1 and 20 mg/kg IV doses, respectively. The 28-d time-average target coverage percentages (defined as the inverse of time-average free target percentage) were calculated to be 38% and 97% for 1 and 20 mg/kg IV doses, respectively.
Projection of pharmacologically active dose in human
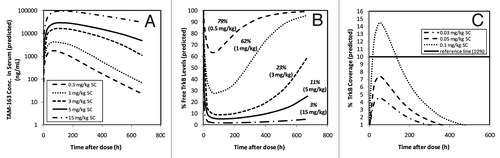
The TMDD model parameters developed using monkey data and the target occupancy/PD link were scaled to human to predict the human doses that will result in an appropriate level of pharmacological activity (e.g., body weight change). In the clinic, the SC dosing of TAM-163 is the intended route of administration. Correspondingly, the scaled model took into account the antibody absorption kinetics and bioavailability estimated from the monkey data. Ultimately, the scaled TMDD model (with projected human PK and biomeasures) was used to predict human SC doses that would produce an average target coverage similar to that for the IV doses in monkeys at which a statistically significant body weight gain was observed (, ). Specifically, a single SC dose of 1 mg/kg in humans is projected to achieve 62% time-averaged free levels of target or 38% time-averaged target coverage (), which is identical to the estimated target coverage at the minimally pharmacologically active dose in lean monkeys (i.e., 1 mg/kg IV). A single SC dose of 15 mg/kg SC was predicted to achieve 3% time-averaged free target levels or 97% time-averaged target coverage in humans, which is identical to the estimated target coverage at the fully pharmacologically active dose in lean monkeys (i.e., 20 mg/kg IV). Consequently, 1 mg/kg SC dose was defined as the minimally pharmacologically active dose in human, whereas 15 mg/kg SC dose was predicted to achieve close to maximal pharmacological effect in single dose human studies.
Figure 6. Projection of target coverage profiles after a single SC dose of TAM-163 administered to humans. (A) Serum TAM-163 concentrations in healthy humans at 0.5, 1, 3, 5 and 15 mg/kg SC doses were projected using the model parameters specified in . (B) Projected % free target levels at various SC doses in human estimated with the human TMDD model scaled from monkey model parameters. The estimated average free target percentages over 28 d (i.e., 672 h) after single SC doses to humans were 79%, 62%, 23%, 11% and 3% for 0.5, 1, 3, 5 and 15 mg/kg doses, respectively. Conversely, the estimated 28-d time-average % target coverage after single SC doses to humans were 21%, 38%, 77%, 89% and 97% for 0.5, 1, 3, 5 and 15 mg/kg doses, respectively. (C) % Target coverage in humans projected at single SC doses of 0.03, 0.05 and 0.1 mg/kg. Conc., concentration.
Projection of starting dose in human
The TMDD model scaled to human was utilized to project starting doses for FIH trials based on the MABEL (minimum anticipated biological effect level) approach.Citation19 As a surrogate measure of minimal pharmacological effect, predicted receptor coverage has been suggested and utilized in recent published studies to guide starting doses for human trials.Citation17,Citation20 Adopting this approach, we applied the human TMDD model of TAM-163 to predict a SC starting dose that will result in a target coverage value of < 10% at all time after dosing. presents the target coverage profile for SC doses of 0.03, 0.05 and 0.1 mg/kg. At these doses, corresponding peak target coverage is projected to be 4.5%, 7.4% and 14.4%, respectively, and the average coverage over a month is projected to be 1.3%, 2.2% and 4.5%, respectively. Based on these model predictions, the 0.05 mg/kg SC (3 mg for a 60 kg human) was recommended as the starting dose with < 10% coverage at all times post-dose. Note that at this dose, the peak target coverage is estimated as 7.4% and thus is below 10% level and the average level of target coverage over a 28-d period is 2.2% and is well below the level of 38% at which statistically significant body weight gain was seen in monkeys. Thus, a single dose of 0.05 mg/kg SC of TAM-163 is expected to result in minimal biological effect in humans.
Discussion
To guide the dose selection of first-in-human studies of TAM-163, we developed a mechanistic PK/PD modeling approach to analyze longitudinal serum concentration and body weight data following single administration of an anti-TrkB agonist antibody TAM-163 to lean (0.3–60 mg/kg dose range) and obese (1–10 mg/kg dose range) monkeys. Specifically, we estimated TrkB receptor occupancy (driven by PK and TrkB/TAM-163 biomeasures) and correlated the receptor occupancy data with the maximal PD effect. This correlation was translated to human system to guide the FIH dose selection.
The Emax-based analyses of mean PD time-course indicated that single administration of TAM-163 resulted in statistically significant dose-dependent body weight gain in both lean cynomolgus and obese rhesus monkeys at doses of greater or equal to 1 mg/kg, with Emax values of ~5–22% across monkey species. Based on the Emax analysis, doses of 1 and 20 mg/kg were defined as minimally and fully pharmacologically active doses, respectively. The PD effect appeared to be driven by the AUC rather the Cmax, since time to reach Emax ranged from 17 to 28 d. Note that the results from the Emax-based PD analysis were consistent with a population modeling analysis of body weight-time course that established PK/PD relationships for body weight effect based on the individual cynomolgus monkey data (Supplemental Data). For the purposes of guiding dose selection of human trials, a simpler Emax model was chosen to correlate pharmacological activity to TrkB occupancy (estimated via TMDD modeling as discussed below) in monkeys and humans.
Initial exploratory analysis using non-compartmental modeling of monkey serum concentration data showed (1) nonlinear PK profile of TAM-163 at the 0.3–20 mg/kg dose range and (2) similarity of TAM-163 PK profiles in lean cynomolgus and obese rhesus monkeys. To confirm that a faster elimination of TAM-163 observed at lower doses was not due to the presence of ADAs, a bridging ELISA method was used to assess the formation of ADAs in both non-human primate species. The incidence of ADAs was low (less than 5%), and there was no apparent difference between dose groups or species, suggesting that ADAs are unlikely to account for nonlinear PK profile of TAM -163.
The dose-dependent decrease in CL described above for TAM-163 is a hallmark of target mediated clearance observed for various antibodies against surface expressed, as well as some soluble targets.Citation16,Citation18,Citation21 The observation of such target mediated process suggests target engagement in vivo, and consequently, the observed nonlinear PK profiles are quite informative about the target expression levels in the body and corresponding target coverage upon antibody dosing. Thus, we developed a TMDD model approach using the nonlinear serum concentration data from NHP studies.
To avoid the problem of over-parameterization of the model leading to non-identifiability of key parameters required for target coverage estimation, we performed experimental determination of key kinetic constants driving the target mediated clearance. First, Kon, Koff and Kd for TAM-163/TrkB binding were measured in vitro by the SPR technology and used as the estimates of the corresponding parameters in vivo. Second, internalization rate of TrkB upon TAM-163 binding (Kint) was measured in a human neuroblastoma cell line (SH-SY5Y) and likewise used as an estimate of the corresponding in vivo parameter. With the experimental estimation of these three biomeasures (Kon, Koff, and Kint) the number of parameters that needed to be estimated by the model was reduced by three, since these parameters were fixed during data fitting. An additional assumption was made that the Kdeg (the turnover rate of TrkB when not in complex with TAM-163) was the same as Kint, because the receptor is known to undergo internalization under the constant presence of endogenous ligand BDNF that binds TrkB with a similar Kd value as TAM-163 (~7nM).Citation22
With the experimentally determined biomeasures, the TMDD model was used for simultaneous fitting of lean and obese monkey data toward estimating the target (TrkB) coverage at various doses of TAM-163. The model predicted TrkB coverage by TAM-163 was subsequently linked to the observed body weight gain. The modeling results showed that, in monkeys at a minimally pharmacologically active dose of 1 mg/kg IV, the predicted TrkB target coverage (average over a 28-d period) was 38%, whereas at a fully pharmacologically active dose of 20 mg/kg IV, the predicted time-averaged target coverage was 97%. A low level of target coverage (38% at 1 mg/kg) resulting in a pharmacological activity is consistent with the agonistic action of TAM-163 upon TrkB binding. As reported previously, high potency agonists generally require low level of target coverage, as opposed to the antagonists that require 60–90% or higher target coverage to generate a pharmacologically meaningful effect.Citation23
To translate the monkey model to human settings, monkey parameters were scaled to obtain a human specific TMDD model. First, allometric scaling of the linear components of monkey PK parameters was conducted to predict the corresponding human PK parameters. The absorption rate (Ka), the central elimination rate (Kel), the central to peripheral distribution rate (K12), and the peripheral to central distribution rate (K21) of TAM-163 were scaled from monkeys to human with an exponent of -0.25 (). The central volume of distribution (V1) was scaled using an exponent of 1. The bioavailability (F) though SC route was assumed to be the same in monkeys and human (0.644 or 64.4%). Several recent studies on biotherapeutics report recommendations on the ideal exponent value to be used for the clearance and volume parameters,Citation6,Citation7,Citation9,Citation14,Citation24,Citation25 but these recommendations differ from one another and likely to be influenced by the limited number of drugs that were included in the analysis. Our use of -0.25 exponent for first-order kinetic constants and 1.0 for volume is consistent with the fundamental allometric principles,Citation7 and supported by the recent study on the scaling of antibodies exhibiting nonlinear PK.Citation14 Moreover, an exponent of 0.85 instead of 0.75 for scaling does not lead to any meaningful changes in our dose projections (data not shown). For example, a dose of 15 mg/kg SC is projected to achieve a coverage value of 96% using exponent of 0.85 vs. 97% using exponent of 0.75. Ultimately, availability of human PK will provide the necessary data to test the adequacy of these exponents in predicting human exposures.
Target specific parameters (Kint and Kd) were assumed to be the same between monkeys and humans, given the absence of any experimental evidence pointing to differences in TrkB kinetics between monkeys and humans. The assumption of similar Kint between monkeys and human is supported by a recent study on an IgG2 antibody against AKL1 receptor, in which authors reported similar complex internalization rates between monkeys and humans.Citation12 The Kd assumption is supported by 100% identity in amino acid sequence of extracellular binding domain of TrkB is 100% between monkeys and humans.
Using the human-specific TMDD model described above and assuming similar target coverage/PD relationships in monkeys and humans, we projected 1mg/kg and 15 mg/kg SC to be the minimally and fully pharmacologically active human doses, respectively. Additionally, to provide guidance on the starting dose for the FIH study, the model was utilized to predict a SC dose that results in < 10% target coverage at the projected human exposures, in agreement with the ICH6 guidelines for MABEL dose.Citation19 Our analysis suggested that a starting dose of ~0.05 mg/kg SC (or 3 mg for a 60 kg human) would be consistent with MABEL guidelines.
In summary, our modeling results based on monkey PK/PD data suggest that the FIH study of TAM-163 should start with the 0.05 mg/kg SC dose cohort and include dose levels ≥ 1mg/kg SC to observe pharmacological activity. Additionally, doses greater than 15 mg/kg SC are unlikely to show improvement in the pharmacological activity because nearly maximal target coverage (~97%) is projected to be already achieved at this dose. The top dose selection, however, would require integration of safety and pharmacology data, and may be lower than 15 mg/kg SC.
In conclusion, this report exemplifies the use of mechanistic modeling to guide human dose projections of therapeutic proteins with complex PK/PD relationship, especially when the physiological basis of this complexity is only partially understood. As the biology of the pathway is further elucidated, more complex systems pharmacology models may be used for future analyses of anti-TrkB antibody pharmacology data across species.
Materials and Methods
Materials
TAM-163 IgG1 antibody was generated via immunization of BALB/c mice with recombinant human TrkB-extracellular domain (rhTrkB- ECD) (R and D Systems, Minneapolis, MN) and humanized at Pfizer Inc. (previously Wyeth Research). The antibody was produced using stable Chinese hamster ovary (CHO) cell line and formulated with either 10 mM L histidine, 5% sucrose, pH 6.0 or 20 mM l-histidine, 7.5% sucrose, 0.01% polysorbate 80, pH 6.0.
Animals
Naïve 3–6 y old male or female cynomolgus (lean) or male rhesus (obese) monkeys were used for all studies (as indicated in ). Cynomolgus monkeys were given a daily food ration of Teklad Certified Global Primate Diet biscuits (with additional biscuits offered if all were consumed), and were allowed water ad libitum. Rhesus monkeys were on a 45% high-fat diet for 9 mos, and were then acclimated to two meals of 1.5 to 2.5 h duration per day. It was ensured that animals had excess biscuits for each meal. In-life portions of the cynomolgus and rhesus monkeys studies were conducted at Pfizer Inc. and Charles Rivers Inc., respectively, and the respective Institutional Animal Care and Use Committees approved all aspects of these studies.
PK/PD study design
summarizes designs of the studies that were conducted in cynomolgus and rhesus monkeys. For each study with multiple dose groups, animals were weighed and assigned to groups in a manner that minimized mean body weight differences among groups. The test article was administered via a single IV or SC injection at the indicated dosages (mg/kg), volumes (mL/kg), and formulation buffers (). The administered doses (in mgs) were based on the most recent body weights prior to dosing. For IV administration, the test article was administered as a single bolus dose into the saphenous vein.
For collection of serum for test article serum concentrations, all animals were bled from the cephalic, saphenous or femoral vein at time points indicated in . Whole blood aliquots were collected in tubes without anticoagulant, allowed to clot at room temperature, processed for serum by centrifugation, and stored at -70°C until bioanalytical analyses. Body weights were taken at pre-dose (at least twice for the baseline body weight determination) and then at time points indicated in and in the figures. Additional serum samples for determination of anti-drug antibodies (ADA) were taken at pre-dose, day 14, and until the end of the study.
ELISA for determination of TAM-163 serum concentrations
TAM-163 in serum samples was captured onto a microtiter plate pre-coated with rhTrkB- ECD. The bound TAM-163 was detected with a mouse anti-human IgG mAb conjugated to horseradish peroxidase (HRP). The enzyme substrate, 3, 3′, 5,5′-tetramethylbenzidine (TMB), was used to produce a colored end product. Optical densities (OD) were measured using a microplate spectrophotometer at 450 nm. Sample concentrations were determined by interpolation from a calibration curve that was fit using a 4 parameter logistic equation (Softmax Pro, version 4.3.1, Molecular Devices and Watson LIMS, version 7.0.0.01, Thermo Electron Corporation). The lower limit of quantitation (LLOQ) was 14.6 ng/mL.
ELISA for determination of anti-TAM-163 antibodies
Anti-TAM-163 antibodies in serum samples were captured onto a microtiter plate pre-coated with TAM-163. Bound antibodies were detected with biotinylated TAM-163 and Avidin D conjugated to HRP and ODs were determined as described above. A positive control (goat anti-human IgG) was used to monitor the performance of the assay. Normal cynomolgus monkey serum was used as the negative control and also to determine the assay cutpoint OD value, which was defined as twice the mean of the OD value of the negative control for each plate. Samples were screened at dilutions of 1:25 and 1:75. Samples generating an OD less than the cutpoint OD were considered negative.
Body weight data analysis using an Emax approach
Body weights were monitored over the time period indicated in and expressed as the percentage change from baseline body weight for each animal. Baseline was defined as the latest pre-dose value for all groups, except for the 0.3 and 1 mg/kg cynomolgus monkey groups, for which an average of pre-dose values over the one-month monitoring period was used. The mean body weight change time-course for each dose group was used to obtain Emax values (the maximal observed body weigh change over the duration of the study) and TmaxE (the time point at which Emax was observed).
Serum concentration analysis using a non-compartmental model
PK parameters for individual serum concentration profiles were determined using Model 201 for IV route and Model 200 SC routes of the pharmacokinetic software package WinNonlin, ver. 5.1 (Pharsight). The area under the serum concentration vs. time curve (AUC) was calculated using the linear trapezoidal method. The slope of the apparent terminal phase was estimated by log-linear regression using at least 3 data points and the terminal rate constant (λ) was derived from the slope. AUC0-∞ was estimated as the sum of the AUC0-t (where t is the time of the last measurable concentration) and Ct/λ. The apparent terminal half-life (t½) was calculated as 0.693/λ.
Biomeasures Assays
Kinetic parameters (i.e., association and dissociation rate constants) for TAM-163/TrkB interactions were determined by the surface plasmon resonance (SPR) method. Human TrkB ECD was immobilized at a low density on a CM5 chip (41 and 30 response units, respectively) and then various concentrations of TAM-163 were injected over the surface using a 2 min association and 4 min dissociation. The surface was regenerated with 4 M MgCl2 between injection cycles. Data analysis was done using a 1:1 fit model and the association rate constant, dissociation rate constant) and KD (affinity) were calculated.
The TrkB internalization was detected by measuring cell surface associated TrkB in the presence or absence of TrkB activation as previously described.Citation26
Serum concentration analysis using a TMDD model
TAM-163 serum concentration in NHPs were analyzed using a TMDD model that incorporates the binding equilibrium and in-vivo turnover rates of TAM-163 antibody, free target (e.g.,., TrkB receptor) and the drug-target (e.g., TAM-163/TrkB) complex as depicted in . MONOLIX v3.2 software was used to perform all the data fitting and model estimation.Citation27 This modeling framework is similar to the approximation of target-mediated drug disposition models originally described by Mager and coworkersCitation16,Citation28 and recently expanded by Gibiansky and coworkers.Citation15,Citation29 The following set of equations describes the in-vivo kinetics of drug, target, and drug-target complexes:
Here, Equations 1, 2 and 3 describe the kinetics of drug amount in the SC compartment (ASC), central (Ac), and peripheral compartments (Ap), respectively. Equations 4 and 5 describe the dynamics of free receptor (R) and drug-receptor complex (RC) in concentration terms. Equation 6 takes into account the physiological balance between the receptor synthesis (Ksyn) and degradation rate prior to the antibody dosing. Equation 7 was used to convert antibody amount in the central compartment to the antibody concentration (Cobs) to fit the observed serum concentrations.
Ka and Kel denote first order antibody absorption and elimination rate constants, respectively, whereas K12 and K21 are rate constants for antibody distribution from central-to-peripheral and peripheral-to-central compartments, respectively. Central volume of distribution is denoted by V1. Receptor-drug binding kinetics is governed by association (Kon) and dissociation rate (Koff) constants. Physiological degradation rate of free receptor and complex is denoted as Kdeg and Kint, resepcitvely. Ro parameter denotes the baseline levels of the target prior to dosing.
V1 was estimated separately for cynomolgus and rhesus monkeys. Inter-individual variability (IIV) was estimated for only Ka and V1, and expressed using an exponential error model (Eqn. 8).
Where PRi is the individual value of ith model parameters with a population mean of PRpop. ńi is a random variable for the ith parameter with a mean of 0 and variance of ùCitation2i for ith parameter. The residual variability in the observed data was modeled using an combined error model with the proportionality constant (a) and additive constant (b).
Additional material
Download Zip (358 KB)Acknowledgments
Authors thank Chandrasekhar Udata, Pam Szklut, Lei Sun, Beth Leary, Chris Shea, Bonnie Rup, Janet Paulsen, Ying Sun, Kim Marquette, Guo Feng, Karissa Adkins, William McWilliams and Xin Xu for their valuable contributions to data generation and interpretation.
Disclosure of Potential Conflicts of Interest
All authors were employees of Pfizer, Inc. at the time of study completion.
References
- Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci 2006; 126-127:30 - 8; http://dx.doi.org/10.1016/j.autneu.2006.02.027; PMID: 16632412
- Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol 1996; 148:1807 - 18; PMID: 8669468
- Lin JC, Tsao D, Barras P, Bastarrachea RA, Boyd B, Chou J, et al. Appetite enhancement and weight gain by peripheral administration of TrkB agonists in non-human primates. PLoS One 2008; 3:e1900; http://dx.doi.org/10.1371/journal.pone.0001900; PMID: 18382675
- Tsao D, Thomsen HK, Chou J, Stratton J, Hagen M, Loo C, et al. TrkB agonists ameliorate obesity and associated metabolic conditions in mice. Endocrinology 2008; 149:1038 - 48; http://dx.doi.org/10.1210/en.2007-1166; PMID: 18063676
- Hünig T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nat Rev Immunol 2012; 12:317 - 8; http://dx.doi.org/10.1038/nri3192-c2; PMID: 22487653
- Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol 2009; 49:1382 - 402; http://dx.doi.org/10.1177/0091270009337134; PMID: 19837907
- Mahmood I. Pharmacokinetic allometric scaling of antibodies: application to the first-in-human dose estimation. J Pharm Sci 2009; 98:3850 - 61; http://dx.doi.org/10.1002/jps.21682; PMID: 19177515
- Mordenti J, Chen SA, Moore JA, Ferraiolo BL, Green JD. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm Res 1991; 8:1351 - 9; http://dx.doi.org/10.1023/A:1015836720294; PMID: 1798669
- Oitate M, Masubuchi N, Ito T, Yabe Y, Karibe T, Aoki T, et al. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metab Pharmacokinet 2011; 26:423 - 30; http://dx.doi.org/10.2133/dmpk.DMPK-11-RG-011; PMID: 21606605
- Vugmeyster Y, Szklut P, Tchistiakova L, Abraham W, Kasaian M, Xu X. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of humanized monoclonal anti-IL-13 antibodies with different IL-13 neutralization mechanisms. Int Immunopharmacol 2008; 8:477 - 83; http://dx.doi.org/10.1016/j.intimp.2007.12.004; PMID: 18279802
- Betts AM, Clark TH, Yang J, Treadway JL, Li M, Giovanelli MA, et al. The application of target information and preclinical pharmacokinetic/pharmacodynamic modeling in predicting clinical doses of a Dickkopf-1 antibody for osteoporosis. J Pharmacol Exp Ther 2010; 333:2 - 13; http://dx.doi.org/10.1124/jpet.109.164129; PMID: 20089807
- Luu KT, Bergqvist S, Chen E, Hu-Lowe D, Kraynov E. A model-based approach to predicting the human pharmacokinetics of a monoclonal antibody exhibiting target-mediated drug disposition. J Pharmacol Exp Ther 2012; 341:702 - 8; http://dx.doi.org/10.1124/jpet.112.191999; PMID: 22414855
- Aston PJ, Derks G, Raji A, Agoram BM, van der Graaf PH. Mathematical analysis of the pharmacokinetic-pharmacodynamic (PKPD) behaviour of monoclonal antibodies: predicting in vivo potency. J Theor Biol 2011; 281:113 - 21; http://dx.doi.org/10.1016/j.jtbi.2011.04.030; PMID: 21557949
- Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, et al. Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet 2011; 50:131 - 42; http://dx.doi.org/10.2165/11537430-000000000-00000; PMID: 21241072
- Gibiansky L, Gibiansky E. Target-mediated drug disposition model for drugs that bind to more than one target. J Pharmacokinet Pharmacodyn 2010; 37:323 - 46; http://dx.doi.org/10.1007/s10928-010-9163-3; PMID: 20669044
- Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol 2006; 72:1 - 10; http://dx.doi.org/10.1016/j.bcp.2005.12.041; PMID: 16469301
- Yu J, Karcher H, Feire AL, Lowe PJ. From target selection to the minimum acceptable biological effect level for human study: use of mechanism-based PK/PD modeling to design safe and efficacious biologics. AAPS J 2011; 13:169 - 78; http://dx.doi.org/10.1208/s12248-011-9256-y; PMID: 21336535
- Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010; 49:633 - 59; http://dx.doi.org/10.2165/11535960-000000000-00000; PMID: 20818831
- Muller PY, Milton M, Lloyd P, Sims J, Brennan FR. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr Opin Biotechnol 2009; 20:722 - 9; http://dx.doi.org/10.1016/j.copbio.2009.10.013; PMID: 19896825
- Agoram BM. Use of pharmacokinetic/ pharmacodynamic modelling for starting dose selection in first-in-human trials of high-risk biologics. Br J Clin Pharmacol 2009; 67:153 - 60; http://dx.doi.org/10.1111/j.1365-2125.2008.03297.x; PMID: 19076987
- Lowe PJ, Tannenbaum S, Gautier A, Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br J Clin Pharmacol 2009; 68:61 - 76; http://dx.doi.org/10.1111/j.1365-2125.2009.03401.x; PMID: 19660004
- Chen ZY, Ieraci A, Tanowitz M, Lee FS. A novel endocytic recycling signal distinguishes biological responses of Trk neurotrophin receptors. Mol Biol Cell 2005; 16:5761 - 72; http://dx.doi.org/10.1091/mbc.E05-07-0651; PMID: 16207814
- Grimwood S, Hartig PR. Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol Ther 2009; 122:281 - 301; http://dx.doi.org/10.1016/j.pharmthera.2009.03.002; PMID: 19306894
- Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned?. MAbs 2011; 3:61 - 6; http://dx.doi.org/10.4161/mabs.3.1.13799; PMID: 20962582
- Kagan L, Abraham AK, Harrold JM, Mager DE. Interspecies scaling of receptor-mediated pharmacokinetics and pharmacodynamics of type I interferons. Pharm Res 2010; 27:920 - 32; http://dx.doi.org/10.1007/s11095-010-0098-6; PMID: 20232116
- Zheng J, Shen WH, Lu TJ, Zhou Y, Chen Q, Wang Z, et al. Clathrin-dependent endocytosis is required for TrkB-dependent Akt-mediated neuronal protection and dendritic growth. J Biol Chem 2008; 283:13280 - 8; http://dx.doi.org/10.1074/jbc.M709930200; PMID: 18353779
- Chan PL, Jacqmin P, Lavielle M, McFadyen L, Weatherley B. The use of the SAEM algorithm in MONOLIX software for estimation of population pharmacokinetic-pharmacodynamic-viral dynamics parameters of maraviroc in asymptomatic HIV subjects. J Pharmacokinet Pharmacodyn 2011; 38:41 - 61; http://dx.doi.org/10.1007/s10928-010-9175-z; PMID: 21088872
- Mager DE, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res 2005; 22:1589 - 96; http://dx.doi.org/10.1007/s11095-005-6650-0; PMID: 16180117
- Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn 2008; 35:573 - 91; http://dx.doi.org/10.1007/s10928-008-9102-8; PMID: 19005743