Abstract
Transgenic mice expressing human neonatal Fc receptor (FcRn) instead of mouse FcRn are available for IgG antibody pharmacokinetic (PK) studies. Given the interest in a rodent model that offers reliable predictions of antibody PK in monkeys and humans, we set out to test whether the PK of IgG antibodies in such mice correlated with the PK of the same antibodies in primates. We began by using a single research antibody to study the influence of: (1) different transgenic mouse lines that differ in FcRn transgene expression; (2) homozygous vs. hemizygous FcRn transgenic mice; (3) the presence vs. absence of coinjected high-dose human intravenous immunoglobulin (IVIG), and (4) the presence vs. absence of coinjected high-dose human serum albumin (HSA). Results of those studies suggested that use of hemizygous Tg32 mice (Tg32 hemi) not treated with IVIG or HSA offered potential as a predictive model for PK in humans. Mouse PK studies were then done under those conditions with a panel of test antibodies whose PK in mice and primates is not significantly affected by target binding, and for which monkey or human PK data were readily available. Results from the studies revealed significant correlations between terminal half-life or clearance values observed in the mice and the corresponding values reported in humans. A significant relationship in clearance values between mice and monkeys was also observed. These correlations suggest that the Tg32 hemi mouse model, which is both convenient and cost-effective, can offer value in predicting antibody half-life and clearance in primates.
Introduction
Having a convenient and inexpensive means to better predict the pharamcokinetics (PK) of IgG antibodies prior to performing studies in non-human primates or humans could offer several advantages. At the discovery stage, the predicted PK of lead candidates could be factored into decisions regarding which candidates to progress forward. Both toxicology studies and Phase 1 clinical studies could be designed with greater precision, most likely reducing both the number of dosage groups required and overall timelines. For complex drugs like IgG antibodies, even the most sophisticated in silico modeling programs currently available have serious limitations, in large part because of the many factors that can influence antibody PK. Although we and others have observed a significant range of affinities across different IgG antibodies for recombinant FcRn, the receptor that is critical to the long half-life of IgG antibodies, a correlation between affinity for FcRn and PK has not been observed among panels of unrelated antibodies, which is again attributable to the influence of other factors.Citation1-Citation3 These other factors may include immunogenicity of the antibody, target binding, molecular charge, certain post-translational modifications, Fab glycosylation, hydrophobicity (our unpublished observations), and proteolysis. What may be needed for greater predictive value is an in vivo model that allows more of these additional factors to manifest. We decided to perform our studies using human (hu) FcRn transgenic mice with the belief that these mice may be a better surrogate for preclinical evaluation of therapeutic antibodies.Citation4-Citation6
FcRn is the neonatal Fc receptor known for its role in IgG homeostasis.Citation7,Citation8 It is part of the major histocompatibility (MHC) I family and a heterodimer consisting of an Fc-binding α chain and a β2m subunit. Most of the FcRn resides in acidic endosomes within various cell types such as epithelial cells, endothelial cells, and macrophage/monocytes. In addition to mediating transcytosis of IgG antibodies across epithelial cell layers, FcRn mediates the recycling of internalized IgG due to its good affinity for IgG at the endosomal pH of 6.0, but undetectable affinity at the blood pH of 7.4. In this way, those IgG molecules that bind FcRn after cellular uptake by non-specific pinocytosis get escorted back to the plasma membrane and released at neutral pH into the extracellular environment. Such recycling results in long circulating half-lives and high serum levels of IgG antibodies. IgG that does not bind FcRn in this manner is subsequently degraded in lysosomal compartments.
The mice used in our studies lack the endogenous FcRn α chain and are transgenic for the human FcRn α chain. The human transgene is under the control of either the native huFcRn α gene promoter (Tg32 mice) or a chicken β–actin gene promoter (Tg276 mice).Citation4,Citation5 The FcRn receptor in such mice has human α chain associated with endogenous mouse β2m chain, an apparent fully-functional 2-species hybrid receptor. The FcRn α chain depends on co-expressed β2m for its appropriate expression and localization. Consequently, both FcRn α chain knockout mice and β2m knockout mice are deficient in FcRn. A potentially important difference in the two knockout types is that β2m knockouts are also deficient in MHC class I molecules and several other FcRn homologs that also depend on β2m for their expression. In these huFcRn mice, endogenous murine IgG bind with very low affinity to huFcRn, and as a result serum levels of murine IgG are lower than in normal mice.Citation9
We began our studies by evaluating the effects of four variables that could theoretically affect the PK of IgG antibodies and used results of those evaluations to settle on a potential huFcRn transgenic model. The considerations included the following: (1) the strain of FcRn transgenic mice; (2) FcRn transgene dosage (homozygous vs. hemizygous mice); (3) whether to include human IVIG or human serum albumin in the mouse model, (4) and mouse breeding vigor. After assessing these characteristics, a mouse model was then tested by performing PK evaluations with a panel of eight monoclonal IgG1 antibodies selected based on (a) availability of the antibody, (b) molecules were human IgG1 isotype, (c) lack of a significant influence on PK by target binding (in either primates or mice) and (d) availability of primate PK data. The mouse PK data was compared with existing monkey and human PK data for those same antibodies and statistical analyses were used to test for correlations.
Results
The availability of huFcRn-transgenic mice and the demonstration of the functionality of the human receptor in those mice made them an attractive starting place for our search for a PK model with predictive capabilities,Citation4 but preliminary questions included: (a) which of two available transgenic mouse lines should be used?; (b) should homozygous (homo) or hemizygous (hemi) mice be used?; (c) would coinjection of high-dose IVIG to better mimic competitive binding conditions in humans offer an advantage?; and (d) would coinjection of high-dose HSA to better mimic conditions in humans offer an advantage?
Comparison of two human FcRn transgenic mouse lines
Expression of the human FcRn α transgene in the Tg32 mice is driven by its native human gene promoter, whereas its expression in the Tg276 mice is driven by the chicken β-actin promoter.Citation4,Citation5 To gauge whether one mouse strain might offer advantages over the other, PK evaluations in Tg32 homo and Tg276 homo mice were done with a human IgG1 monoclonal antibody, B21M WT, that has native (wild-type) Fc sequences and a variant, B21M-Fc2, that shows enhanced affinity for FcRn at pH 6 due to a single, proprietary Fc mutation. In both of the two studies that were performed, B21M WT showed similar half-life values in Tg32 homo and Tg276 homo mice (for example, 9.3 ± 2.5d in Tg32 homos and 10.3 ± 1.9d in Tg276 homos for the study shown in ; ± represents the SEM). Likewise, B21M-Fc2, while showing a 2.7-fold longer half-life than B21M WT, had very similar half-lives in the two strains of transgenic mice (t1/2 values of 24.9 ± 5.2d and 27.8 ± 7.1d, respectively, ). These data did not suggest a reason for favoring one transgenic line over the other.
Figure 1. Comparison of human FcRn transgenic mouse lines. Tg32 and Tg276 mice were IV-injected via tail vein with 2 mg/kg doses of either (A) B21M WT or (B) B21M-Fc2, a variant of B21M WT with enhanced affinity for FcRn at pH 6. Serum concentrations are shown for the 21 d study. (C) PK profile of B21M WT in Tg32 homo vs. Tg32 hemi mice. (D) Results of measuring endogenous mouse IgG levels by ELISA in sera of different mice that had not been injected with human IgG. (E) PK profiles in Tg32 mice IV-dosed at 2 mg/kg with B21M WT in the presence or absence of a coinjected 200 mg/kg dose of human IVIG. For Tg32 homo mice, t1/2 values of the group without IVIG were derived from 2 mice, whereas values from the group with IVIG was derived from 4 mice. (F) Results of measuring endogenous mouse serum albumin levels by ELISA in sera of different mice that had not been injected with human IgG. In (A), (B), (C) and (E), B21M serum levels were measured at different time points by ELISA, and results are shown as the mean ± SEM of 4 animals/group unless stated otherwise. Half-life values are reported with ± 1 standard deviation in parentheses, except for Tg32 mice in (E) which are reported as mean values.
Effect of homozygous vs. hemizygous transgenic mice
The mice may be either homozygous or hemizygous for the transgene – the latter being obtained by crossing homozygous mice with FcRn knockout mice and collecting the offspring. To determine whether transgene zygosity would affect the half-life of an IgG1 antibody, a 2 mg/kg dose of B21M WT was IV-injected into either Tg32 homo or Tg32 hemi mice. Analyses of the PK data revealed that the t1/2 values for B21M WT were similar in Tg32 homo and hemi mice (9.3 ± 2.5d and 10.1 ± 1.0d, respectively, ). When B21M WT was given IV at the same dose to Tg276 homo or hemi mice, the results were different, with the homo mice appearing to show a longer half-life than the hemi mice (8.3 ± 0.9d vs. 6.8 ± 1.4d, respectively, data not shown). The results suggest that, under our experimental conditions, transgene zygosity had no discernible effect on antibody half-life in Tg32 mice, but did seem to have a measurable effect in Tg276 mice.
Effect of IVIG in transgenic mice
Human monoclonal antibodies injected into the huFcRn-transgenic mice may get little competition for FcRn binding from endogenous mouse IgG because mouse IgG has much lower affinity than human IgG for human FcRn. Therefore, low levels of endogenous IgG are predicted in these mice.Citation4,Citation8,Citation9 To confirm that the untreated transgenic mice we used had low levels of endogenous IgG compared with normal mice, IgG serum levels were measured with a mouse IgG-specific ELISA. The results, depicted in , revealed levels of 1.17 ± 0.07 mg/ml for normal C57BL/6, 0.54 ± 0.18 mg/ml for Tg32 homo, 0.23 ± 0.10 mg/ml for Tg32 hemi, 0.22 ± 0.05 mg/ml for Tg276 homo, and 0.12 ± 0.05 mg/ml for Tg276 hemi mice. In addition, serum levels were 0.09 ± 0.01 mg/ml for FcRn α chain knockouts and 0.03 ± 0.01 mg/ml for β2m knockouts. With most adult humans having a mean range of 6–12 mg/ml of circulating IgG,Citation10 the particularly low levels of the low-binding mouse IgG suggested that having high levels of polyclonal human IgG present to act as competitor may be important to attain a representative model. Therefore, the effects on antibody PK of cotreating with high-dose human IVIG was tested in these mice.
The amount of IVIG that could be given to mice was determined by dose-escalation and visual monitoring for signs of protein toxicity. We found that Tg32 mice dosed with up to 5000 mg/kg showed no adverse effects. From dose-escalating studies, a dose of 200 mg/kg IVIG was chosen for subsequent studies with corresponding IgG levels of approximately 8 mg/ml, which was within the normal range for humans. The effects of coinjecting 200 mg/kg IVIG with a 2 mg/kg dose of B21M WT was tested in these FcRn transgenic mice. Serum levels of B21M WT were measured using a pair of anti-Id antibodies specific for B21M even in the presence of a large excess of human IgG. High-dose IVIG had very little effect on serum levels of B21M WT for the 28 d study in Tg32 homo mice (t1/2 values of 10.4 ± 2.0d for WT and 11.1 ± 7.3d for WT + 200 mg/kg IVIG, ) and in Tg276 homo mice (data was similar, and not shown). Therefore, to create an even more competitive environment, Tg32 hemi mice expressing less FcRn were IV-injected with the same amounts of IVIG and B21M WT. This time B21M WT showed a significantly shorter half-life when coinjected with high-dose IVIG (t1/2 values of 9.5 ± 0.7d for WT and 3.4 ± 0.8d for WT + 200 mg/kg IVIG, ). Although we did not evaluate high-dose IVIG for PK in Tg276 hemi mice, we did observe that high-dose IVIG had little effect in Tg276 homo mice.
Effect of human serum albumin in transgenic mice
Albumin also owes its long circulating half-life and high serum levels to pH-dependent binding to FcRn, the same receptor that binds IgG. Although it has been reported that IgG and albumin bind to distinct sites on FcRn, and that IgG bound to FcRn does not affect binding of that same FcRn to albumin, definitive data on whether albumin affects IgG binding to FcRn may still be lacking.Citation11 The endogenous levels of serum albumin in untreated normal and FcRn-related mouse lines were measured by a mouse serum albumin ELISA kit. Albumin levels in Tg32 homo, Tg32 hemi, Tg276 homo and normal C57BL/6 mice were all similar, whereas albumin levels in FcRn α chain knockout were only 50% as high as levels in C57BL/6 mice, and levels in β2m knockout mice were only 40% as high as levels in C57Bl/6 mice (). These data demonstrate that the FcRn receptor in the Tg32 and Tg276 mice efficiently maintains mouse serum albumin levels.
The effect of HSA on human IgG levels was also examined. Mice were administered 50 mg/kg, 100 mg/kg and 200 mg/kg HSA along with 100 mg/kg human IVIG. Blood was collected from mice up to 14 d after injections, serum prepared, and the levels of human IgG and HSA determined by ELISA. Results showed that human IgG levels were essentially the same regardless of how much HSA was injected, implying that there would be little value in coinjecting HSA when studying the PK of human IgG in these mice (data not shown). Since mouse albumin levels were similar among the transgenic mice and normal C57BL/6 mice, and human IgG levels were not affected by increasing amounts of HSA, these results suggested that coinjecting human albumin would not offer an advantage.
Selection of the huFcRn-transgenic mouse model
The above observations, the fact that the FcRn transgene in Tg32 mice is driven by its native promoter rather than a β–actin promoter, and the fact that temporary breeding challenges limited the availability of Tg276 mice, all led toward a decision to focus on the Tg32 mice. Hemizygous mice, with reduced FcRn gene dosage, were preferred because it was believed that antibody Fc variants would show better differential clearance rates in hemizygous mice than in homozygous mice. Although inclusion of high-dose IVIG would more closely mimic conditions in human patients, the decision (based on data discussed below) was to not include IVIG because the rank order of half-lives observed across different antibodies did not appear to be obviously affected by the presence or absence of IVIG. Since there was little effect on human IgG levels with large amounts of HSA, coinjecting human albumin was not thought to be necessary. Therefore, the selected mouse model for our subsequent PK studies was Tg32 mice hemizygous for the FcRn transgene, without coinjected IVIG or HSA.
Evaluation of a panel of antibodies in Tg32 hemi mice
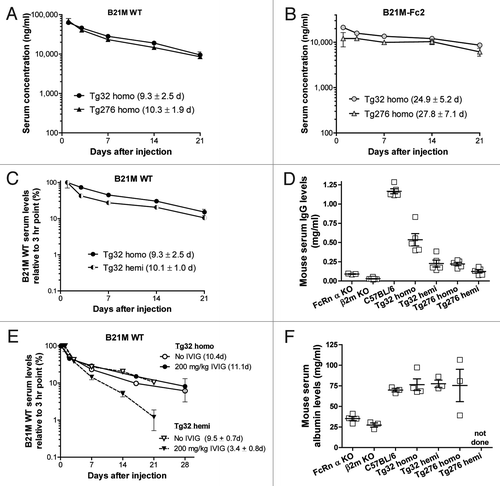
The above-described mouse model was used to evaluate a panel of unrelated human IgG1 clinical antibodies whose PK was not significantly affected by target binding, and for which monkey or human PK data was available. In the initial PK study, Tg32 hemi mice were injected IV (4 mice/group) with a 2 mg/kg dose of one of eight different clinical antibodies. Serial retro-orbital bleeds were obtained at 1, 3, 7 and 14 d after injection and a terminal bleed 21 d after injection. Sera were prepared from each sample and the amount of human IgG determined by ELISA. The serum IgG concentration vs. time profiles of the eight antibodies, expressed as percent of day 1 serum levels, showed that most curves displayed a linear decline over time on the semi-log plot shown in . Data points from mice with high immune serum titers, demonstrated by detection of mouse anti-human antibodies of greater than 1 to 1000 at 7 d after dosing and corresponding usually to abrupt changes in PK profile, were excluded from the analyses. As an example, mice dosed with CNTO 7 showed anti-human antibody titers from days 14–21 in all animals which would account for the noticeable drop in serum levels at day 21. Data from this study were compared with published human PK data and with Johnson and Johnson (J&J) clinical results (). A Pearson analysis revealed a significant correlation (p = 0.026) between the half-life values in humans and the half-life values in Tg32 mice.
Figure 2. Mouse PK and comparison to human PK data. (A) PK profile from an initial study using 8 clinical antibodies. Data are shown relative to day 1 serum levels and each point is the mean serum level ± SEM of 4 mice/group. (B) Comparison of half-life values from Tg32 hemi mice with half-life values from available human clinical data. Data points from mice with high immune response to the injected antibody, demonstrated by mouse anti-human antibody titers of greater than 1 to 1000 and corresponding to abrupt PK changes, were excluded in the analyses. The relationship between human and mice half-life values showed a significant correlation, Pearson r = 0.92, p = 0.026.
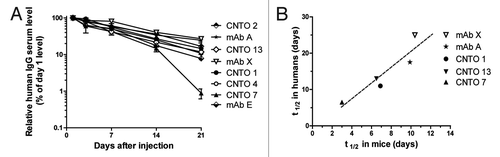
These same antibodies were tested in a PK study (4 mice/group) involving coinjection of high-dose IVIG. To measure human monoclonal antibody levels in the presence of human IVIG, the clinical antibodies were radiolabeled with 125I to a specific activity of approximately 10 µCi/µg. The final 0.2 mg/ml formulation contained a 1 to 40 ratio of 1251-labeled antibody to unlabeled antibody in order to limit the dose of radioactivity to less than 10 µCi per animal. Bleeds were collected 2, 4, 7 and 9 d post-injection. shows the PK profiles expressed as the percent of the serum level on day 2. Three animals were excluded (each from a different test group) because initial serum concentrations were about 3-fold lower than other animals in the group (suggesting those mice were not administered the intended dose). CNTO 4 was tested in the presence and absence of IVIG, and the calculated mean clearance (CL) values with or without IVIG were 58.9 ml/d and 42.3 ml/d, respectively. This 1.4-fold difference was consistent with early observations that coinjected IVIG could accelerate antibody clearance. shows the human vs. mouse half-life relationship using human PK data that were available and data from Tg32 hemi mice that were administered 1251-labeled antibodies. The correlation was significant, with p = 0.030.
Figure 3. Mouse PK in the presence of coinjected IVIG and comparison of observed half-life values to human data. (A) PK profiles are shown using 125I-labeled antibodies in Tg32 hemi mice. Serum concentration is reported as mean ± SEM of 4 mice/group, calculated from the cpms/ml and specific activity of the 125I-labeled test antibody. (B) Relationship between human half-life values that were available and mice half-life values showed a significant correlation, Pearson r = 0.86, p = 0.030.
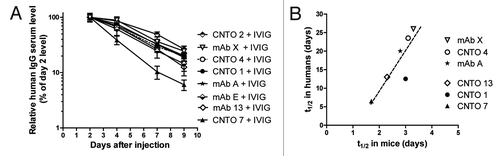
Results of these two studies revealed a correlation between antibody half-life in humans and antibody half-life in mice transgenic for huFcRn. Another study was done to retest for the correlation without IVIG, and to include two additional antibodies, CNTO 14 and CNTO 8 (with 4 mice/group). Results in show the PK profile expressed as percent initial serum level at day 2. Animals with immune titers over 1:1000 against the IV dosed antibody were omitted from the analyses. shows the half-life correlations between mouse PK and available human PK. The half-life correlation was significant, p = 0.027.
Figure 4. Mouse PK data in the absence of coinjected IVIG and comparison of observed half-life values to human data. (A) PK profiles in a study done in the absence of coinjected IVIG. Serum levels normalized to day 2 are reported as mean ± SEM of 4 mice/group. (B) The comparison of half-lives from available human data vs. half-lives in mice are shown. Outliers with immunogenicity, demonstrated by an abrupt drop in the PK profile, were excluded from both graphs. The correlation was significant: Pearson r = 0.81, p = 0.027.
Correlation of PK in mice vs. nonhuman primates
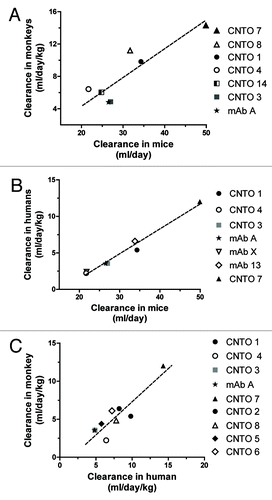
PK data analyses for the study shown in revealed an impressive correlation with monkey data that had been collected from past toxicology studies, either internal or published by others.Citation12,Citation13 The correlation was especially striking when clearance values from this study were averaged with clearance values from the study shown in , the other study in FcRn transgenic mice done in the absence of human IVIG. From a data set of 7 antibodies, a Pearson correlation test showed that the relationship between monkey clearance and mouse clearance values was significant (, p = 0.008).
Figure 5. Relationships between clearance values in monkeys, humans, and mice. (A) Correlation between monkey and mouse CL data are shown from the average values in the two studies that did not include coinjected IVIG, (Pearson r = 0.88, p = 0.008). (B) PK correlation between human and mouse data are shown from the average values of these studies (Pearson r = 0.99, p < 0.001). (C) Correlation between monkey and human data are shown from the average values collected from internal studies and the literature (Pearson r = 0.90, p = 0.001).
Correlation of PK in mice vs. humans
The averaged PK data from the studies described in and were also compared with human data from J&J clinical trials and from published data.Citation12-Citation14 For the 7 antibodies in which human PK data were available, the correlation test showed that the relationship between human clearance and mouse clearance was significant (, p < 0.001).
Correlation of PK in monkeys vs. humans
An additional comparison was made between monkey antibody PK values from internal or published data, and the data from J&J clinical trials.Citation12,Citation13 For 9 antibodies for which human PK data were available, the correlation tests showed that the relationship between monkey clearance and human clearance was significant (, p = 0.001). These results are consistent with the observation that there is an excellent correlation of human antibody PK between mice and non-human primates, and between mice and humans.
Discussion
Having set out to test whether a convenient in vivo model capable of predicting IgG antibody PK in monkeys and humans might be identified using mice, we report here a direct correlation between PK in FcRn-transgenic mice vs. monkeys and humans for a panel of antibodies whose target binding does not significantly affect their clearance. While not being certain whether use of mice transgenic for huFcRn would offer a greater chance of success than use of normal mice, we opted to focus on the transgenic mice for three reasons: a) observed antibody-to-antibody differences in affinity for huFcRn may not correspond to any differences in affinity for muFcRn; b) we were simultaneously studying the PK of human Fc-mutant antibodies (for which binding to huFcRn is markedly different than binding to muFcRn) in the FcRn-transgenic mice, and so there was convenience in using the same mice; and c) a humanized mouse model had greater appeal.
We began by considering the possible influence of several variables: namely, two distinct transgenic lines differing in the tissue and cellular expression patterns of the huFcRn; FcRn expression level as dictated by transgene dosage; the cotreatment of mice with high-dose human IVIG, and the cotreatment of mice with high-dose HSA. The results of those evaluations prompted us to choose the use of hemizygous Tg32 mice without high-dose IVIG and HSA for the subsequent PK evaluations of a panel of clinical IgG antibodies. Our measurements of relative endogenous IgG and HSA in FcRn-transgenic mice were consistent with values reported recently by Stein et al.Citation15 It is not known why we did not see a clear difference between PK in Tg32 mice vs. Tg276 mice, as was observed by Petkova et al.Citation4 One difference was that the comparison we described was made using mice homozygous for the FcRn α chain transgene, whereas the only Tg32 data reported by Petkova et al. was from Tg32 hemi mice, for comparison to their Tg276 hemi mice data. Because expression of functional FcRn heterodimer in these mice is dependent on a limited amount of endogenous β2m subunit, it is possible that, while Tg32 hemi mice may express significantly more functional FcRn than Tg276 hemi mice, the Tg32 homo mice that we used may not express much more FcRn than Tg276 homo mice due to β2m expression levels being a limiting factor in Tg32 homo mice. It also seems likely that differences between antibodies contribute to the observed discrepancies in the data, potentially related to the wide-ranging affinities that different human IgG1 antibodies with native Fc sequences have for recombinant FcRn, and potentially related to differences in antibody half-lives. Our test antibody showed a terminal half-life of 10.3 ± 1.9d in Tg276 homo mice whereas Petkova reported the Hu4D5-IgG1 antibody showed a half-life of only 5.79 ± 0.34d in Tg276 homo mice. The PK of their shorter half-life antibody may be more enhanced in going from Tg276 hemi to Tg32 hemi mice (due to greater FcRn expression in Tg32 hemi mice), analogous to the dramatic gain in half-life that they observed in going from Tg276 hemi (1.7d) to Tg276 homo mice (5.8d).Citation4
The decision to not coinject IVIG in the chosen model, while being convenient in that it meant not having to contend with potential safety risks from the human blood-derived product, was not anticipated at the start of the studies. While having high serum levels of IVIG clearly reduced the half-life of B21M WT, as would be expected, the correlations with primate data were just as good whether IVIG was used or not, implying that differences in PK among the test antibodies were not dependent on establishing a greater degree of competition from endogenous IgG. The extent of the effects of IVIG, however, did appear to depend on the affinity of an antibody for FcRn (unpublished data) because the IVIG dose that we used had little or no effect on half-life of a B21M-Fc2 variant that had enhanced affinity for FcRn, but had a dramatic effect on another B21M variant that had very weak affinity for FcRn.Citation16 The decision to not coinject HSA was anticipated at the start of the studies, and was consistent with reports that albumin and IgG bound to different sites on FcRn,Citation17 but we did not want to rule out the possibility that the presence of high levels of HSA in vivo might influence the PK of the antibodies. From these studies and many others not described here, Tg32 hemi mice not treated with high-dose IVIG or albumin appeared to offer potential as a predictive rodent model for human PK. The work described follows up on the hypothesis.
The antibodies included in the PK studies represented an assortment of IgG1 antibodies that have shown a range of half-lives in humans, making it easier to test for correlations with mouse PK data. They were the only antibodies that met all criteria for inclusion in our analyses, i.e., IgG1 isotype, antibody availability, primate PK data availability, and PK not being significantly influenced by target binding in either primates or mice (e.g., due to antibody internalization following binding to cell-surface antigen). Except for two of the antibodies having the E356M358 (EU numbering) allotypic sequence instead of the D356L358 sequence that was present in the other seven, their Fc sequences were identical. For all PK studies, serum samples were tested for mouse anti-human IgG immune titers to avoid skewing PK data due to test article immunogenicity.
Statistical correlations were compared on the PK data obtained from our studies with the PK data obtained with internal and published data. Data in our three studies included only animals with little to no immune titers to avoid complications of potential rapid antibody-complex clearance. Half-life and CL values were calculated and compared in a correlation test with values that were known or published for the antibodies. In cases where a range was reported, the midpoint value was used in the calculations. Our data revealed important PK trends using FcRn-transgenic Tg32 mice, monkeys and humans. Notable observations were: (1) the correlation between human antibody PK in Tg32 mice and monkeys was significant (p < 0.008); (2) the correlation between Tg32 mice and humans was significant (p ≤ 0.001); (3) the correlation between humans and monkeys was significant (p = 0.001).
Overall results reveal the potential value of this mouse model and suggest that additional studies are warranted with more antibodies, including those of other IgG isotypes. Although we might be far away from using mice instead of non-human primates for preclinical drug testing, these results would undoubtedly be useful along with interspecies scaling analyses for PK drug modeling.Citation12,Citation18 Other model improvements may be possible, e.g., by having the mice transgenic for human IgG, or transgenic for human β2m subunit in addition to FcRn α. As more human antibodies and primate PK data become available, it should be possible to more rigorously test this mouse model for its predictive capability. Meanwhile, Tg32 mice hemizygous for the FcRn transgene may well serve as a convenient, cost-effective surrogate for in vivo prediction of human IgG antibody half-life and clearance in primates.
Materials and Methods
Animals
Transgenic animals used in these studies are derived from C57BL/6 mice. Tg32 (B6.Cg-FcgrttmlDcrTg(FCGRT)32Dcr) and Tg276 (B6.Cg-Fcgrttm1DcrTg(FCGRT)276Dcr) licensed from The Jackson Laboratory (Bar Harbor) have their endogenous mouse FcRn α gene knocked out and are transgenic with the human FcRn α gene under the control of the native human gene promoterCitation5 or the chicken β–actin promoter.Citation4 Both human FcRn transgenic strains show clinical chemical parameters similar to those found in wild-type mice with the exception of endogenous IgG levels, which are greatly reduced in these mice.Citation15 Tg32 hemi and Tg276 hemi refer to mice hemizygous for the FcRn transgene, the latter derived by mating homozygous transgenic mice with FcRn α knockout mice (transgene copy number reduced by half). β2m knockout mice, referred to as FcRn knockout mice (B6.129P2-B2mtm1Unc) were obtained from The Jackson Laboratory. All mouse breeding was done at Sigma-Aldrich Ace Animals. For the studies, 6–8 weeks old mice were used.
Antibodies
B21M is a human IgG1,κ monoclonal antibody specific for F glycoprotein of respiratory syncytial virus (RSV). The designation B21M WT refers to wild-type B21M to distinguish it from sequence variants. B21M-Fc2 is an Fc variant of B21M WT containing a single, proprietary amino acid substitution that results in greater affinity for FcRn at pH 6. Human intravenous immunoglobulin, IVIG, was purchased from OctaPharma Pharmazeutika. Clinical human IgG1 antibodies were purchased commercially and coded with a mAb number. J&J antibodies were coded with a CNTO number.
Preparation of test articles
Test antibodies were formulated in phosphate-buffered saline and filtered through 2 micron sterile filters. In preparations containing IVIG, each test antibody was prepared as a 2x solution and combined equally with 2x IVIG for the final formulation.
For one study, test articles were radiolabeled with 125I (Perkin-Elmer) using the method described in the package insert for pre-coated iodination tubes (Thermo Scientific). Labeled antibody showed specific activities of 9–12 µCi/µg and demonstrated functional binding to recombinant huFcRn (expressed at Janssen Research and Development). 125I-labeled antibodies were formulated in PBS containing unlabeled antibody such that the final specific activity of the test article was approximately 50 µCi/ml and 3 x 105 cpm/µg. A typical 0.18 ml administered dose would contain less than 10 µCi per animal.
Mouse PK studies
For most antibody PK studies, female mice between 6 to 8 week-old, were injected with test antibody intravenously via tail vein at a dose of 2 mg/kg into 4 animals per group. Serial retro-orbital bleeds were obtained from CO2-anesthesized mice at indicated time points and terminal bleed was taken cardiac puncture. After 30 min at room temperature, blood samples were centrifuged at 2500 rpm for 15 min and serum collected for analyses. All PK studies were approved by the Institutional Animal Care and Use Committee at Janssen Research and Development, LLC.
Mouse serum analyses
Concentrations of human IgG in the serum samples were determined by an enzyme-linked immunoassay (ELISA) using human IgG-specific binding reagents (Jackson ImmunoResearch). Maxisorp 96-well plates (Nalge Nunc) were coated with polyclonal goat anti-human F(ab’)2 IgG at 10 µg/ml in PBS at 4°C overnight. Plates were washed with wash buffer (phosphate buffered saline containing 0.05% Tween 20, PBST) and blocked with Superblock (Thermo Scientific). Standards and samples were diluted in sample buffer (PBST with 1% bovine serum albumin) and added to plates. After a 2 h incubation, plates were washed and bound antibody was detected with horseradish peroxidase-conjugated goat anti-human IgG at 1:15:000 dilution in sample buffer. After a final 1 h incubation, plates were washed and developed using TMB (Fitzgerald Industries) or SigmaFAST OPD (Sigma-Aldrich), and color development was stopped by addition of 3N HCl. Absorbance at 450 or 492 nm was measured on the SpectraMax Plus 384 microplate reader (Molecular Devices). Concentrations of test samples (serially diluted 6–8 times for a mean value) were determined from a standard curve using a 4-parameter nonlinear regression program in Softmax Pro software (Molecular Devices).
To determine whether the PK serum samples had notable immune titers that could affect the PK of test samples, an ELISA was performed on maxisorb plates coated with the respective test article at 10 µg/ml. Serum samples were diluted in 1% BSA-PBS and incubated on the plates. Horseradish peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch) was used to detect captured antibody; followed by OPD or TMB addition for substrate development. Plates were read and spectrophotometer readings that were three times greater than buffer or control sera values were considered positive. Immune titers were expressed as 1/serum dilution.
For detection of B21M antibodies in the presence of IVIG, serum samples were analyzed using a dual anti-id ELISA. Mouse serum samples and appropriate reference standards were incubated on plates coated with an anti-id antibody at 2 µg/ml in PBS overnight at 4°C, washed and blocked with Superblock. Serum samples were diluted in sample buffer, 1% BSA-PBS, and incubated on plates for 2 h at room temperature and washed. Bound B21M antibody was detected using a different anti-id antibody that was biotinylated at 2 µg/ml for 1 h followed by incubation with horseradish peroxidase-conjugated streptavidin (1:20,000; Jackson ImmunoResearch) for 1 h. Plates were developed with OPD or TMB substrate, reactions were stopped by addition of 3N HCl, and plates read in the plate reader. Serum B21M antibody concentrations were determined with a standard curve as described.
Endogenous mouse IgG was measured with a standard mouse IgG ELISA. Maxisorb plates were coated with goat F(ab’)2 anti-mouse IgG at 10 µg/ml. Serum samples diluted in 1% BSA-PBS were incubated on plates for 2 h, and bound antibody was detected with a 1 h incubation using horseradish peroxidase-conjugated donkey anti-mouse IgG. OPD substrate was added to washed plates and the reaction stopped with 3N HCl. Plates were read in the plate reader and concentrations were determined by comparison with a standard curve using purified murine IgG from C57Bl/6 mice.
Human serum albumin levels were detected using ELISA reagents from Cygnus Technologies. Endogenous mouse serum albumin levels were determined using a mouse serum albumin ELISA kit from Genway.
In certain assays, an electrochemiluminescent immunoassay was used to measure antibody concentration in mouse sera. Briefly, Streptavidin Gold multiarray 96-well plates (Meso Scale Discovery) were coated with 50 µl/well of 1 µg/ml biotinylated F(ab')2 goat anti- human IgG (H+L, Jackson Immunochemical) in Starting Block T20 (Thermo Scientific) overnight at 4°C and washed with Tris-buffered saline with 0.05% Tween (TBST). Standards and serum samples were prepared in sample buffer, 1% BSA in TBST and 20 mM EDTA, added to plates and incubated for 1 h at room temperature on a shaker. Plates were washed and incubated for 1 h with 1 µg/ml MSD-TAG (ruthenium)-labeled anti-human IgG mAb, R10Z8E9.Citation19 Plates were washed, Read buffer T (Meso Scale Discovery) was added and plates were read on the MSD Sector Imager 6000.
Data analyses
Pharmacokinetics
Terminal half-life (t1/2) calculations of the elimination phase (β phase) for PK studies were determined using the 1-phase exponential decay model fitted by linear regression of natural log concentration vs. time using Prism version 5.01 software (GraphPad Software, Inc.). Two-phase models were ruled out because, for each test article, the best-fit model was a 1-phase exponential decay model as determined by nonsignificance of the extra sum of squares F test (p > 0.05) for the majority of animals. The least squares nonlinear decay model was weighted by 1/fitted concentration. Half-life calculations of the elimination phase (β phase) were determined using the formula t1/2 = ln2/β, where β is the –slope of the line fitted by the least square regression analysis starting after first dose.
In the three PK studies described here, the terminal half life value for an antibody was determined by taking the average of the t1/2 values calculated for each animal within the test group. In PK studies reported from elsewhere, the mid-range of the terminal half-life values was used. For example, if the reported range is t1/2 = 10–16 d, then 13 d was the value used in our analyses.
Area under the curve, AUC(0-infinity) was calculated based on linear regression analyses (with slope = β, and Y-intercept = α): AUC(0-infinity) (µg · day/ml) = exp(α)/β . Clearance (CL) was calculated with the formula: CL (ml/day) = IV dose (µg)/ AUC(0-infinity) (µg · day/ml) (or CL expressed as ml/day/kg if weight-adjusted).
Pearson Correlation coefficients r values were significant when p < 0.05. All statistical analyses were performed using the Prism Software version 5.01.
Outliers in our studies were identified as animals either showing a mouse anti-human IgG titer greater than a 1 to 1000 about 7 d after dosing (and corresponding to an abrupt drop in the serum levels) or an initial serum value that was more than 2-fold lower than values for other mice in the group, perhaps due to not being fully dosed.
Radioactivity calculations
125I-labeled IgG concentrations were determined from the specific activity of serum samples. Radioactivity of each serum sample (cpms/ml) and the corresponding 125I-labeled test article (cpms/ml in the 0.2 mg/ml test article) were measured in the gamma counter on the same day. Concentration was determined as follows: 125I-labeled IgG concentration (µg/ml) = sample (cpm/ml)/specific activity(cpm/µg).
| Abbreviations: | ||
| mAb | = | monoclonal antibody |
| CL | = | clearance |
| FcRn | = | neonatal Fc receptor |
| hemi | = | hemizygous |
| homo | = | homozygous |
| HSA | = | human serum albumin |
| Id | = | idiotype |
| IgG | = | immunoglobulin G |
| IV | = | intravenous |
| IVIG | = | intravenous immunoglobulin |
| RSV | = | respiratory syncytial virus |
| p | = | probability-value |
| PK | = | pharmacokinetics |
| SEM | = | standard error of the mean |
| t1/2 | = | terminal half-life |
Acknowledgments
Janssen Research and Development, LLC provided funding for all research. All authors are employees of Janssen Research and Development, LLC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol 2006; 176:346 - 56; PMID: 16365427
- Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Jiang W, Wroblewski VJ. Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: relationship to pharmacokinetics in mice and primates. Drug Metab Dispos 2007; 35:86 - 94; http://dx.doi.org/10.1124/dmd.106.011734; PMID: 17050651
- Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos 2011; 39:1469 - 77; http://dx.doi.org/10.1124/dmd.111.039453; PMID: 21610128
- Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol 2006; 18:1759 - 69; http://dx.doi.org/10.1093/intimm/dxl110; PMID: 17077181
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol 2003; 170:3528 - 33; PMID: 12646614
- Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 2003; 197:315 - 22; http://dx.doi.org/10.1084/jem.20021829; PMID: 12566415
- Ghetie V, Ward ES. FcRn: the MHC class I-related receptor that is more than an IgG transporter. Immunol Today 1997; 18:592 - 8; http://dx.doi.org/10.1016/S0167-5699(97)01172-9; PMID: 9425738
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715 - 25; http://dx.doi.org/10.1038/nri2155; PMID: 17703228
- Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol 2001; 13:1551 - 9; http://dx.doi.org/10.1093/intimm/13.12.1551; PMID: 11717196
- Stoop JW, Zegers BJM, Sander PC, Ballieux RE. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol 1969; 4:101 - 12; PMID: 4182354
- Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry 2006; 45:4983 - 90; http://dx.doi.org/10.1021/bi052628y; PMID: 16605266
- Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol 2009; 49:1382 - 402; http://dx.doi.org/10.1177/0091270009337134; PMID: 19837907
- Keizer RJ, Huitema ADR, Schellens JHM, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010; 49:493 - 507; http://dx.doi.org/10.2165/11531280-000000000-00000; PMID: 20608753
- Wolfe EJ, Cavacini LA, Samore MH, Posner MR, Kozial C, Spino C, et al. Pharmacokinetics of F105, a human monoclonal antibody, in persons infected with human immunodeficiency virus type 1. Clin Pharmacol Ther 1996; 59:662 - 7; http://dx.doi.org/10.1016/S0009-9236(96)90006-5; PMID: 8681491
- Stein C, Kling L, Proetzel G, Roopenian DC, de Angelis MH, Wolf E, et al. Clinical chemistry of human FcRn transgenic mice. Mamm Genome 2012; 23:259 - 69; http://dx.doi.org/10.1007/s00335-011-9379-6; PMID: 22193411
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fc γ RI, Fc γ RII, Fc γ RIII, and FcRn and design of IgG1 variants with improved binding to the Fc γ R. J Biol Chem 2001; 276:6591 - 604; http://dx.doi.org/10.1074/jbc.M009483200; PMID: 11096108
- Andersen JT, Sandlie I. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab Pharmacokinet 2009; 24:318 - 32; http://dx.doi.org/10.2133/dmpk.24.318; PMID: 19745559
- Han C, Zhou H. Monoclonal antibodies: interspecies scaling with minimal preclinical information. Ther Deliv 2011; 2:359 - 68; http://dx.doi.org/10.4155/tde.11.1; PMID: 22834006
- Stubenrauch K, Wessels U, Lenz H. Evaluation of an immunoassay for human-specific quantitation of therapeutic antibodies in serum samples from non-human primates. J Pharm Biomed Anal 2009; 49:1003 - 8; http://dx.doi.org/10.1016/j.jpba.2009.01.030; PMID: 19250787