Abstract
Antibody-drug conjugates (ADCs), formed through the chemical linkage of a potent small molecule cytotoxin (drug) to a monoclonal antibody, have more complex and heterogeneous structures than the corresponding antibodies. This review describes the analytical methods that have been used in their physicochemical characterization. The selection of the most appropriate methods for a specific ADC is heavily dependent on the properties of the linker, the drug, and the choice of attachment sites (lysines, inter-chain cysteines, Fc glycans). Improvements in analytical techniques such as protein mass spectrometry and capillary electrophoresis have significantly increased the quality of information that can be obtained for use in product and process characterization, and for routine lot release and stability testing.
Introduction
Antibody-drug conjugates (ADCs) or immunoconjugates, are becoming an increasingly important class of therapeutic agents undergoing clinical investigations for treatment of cancer.Citation1 ADC products in late stage clinical development include brentuximab vedotin (SGN-35; Seattle Genetics) for treatment of CD30-positive malignancies such as Hodgkin's lymphoma, inotuzumab ozogamicin (CMC-544; Pfizer) for CD22-positive B cell malignancies such as non-Hodgkin lymphoma, and trastuzumab emtansine (T-DM1; Genentech/Roche/ImmunoGen) for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer.Citation1–Citation5
ADCs as a class harness the exquisite selectivity of monoclonal antibodies (mAbs) to achieve targeted delivery of cytotoxic drugs.Citation6–Citation8 As a result of this targeted delivery, ADCs selectively eliminate tumor cells that overexpress the target antigen while limiting drug toxicity to normal, healthy tissues.Citation6,Citation9–Citation11 Critical to the clinical efficacy of an ADC are the target site-specificity and binding properties of the antibody, the in vitro and in vivo stability of the linker and drug species, the potency of the drug, and both the distribution and average number of drug species on the antibody.Citation6 These requirements highlight the importance of understanding the physicochemical properties of ADCs and choosing the appropriate analytical and bioanalytical techniques to assess and monitor them during manufacturing and subsequent storage.
ADCs are constructed from three components: a mAb that is specific to a tumor antigen, a highly potent cytotoxic agent and a linker species that enables covalent attachment of the cytotoxin to the mAb through either the protein or the glycan. The primary sites used for protein-directed conjugation are the amino groups of lysine residues or the sulfhydryl groups of the inter-chain cysteine residues. Conjugation typically starts with functionalizing the mAb through either attachment of a bifunctional linker, reduction of inter-chain disulfides or oxidation (for carbohydrate conjugation), followed by reaction with the cytotoxic drug (such as the thiol-containing DM1), or with a preformed drug-linker species (such as maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl-MMAE, “vc-MMAE”). The conjugation technology, regardless of the site and process used for linkage, results in an ADC molecule that is heterogeneous with respect to both the distribution and loading of cytotoxic drug species on the mAb.Citation1 This heterogeneity is challenging both from a process control and an analytical development perspective. Recent efforts to minimize this heterogeneity have included both process development strategiesCitation12 and the use of protein engineering. To this end, inter-chain cysteines have been selectively replaced with serine residues,Citation13 and cysteines have been introduced at sites that were optimized for both drug conjugation with well-defined stoichiometry and their having minimal disruption to the mAb structure and epitope binding.Citation14
Examples of cytotoxic drugs that have been conjugated to mAbs are shown in .Citation6 These include molecules that bind DNA (e.g., doxorubicin), alkylate DNA (e.g., calicheamicin, duocarmycin) or inhibit tubulin polymerization (e.g., maytansinoids, auristatins). The ADCs farthest along in clinical development contain bound maytansines, auristatins and calicheamicins,Citation1,Citation6 although other drugs are being evaluated both pre-clinically and clinically. For any given ADC, the chemical properties of the cytotoxin and linker, combined with selection of linkage site (the ADC “architecture”), will dramatically affect the physicochemical attributes, and the selection of analytical methods to assess these attributes will depend on this architecture. Assays used for the parent mAb may not work for its corresponding ADC or assays used for one type of ADC, may not be applicable to an ADC with a different architecture. Depending on the ADC, the same assay method (e.g., a charge-based assay or one that assesses ADC structure under denaturing conditions) may provide different information. This review summarizes the published approaches and methods that have been used for analytical characterization of ADCs. In some cases, these methods may also be used for routine lot-release and stability testing of a product for use in the clinic. Biophysical characterization tools for monitoring higher order structures of ADCs and the challenges associated with such efforts are also discussed. Although bioanalytical methods, including ELISAs to assess antigen binding and cell-based assays to demonstrate target-dependent cytotoxicity, are used to determine potency and are critical to the characterization and quality control of ADCs, they are out of the scope of this review. Many of the references cited below also include descriptions of these methods and a recently submitted review focuses more specifically on these techniques (Stephan JP, et al. In press, Bioanalysis).
Drug to Antibody Ratio (DAR)
One of the most important quality attributes of an ADC is the average number of drugs that are conjugated because this determines the amount of “payload” that can be delivered to the tumor cell and can directly affect both safety and efficacy. A variety of methods have been used to measure this attribute, depending on the properties of the drug and how it is linked to the protein (i.e., site of conjugation and structure of the linker).
The simplest technique relies on a UV/VIS spectroscopic analysis of the ADC. This method requires that the UV/VIS spectra of the drug and of the antibody have different Amax values. Using the measured absorbances of the ADC and the extinction coefficients of the mAb at its Amax of ∼280 nm and the drug at its Amax, the individual concentrations of mAb and drug can be determined by the solution of two simultaneous equations. From this, the molar ratio (moles of drug per mole of antibody) can be calculated. This technique has been used widely, even for ADCs with a relatively small difference in Amax between protein and drug, such as with the conjugated vinca alkaloid, DAVLB (4-desacetylvinblastine) where the Amax of the drug is 270 nm.Citation15 Other conjugated drugs for which this technique has been applied include the maytansinoid DM1,Citation16 methotrexate,Citation17 CC-1065 analogues,Citation18 adriamycin,Citation19 doxorubicin (DOX),Citation20 calicheamicin analoguesCitation21,Citation22 and dipeptide-linked auristatins such as maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl-MMAE (“vc-MMAE”)Citation23 (). In all cases, contribution of the drug or drug-linker to the measured absorbance at 280 nm must be incorporated into the calculation of the protein concentration, as must any contribution of the protein to the absorbance at the drug Amax.Citation15,Citation19,Citation23,Citation24 The application of orthogonal methods to verify the validity of the spectroscopic technique have also been described, including use of radiometric methods (conjugation with radiolabeled drugsCitation21) and chromatographic methods such as hydrophobic interaction chromatography (HIC) separation for quantification of individual drug loaded species as described by Hamblett et al.Citation23 for a conjugate prepared by linkage through the inter-chain disulfide residues (discussed below). Comparisons between UV/VIS and UV-MALDI mass spectrometric methods are also described for a number of drug and chelator conjugates,Citation25 and these include explanations for the differences that are sometimes observed, such as UV photolability of calicheamicin, linker lability and over-quantitation of the UV-DAR method due to high levels of non-covalently bound drug. For lysine-linked conjugates produced using the C225 anti-EGFR antibody (cetuximab) and succinate or glutarate derivatized paclitaxel, MALDI-TOF MS is the only reported method for determining drug loading.Citation26,Citation27
Depending on the chemistry used to attach the drug to the antibody, alternative methods for determining the DAR may be available. Reduction of inter-chain disulfides to produce free sulfhydryl groups allows conjugation at specific residues using maleimide-containing linkers and generates conjugates with a mixture of zero, two, four, six and eight drugs per antibody at a limited number of defined positions.Citation20,Citation28 As a result of the significantly reduced heterogeneity of this linkage chemistry (relative to lysine-linked conjugates), the mixture has proven to be amenable to analysis using hydrophobic interaction chromatography (HIC) as shown in . HIC analysis allows both the characterization of the distribution of drug-linked species and the determination of the average DAR after integration of the observed peaks.Citation23 HIC is performed under non-denaturing conditions at neutral pH with a gradient from high salt to low salt and often includes a low concentration of an organic modifier in the low-salt mobile phase to improve the elution of the mAb loaded with hydrophobic drugs. As a result, even though some of the inter-chain disulfides are disrupted due to the conjugation reaction (while some remain intact), the combination of covalent binding and strong non-covalent forces between the chains are sufficient to keep the mAb intact during analysis. Therefore, each peak observed in corresponds to an intact mAb species with an increasing number of bound drugs. This method has been used to determine the DAR of conjugates containing auristatin drugs linked to inter-chainCitation23 and engineered cysteinesCitation14 via cleavable (peptide) and non-cleavable linkers.Citation29
Drug Distribution
In addition to information about the average DAR, multiple methods have been used to analyze the distribution of drug-linked forms (e.g., fraction of antibodies containing zero, one, two,…, n drugs). This is an important ADC characteristic because different forms may have different pharmacokinetic and toxicological properties.Citation23 Conjugates produced using linkage through surface accessible lysine residues are not easily separated by chromatography due to their high degree of heterogeneity. A number of reports have described the use of mass spectrometry to characterize their drug distribution.
MS analysis of distribution.
In the early 1990s, one of the first reports of mass spectroscopic characterization of ADCs described use of a UV MALDI-TOF instrument.Citation25 Mass spectra of intact mAbs conjugated through lysine residues or through antibody carbohydrates with chelating agents (DTPA, macro-cycle 12N4) or with drugs (calicheamicin, methotrexate, mitoxantrone) were compared with the corresponding unconjugated mAbs. Although the MALDI-TOF method affords relatively poor mass accuracy for large molecules, and its limited resolution does not provide resolution of species with different drug loads, the observed mass shifts of the peak centroids were used to calculate the average drug loads, and the peak shapes were used to mathematically model the distribution. For the reduced lysine-linked conjugates, mass shifts were observed between the unconjugated mAbs and their corresponding ADC light chain and heavy chain, indicating that both chains were modified. Lysine-linked conjugates prepared using activated paclitaxel were analyzed using UV MALDI-TOF MS and this method was also used to determine the average DAR.Citation27 Because UV irradiation leads to degradation of calicheamicin and its derivatives, IR MALDI and ESI-MS were evaluated by Siegel, et al.Citation30 for the analysis of calicheamicin conjugates. IR MALDI in a glycerol matrix, which provides improved resolution for masses above 50 kDa, gave reasonable agreement with UV determinations of average DAR for conjugates with a stable linker. Hydrolytically labile linkers such as those containing a hydrazone were affected by the acidic matrices that are frequently used for MALDI analysis and the acidic LC conditions used for ESI-MS, resulting in calculated average DAR values that were significantly lower than the values by UV due to linker instability.
More recent publications, such as those describing analyses of T-DM1 (trastuzumab-MCC-DM1) and thio-trastuzumab-DM1,Citation31 huN901-SPP-DM1,Citation32 and C242-DM4,Citation33 have used LC-MS with electrospray ionization coupled to time-of-flight (TOF) or triple quadrupole mass detectors. These techniques give much higher mass accuracy and resolution than can be obtained using MALDI. Lazar et al. coupled SEC chromatography with ESI-MS to remove buffer components from the sample and improve the spectroscopic performance.Citation33 shows an example of the deconvoluted mass spectrum of deglycosylated huC242-DM4 obtained using this method. Because the lysine-linked conjugates contain intact disulfides, the antibody chains do not dissociate in the presence of the highly acidic and high percentage organic solvent used to effect ionization as has been observed with the inter-chain cysteine-linked conjugates. Consequently, it is possible to obtain accurate masses for each drug form of the intact ADC. Integration of the deconvoluted mass spectrum can be used to estimate the DAR; however, this assumes equivalent recovery and ionization of all species, which may not always be the case due to the changes in charge and hydrophobicity associated with conjugations to positively charged amines. These factors can affect the accuracy of MS-derived estimations of DAR, therefore assumptions about equivalent ionization and recovery must be verified experimentally.Citation34 Comparisons between UV spectroscopy and ESI-TOF-MS demonstrated that the data are well correlated if the MS method is optimized to capture the signals from the full charge envelope of the ionized product.Citation33
Deglycosylated huN901-DM1, a conjugate where lysine residues were modified with DM1 via a reducible linker, SPP, was analyzed by ESI-TOF-MS both with and without DTT reduction.Citation32 The deconvoluted spectrum of the intact conjugate shows the distribution (from 0–6 drugs) with the expected mass difference (852 Da) between drug-containing forms. For the reduced conjugate, the two chains were separated by reverse-phase HPLC. The number of linkers attached to the light and heavy chains after reduction can be obtained directly from the deconvoluted MS spectra (the linker-drug disulfide bond is broken by DTT treatment). Three prominent peaks in the light-chain spectrum correspond to chains with zero, one and two linkers, while the four prominent peaks in the heavy chain spectrum are for species with zero, one, two and three attached linkers. Therefore, both chains of the antibody are modified with the linker and conjugated with DM1.
Unlike lysine conjugates, LC-MS analysis of cysteine-linked conjugates does not directly produce information about the drug distribution, as the drug-derivatized chains will dissociate under the acidic conditions used for ionization. Nonetheless, LC-MS of reduced conjugates can be used to confirm the peak assignments of RP-HPLC analysis described below (unpublished data).
Chromatographic analysis of distribution.
For conjugates formed by attachment through inter-chain disulfides or specifically engineered sites, drug distribution can be determined chromatographically using HIC.Citation14,Citation23,Citation35 The separation based on HIC also allows for isolation of chromatographically pure species permitting further analysis (e.g., by CE-SDS, ELISA or cell-based bioassay). The drug load of each chromatographic peak can be determined by on-line UV spectrometry if the drug-linker can be distinguished from the protein.Citation23 shows the HIC separation of a mAb-vc-MMAE conjugate along with the corresponding UV spectra of the individual peaks. Conjugation through lysine amine residues will diminish the net positive charge of the mAb by one for each attached drug-linker if the drug-linker is itself uncharged. Charge-based separations such as ion-exchange chromatography (IEC), iso-electric focusing gel electrophoresis (IEF) or capillary iso-electric focusing (cIEF) may be used to estimate the drug distribution for these ADCs. In the case of gemtuzumab ozogamicin (Mylotarg™), which has an average ∼3 to DAR of drugs/mAb (determined by UV spectroscopy), IEC and IEF results have been reportedCitation22,Citation36 demonstrating that this conjugate comprises a mixture where ∼50% of the mAb is unmodified, while the remainder has an average DAR of approximately 6 drugs/mAb as determined by IEC. KunzCitation36 describes the high level (45–65%) of unconjugated mAb (referred to as low conjugate fraction) in gemtuzumab ozogamicin as determined by IEC and that changing conjugation conditions can reduce this significantly (to ∼10%). For a conjugate formed through carbohydrates, the charge of the protein is not affected, but if the drug-linker has a charge, the pI shift derived from the conjugate distribution can be observed using IEF gels.Citation37
RP-HPLC, due to the denaturing effect of the low pH and high organic solvent, can be applied to separating and quantifying light and heavy chain species with different drug loads.Citation12,Citation13 RP-HPLC separation works well for forms that are conjugated at specific sites (the inter-chain cysteines); however, resolution of the more heterogeneous, lysine-linked conjugates using RP-HPLC has not been successfully reported. shows examples of a RP-HPLC separation applied to DTT-reduced samples of a cAC10-vcMMAE conjugate produced under different manufacturing conditions.Citation12 For cysteine-linked conjugates, it is possible to calculate the average DAR by determining chromatographically (using RP-HPLC) the mole fraction for each conjugated heavy and light chain species. The DAR obtained with this approach correlates well with the spectrophotometric method based on the UV absorption of the drug and the antibody.Citation13 RP-HPLC was also used to characterize positional isomers associated with engineered mAbs where the cysteines that form the inter-chain disulfide bonds have been replaced with serine residues.Citation13 Isomeric distribution for the HIC-purified conjugates with two, four and six drugs/mAb was determined by RP-HPLC following reductionCitation35 or by a combination of RP-HPLC and capillary electrophoresis under non-reducing and denaturing condition (CE-SDS).Citation12
Size Variant Analysis of Conjugates
As with any protein therapeutic, the presence of high molecular weight variants (i.e., aggregates) in an ADC has the potential to elicit the production of an anti-therapeutic antibody (ATA) response.Citation38,Citation39 Potential clinical outcomes of an ATA response could be any combination of the following: infusion-associated reactions, a change in the product's pharmacokinetics (i.e., faster clearance) and reduced drug exposure. Many of the drugs that are conjugated to antibodies to produce ADCs are relatively hydrophobic and can increase the likelihood of aggregate formation during manufacturingCitation40 and storage. Therefore, accurate and robust methods to measure the extent of aggregation and fragmentation are needed for both lot-release testing and for stability monitoring.
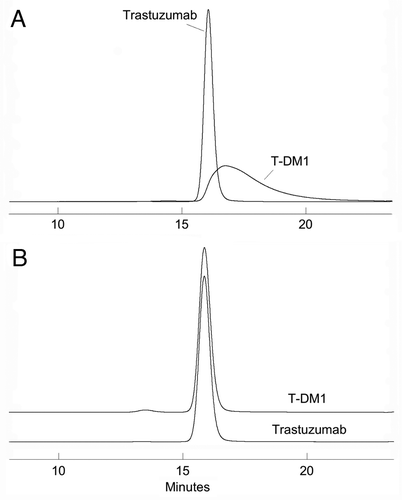
Most published methods for ADCs use the same size-exclusion chromatography analyses that are used for the parent monoclonal antibodies. For example, as reported by Willner et al.Citation20 and King et al.Citation41 conjugates containing DOX were analyzed on a TSK 3000SWXL column eluted with phosphate-buffered saline. This technique, when applied to ADCs, was shown to provide poor peak shape and incomplete resolution of aggregated forms (e.g., dimers) from the monomeric conjugate. In studies performed by Doronina et al.Citation29 the SEC comparison of the conjugated to the starting BR96 mAb demonstrated a slight increase in both retention time and peak tailing for the ADC. This suggests that the attached hydrophobic drugs lead to non-specific interaction between the ADC and the column stationary phase. Addition of an organic modifier to the mobile phase, such as 25% propylene glycol,Citation40 10% DMSOCitation26 or 15% acetonitrileCitation15 has been used successfully to overcome non-specific effects during chromatographic performance. A similar effect of 15% 2-propanol on restoring the peak shape and resolution of T-DM1 analyzed on a TSK 3000SWXL column has been demonstrated in our laboratory (). In addition to eliminating the peak tailing associated with elution of the conjugate and the poor resolution of monomer from dimer, the addition of solvent results in the same retention time for the T-DM1 monomer and unconjugated mAb ().
SEC is performed under non-denaturing conditions and, as described above for HIC analysis, doesn't lead to dissociation of non-covalently linked antibody chains formed by modification of inter-chain disulfides. SEC analysis of ADCs can be used to quantify formation of fragments via cleavage at the antibody hinge, a well-characterized non-enzymatic reaction that is frequently observed in mAbs stored in liquid formulations.Citation42
Analysis of reduced and non-reduced ADCs under denaturing conditions allows structural characterization of the conjugate that cannot be assessed readily at the intact protein level. For antibodies where covalent linkages between chains have been disrupted by conjugation, under non-denaturing conditions (e.g., during SEC and HIC analysis) the high affinity between antibody chains results in the elution of the ADC as an intact 150 kDa molecule. Traditionally, SDS-PAGE has been used for molecular weight-based separations of proteins. Several reportsCitation29,Citation37 have described SDS-PAGE of reduced ADCs to resolve drug-linked antibody chains from the non-conjugated forms.
Anti-CEA-Gal-DOX,Citation37 in which DOX was linked through enzymatically-activated galactose residues on the heavy chain, was characterized using SDS-PAGE under reducing conditions. A slight increase could be observed in the molecular weight of the heavy chain due to conjugation. Western blot analysis developed with rabbit anti-DOX IgG revealed the presence of DOX on the carbohydrate moieties of anti-CEA mAb, but not on the conjugate treated with PNG-ase F, indicating that DOX was specifically coupled to the N-glycan moieties of the heavy chain.Citation37
Cytotoxic drugs conjugated to antibodies through cysteine sulfhydryl groups that are activated by partially reducing interchain disulfide bonds can yield heterogeneous products. The number of drugs on each mAb varies from 0–8 (in increments of 2) with possible positional isomers at each drug substitution level. It is important to understand the isomeric population of each of the drug-loaded species because they could affect efficacy and toxicity of the product.Citation12
Both the light and heavy chains are modified in ADCs where the drugs are linked through the interchain cysteines. Following reduction, SDS-PAGE analysis of AEVB and MMAE conjugates of BR96,Citation29 showed resolution of unconjugated light chain (L0) from the form with one drug (L1) along with partial resolution of unconjugated heavy chain (H0) from the conjugated forms (H1, H2, H3). SDS-PAGE was also used to characterize the cleavage of the protease sensitive MC-vc-PABC linker. McDonagh et al.Citation13 also used SDS-PAGE of reduced and non-reduced vc-MMAE conjugates in characterization of native and engineered cAC10 antibodies and demonstrated resolution of L0 from L1, but only partial resolution of H0 from the heavy chain forms with 1, 2 or 3 equivalents of MMAE.
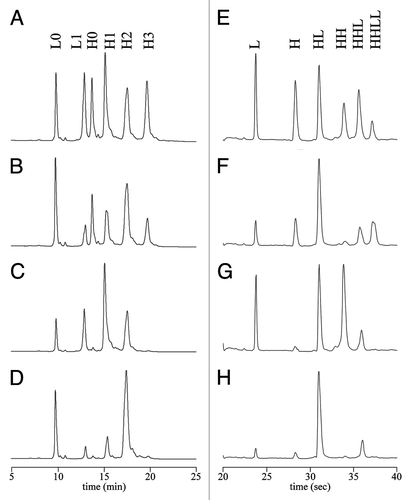
CE-SDS has emerged as a preferred technique that offers clear advantages over SDS-PAGE in terms of speed, reproducibility, resolution, robustness and ease of automation for non-conjugated antibodies.Citation43 One of the earliest applications of this method to ADCs was by Liu et al.Citation44 who used it for the analysis of a BR96-DOX conjugate formed by linkage of drug to inter-chain sulfhydryls. The addition of SDS to the sample dissociates antibody chains that are not covalently linked by intact disulfides due to partial reduction and reaction with linker-drug, and provides information about the sites of conjugation along with a comparison to traditional SDS-PAGE. As shown in , analysis of a cAC10-vc-MMAE conjugate using the Bioanalyzer™, an automated silicon chip-based CE-SDS technology, was reported by Sun et al.Citation12
RP-HPLC, due to the denaturing effect of the low pH and high organic solvent, can be applied to separate and quantify various light and heavy chain species with different drug loads following reduction of the ADC.Citation12,Citation13 RP-HPLC separation works well for forms that are conjugated at specific sites (e.g., the inter-chain cysteines); however, resolution of the more heterogeneous, lysine-linked conjugates by this technique has not been reported. For cysteine-linked conjugates, it is possible to determine the DAR by this approach and it correlates well with the spectrophotometric method based on the absorption of the drug and the antibody.Citation13 Isomeric distribution (i.e., which chains contain the linked drug) of the HIC-purified materials with 2, 4 and 6 drugs/mAb was determined using the RP-HPLC methodCitation35 under reducing conditions or a combination of RP-HPLC and CE-SDS under non-reducing conditions ().Citation12
Charge-Based Separations
Analysis of charge variants such as those resulting from deamidation or formation of N-terminal pyroglutamates may be an important quality attribute of a mAb, especially if the changes affect binding or biological activity.Citation45–Citation47 Depending on the characteristics of the drug, the linker and the conjugation site (i.e., lysines, inter-chain sulfhydryls, carbohydrates), methods that can be applied to the parent mAb may not be applicable to the ADC or may give significantly different information.
Attachment of an uncharged linker and drug through lysine residues decreases the net positive charge by one for each bound drug-linker. In this case, separation based on charge, such as using ion-exchange chromatography (IEC) or iso-electric focusing (IEF), results in profiles that characterize the drug load, rather than proving information about the underlying mAb.Citation22 For gemtuzumab ozogamicin, the IEC profile showed that most of the calicheamicin was on approximately half of the antibody while 45–65% of the product was a low conjugated fraction, essentially unconjugated antibody.Citation22,Citation36 A similar distribution can be observed qualitatively using IEF gel electrophoresis.Citation22 Application of slab-gel and capillary isoelectric focusing to the analysis of a naked IgG4 and of a calicheamicin conjugated IgG4 (gemtuzumab ozogamicin) was recently described by Maeda et al. which showed the reproducibility of the highly complex pattern resulting from the conjugate.Citation48 As described above, attachment of the amine of a positively charged drug (DOX) through enzymatically-activated galactose residues on an anti-CEA antibody was also detected qualitatively using IEF gels.Citation37
Other than the application of capillary zone electrophoresis (a charge-based separation) to analysis of a peptide digest,Citation49 there are no other published reports of charge-based assays applied to ADCs. A recently introduced technology, imaged capillary isoelectric focusing (iCIEF), has been successfully applied to analysis of charged variants of mAbs.Citation50 The use of this method for analysis of ADCs has been described by several speakers at recent scientific conferences; however, these have not yet appeared in peer-reviewed literature. The applicability of this method to ADC analysis will depend on the nature of the drug-linker (in particular the charge), the mode of attachment (lysine, cysteine, carbohydrate) and the resulting complexity of the product. In some cases it may be possible to obtain information about charge variants on the underlying antibody.
Analysis of Unconjugated Drug
Another important quality attribute shared by all ADCs is the quantity of free (unconjugated) drug, which poses concerns for differential toxicity and potential safety issues. Residual amounts of unconjugated drug or drug-related impurities may remain in the final product as a result of incomplete removal by purification steps down-stream of the conjugation reaction. Related forms of the unconjugated drug, such as linker-drug species or other degradation products, may also be released during storage of the conjugate. It is therefore essential to develop and apply suitable assays to detect, characterize and quantify free drug species. These assays may also be used as part of characterization of the conjugate's stability in biological matrices such as plasma or cultured tumor cells.
Enzyme-linked immunosorbent assay (ELISA),Citation22,Citation28,Citation51 capillary electrophoresis (CE),Citation49 and high-performance liquid chromatography (HPLC) methods have been used to determine free drug levels in various ADCs.Citation12,Citation15,Citation17–Citation19,Citation24,Citation29,Citation52–Citation55
Competition ELISA was used to determine the free MMAE released from cAC10-vc-MMAECitation28 following incubation with cathepsin B in plasma. Cathepsin B-treated samples and free MMAE quantitation standards were mixed with a fixed concentration of MMAE-conjugated horseradish peroxidase reporter (HRP) and were subsequently added to microtiter plates coated with an anti-MMAE mAb. Free drug in the sample competes with binding of the reporter to the plate, which is detected using a chromogenic reagent. In a similar fashion,Citation51 the amount of the maytansinoid drug released from huC242-SPP-DM1 or huC242-SMCC-DM1 was quantified with a competition ELISA after treating the antigen-positive or bystander cells with the conjugates. In this case, following protein precipitation using ice-cold acetone, samples of the supernatant or a maytansine standard solution were mixed with biotinylated murine anti-maytansinoid mAb. Free maytansinoids in the sample compete with binding of the reporter to a BSA-DM1 coated plate. The bound biotinylated anti-maytansinoid mAb was then detected with streptavidin-HRP to determine the amount of free maytansinoids in the sample.
CE with laser-induced fluorescence (LIF) detectionCitation49 was used for the highly sensitive and selective detection and identification of free DOX and DOX-hydrazone (DOX-HDZ) in a DOX-conjugated BR96 mAb. LIF detection was used because DOX exhibits a strong fluorescent emission at 550 nm with excitation at 490 nm. The intact ADC migrates as a broad, early-eluting set of peaks and is well resolved from the free drug species in the electropherogram. There is no resolution between DOX and the drug-linker complex, DOX-HDZ, by the CE method both are uncharged species at the pH used for the analysis.
HPLC methods can provide excellent precision, sensitivity and selectivity for free drugs. Since cytotoxic drugs are typically low molecular weight, hydrophobic molecules, RP-HPLC has become the most widely employed analytical technique for their separation and quantification. Most commonly, peaks that contain unconjugated drug species are monitored at the absorption maxima of the drug with a UV/Vis detector. For example a vinca-alkaloid conjugateCitation15 was directly injected onto a C-8 RP-HPLC column and eluted isocratically with a mobile phase containing methanol and phosphoric acid. Elution was monitored by UV absorption at 270 nm and the free 4-desacetylvinblastine hydrazide eluted as a moderately broad peak at 9.0 min. The amount of free drug in the post-conjugation test sample was calculated from the HPLC peak area using a standard curve.
One potential issue with direct injection of protein-containing ADC samples onto a reverse-phase HPLC column is column deterioration due to the irreversible binding of proteins to the stationary phase. Accumulation of proteins within an HPLC column can reduce column performance by clogging pores in the stationary phase, thus inhibiting the diffusional mass transport of analytes into the porous packing and decreasing column capacity and efficiency. If significant accumulation of proteins within a column occurs, column backpressure can build up and column performance may be compromised. Guard columns, which can easily be changed periodically, can be installed to prevent contamination and possible damage to the analytical column. For the quantitative analysis of methotrexate (MTX) or its derivatives in samples of MTX-791T/36 conjugates,Citation17 a 3 cm × 4.6 mm guard column was coupled to a 25 cm × 4.6 mm analytical column packed with the same 300 Å pore-size hydrophobic bonded phase and eluted isocratically.
In addition to using guard columns, various sample cleanup procedures have been appliedCitation18,Citation19,Citation54 in order to preserve performance of the RP-HPLC columns. These techniques involve separating the free drug from the protein-drug conjugate in the sample prior to chromatographic analysis. Although the method is somewhat labor-intensive, precipitation of the ADC protein using organic solvents is one reported pretreatment method for the analysis of free drug species, which remain soluble in the organic extract. Conjugates containing adriamycin (ADM) were mixed with an equal volume of acetonitrile, and following removal of the precipitated conjugate, the organic extract was analyzed by RP-HPLC for the presence of unconjugated ADM or ADM derivatives.Citation19
A second method of sample cleanup involves the use of offline solid phase extraction (SPE), where analytes are retained on the stationary phases. For example, traces of free daunomycin and adriamycinCitation54 were removed from the protein fractions by adsorption chromatography on Porapak Q, a porous, polymeric ion-exchange matrix. This material binds free daunomycin and adriamycin very efficiently, while allowing protein and protein-bound drugs to pass through the column unretained. The free drug species were then eluted off the column with acetone or methanol for further analysis. Similarly, a Sep-Pak C-18 cartridgeCitation18 was used to separate the free bis-indolyl-seco-CBI species from the conjugate, and then the free drug species were recovered, analyzed and quantified by HPLC on a reverse-phase C18 column.
To avoid the time-consuming sample cleanup step, Fleming et al.Citation53 reported using a HISEP™ shielded hydrophobic phase column onto which mAb-DM1 conjugate was injected directly without prior sample preparation. This stationary phase combines a hydrophobic core and a hydrophilic outer layer. The hydrophilic layer restricts access of proteins and protein-drug conjugates from interacting with the core, while any free drug molecules present in solution can migrate to the surface and bind to the inner, hydrophobic portion of the stationary phase. Following direct injection of a protein-containing analyte, the protein typically elutes in the unretained “void” volume and the free drug species are eluted with a gradient of increasing organic solvent. As described in this reference, free drug species can be quantified either with or without an external standard. In addition, the A252 and A280 absorbances of the flow-through peak, containing the intact ADC, can be used to estimate the DAR as described previously. As the hydrophilic polymer can reduce mass transfer kinetics of the separation process, these columns may exhibit lower resolution as compared with an “un-shielded” stationary phase.
RP-HPLC, in conjunction with mass spectrometric (MS) analysis, allows the identification and quantitation of various cytotoxic species released from the conjugate. Three major free drug species were observedCitation53 in a mAb-DM1 conjugate stressed at 37°C for 14 days. Mass spectrometric analysis helped to identify them as L-DM1-thiopentanoic acid (drug plus linker), L-DM1-dimer and maysine, a β-elimination degradation product of DM1. Similarly, RP-HPLC/MS can be used to examine in vitro stability of the conjugate and monitor the free drug species released in plasmaCitation52 or in cultured tumor cell-lines.Citation56 Aliquots of cAC10-vcMMAE incubated in human, mouse or dog plasma were analyzed by LC/MS/MS for the release of free MMAE.Citation52 In human and dog plasma, less than 2% of the total drug (as MMAE) was released after ten days. In mouse plasma, less than 5% of the total drug was released during this time. A subsequent qualitative full-scan LC/MS analysis, including UV detection, of the same samples did not reveal the presence of other molecular species identifiable as drug or drug-linker degradation products. The same technology can be applied to ADC manufacturing and storage samples to characterize drug-related impurities that might be present initially or formed over time.
Peptide Mapping Analysis
Peptide mapping is a powerful tool for detecting and characterizing protein modifications such as amino acid substitution, pyroglutamic acid formation, oxidation, deamidation, isomerization, glycation, disulfide shuffling, peptide bond cleavage and crosslinking. Enzymatic cleavage of ADCs can be used to identify drug-containing peptides. For example, peptides labeled with a hydrophobic drug would be expected to elute later than their unmodified forms in the RP-HPLC chromatogram due to increased retention by the column. Protease activity may also be affected by conjugation; for example, trypsin will not cleave at lysines modified by drug, leading to larger and more hydrophobic peptides. Unique chromatographic or electrophoretic profiles of the enzymatic digests of an ADC can be used to establish product identity, characterize the specific sites of drug binding and establish process consistency in a complex ADC product.
Comparative peptide mapping of the chimeric BR96 mAb and the corresponding DOX conjugate formed by linkage through interchain cysteinesCitation49 was performed using capillary electrophoresis (CE) and capillary liquid chromatography (CLC). Both UV absorbance and laser-induced fluorescence (LIF) detection were applied to study enzymatic digests (under both native and reduced/denatured conditions) of the conjugate and of the drug and drug-linker species. As described above, LIF detection can be used for identification of drug-containing peptides based on the fluorescence of the DOX. The peptide maps of BR96 and its conjugate, generated using the analytical procedures described above, resulted in a unique fingerprint for structural analysis.
Chromatographic separations of ADC peptides combined with MS analysis can provide important structural information about the locations of conjugation sites. Although there are no reported examples, this information would be important in instances where modification of residues in the complementarity-determining regions (CDRs) might correlate with an effect on mAb binding to the target antigen.
Since all antibodies have numerous surface accessible residues that can potentially react, conjugation through lysine side-chain amines typically results in a complex mixture of conjugate species that differ in the number of drugs attached as well as in the sites of drug linkage. Structural characterization is challenging due to the heterogeneous nature of the conjugate, the large molecular size of antibodies and possible low abundances of individual modified residues. Trypsin and Asp-N protease digestions were performed as part of the characterization of lysine-modified huN901-DM1.Citation32 Peptide mapping was done following reduction of disulfide bonds and alkylation of the resulting thiols, which cleaved the linker-DM1 bond, resulting in linker-modified peptides. These are harder to identify by HPLC than if the drug were bound. Although trypsin digestion allowed determination of specific sites of modification (because the enzyme cannot cleave at drug- or linker-modified lysines), chromatographic differences were more evident between profiles of unconjugated and conjugated antibody following Asp-N protease digestion. Electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) was used to identify the linker-modified sites based on their characteristic mass difference of 174 Da. Modification sites identified by the two enzymatic digestions were consistent. All observed modifications are partial (i.e., both unmodified and modified versions of peptides were found), with identification of 7 lysine residues in the light chain and 13 lysine residues in the heavy chain. No linker-modified lysine residues in the CDRs of huN901 were detected. The extent of modification at a particular residue was postulated to be affected by the location on the antibody. The detected conjugation sites in huN901-DM1 were mapped in structural models of a human IgG1, and the modified residues were generally found in locations with greater solvent accessibility and structural flexibility.
Conjugates formed through complete reduction of the four antibody interchain disulfides, followed by alkylation with a pre-formed drug-linker maleimide species, will result in conjugates with a homogeneous distribution of ∼8 drugs/mAb.Citation12,Citation20 Hamblett et al.Citation23 reported that cysteine-linked conjugates with an average of four drugs/mAb generated from partially reduced mAbs exhibited a wider in vivo therapeutic index than their counterparts with eight drugs/mAb. Such partially-loaded conjugates are not homogeneous with respect to drug load and contain a distribution of species with different molar ratios of drug to antibody, linked at different sites (see section on Drug Distribution).
Peptide mapping of cysteine-linked BR96-DOX conjugates using CE and CLC (capillary LC) was reported by Liu et al.Citation49 however, detailed conjugated peptide identification was not possible at that time. Peptide mapping of similarly linked vc-MMAE conjugates has been reported in presentations at recent conferences, but none of these have, as yet, appeared in publication. This unpublished data confirms that the drug is linked, as expected, to the inter-chain cysteines of the heavy and light chains and also shows that linkage to the intra-chain cysteines does not occur. Consistent with the hydrophobic nature of vc-MMAE, the drug-containing peptides elute after their unconjugated parent peptides.
Junutula et al.Citation14 described a site-specific conjugation of vc-MMAE to an antibody through engineered cysteine substitutions at positions on light and heavy chains. Tryptic peptide mapping with LC/MS/MS detection of these conjugates identified four drug-conjugated peptides by a characteristic in-source fragmentation ion (m/z 718.5) that is observed in all mass spectra of molecules containing vc-MMAE. All four peptides were identified as complete or partial tryptic cleavage fragments located around the engineered cysteine. The drug-containing peptide masses did not map to any other region of the protein, demonstrating the specificity of the conjugation.
Biophysical Analysis
Many of the cytotoxic drugs being conjugated to antibodies are very hydrophobic (e.g., paclitaxel, DM1, calicheamicin, DOX). Despite the large difference in size between the drug and protein, covalent conjugation of such drugs to antibodies has been observed to exert a significant effect on the ADC's stability, solubility and solution interactions.Citation26,Citation40,Citation41
Effect on higher order structure and stability.
The use of conventional biophysical analytical methods to characterize the biophysical properties of an ADC relative to the unconjugated antibody may be complicated by the presence of the bound drug. Many of the cytotoxic drugs used in ADCs exhibit a strong near-UV absorbance, which can interfere with near-UV-CD and fluorescence spectroscopy methods usually employed for the characterization of protein tertiary structure. The spectroscopic signal of an ADC in the near UV spectrum may depend on the DAR, leading to challenges in differentiating if observed changes in the near-UV spectrum are derived from alteration of tertiary structure or from variation in the DAR. Far-UV-CD, on the other hand, can be employed to probe the effect of drug conjugation on the mAb secondary structure (assuming that the bound drug does not interfere).
Thermal analysis methods such as differential scanning calorimetry can be used to study the effects of drug conjugation on the conformational stability of the antibody.Citation57 Studies with T-DM1 suggest that the conformational stability of the Fc domain is particularly affected due to modification and conjugation to lysine residues. As a result, the impact of conjugation on the conformational and colloidal stability of an antibody should be carefully studied during formulation development.
Effect on solubility.
The solubility of an antibody can be significantly altered upon conjugation with a hydrophobic drug. Solubility decrease may be either due to reduction of net surface charge when the conjugation of the drug is through surface exposed lysines, or due to the hydrophobicity of the conjugated drug and linker. The effect on solubility can be expected to increase with the degree of conjugation. Several authors have reported that high DAR ADC's tend to precipitate or aggregate preferentially during conjugation.Citation40 For this reason, several reports in the literature refer to the use of highly branched, hydrophilic linkers to overcome the aggregation caused by high drug loads.Citation26,Citation41
Because high salt concentrations can lead to an increase in hydrophobic interactions, the solubility of an ADC may be more sensitive to the effects of high salt as compared to the unconjugated antibody. Storage of solutions containing ADCs (e.g., ion-exchange process chromatography streams) may result in increased aggregation or selective precipitation of ADC species with high DARs leading to the formation of protein particulates.
Conclusions
The last ten years have seen an increasing interest in ADCs as a way to extend the therapeutic potential of mAbs. This trend has been driven by the clinically documented efficacy that is being reported in the literature for several of these drugs. As compared to the parent “unconjugated” antibody, the ADC has an increased level of complexity that arises from the heterogeneity of the conjugation, layered on top of the starting variability associated with the antibody. Improvements in analytical technology have provided the tools to better characterize these products, giving ADC developers the information needed for process and formulation development, and for identifying the methods needed for QC lot-release and stability testing.
Developments in mass spectrometry of biological molecules—including better TOF analyzers, as well as MS/MS and MS/MS/MS capabilities—have permitted more accurate characterization of drug distribution and identification of conjugation sites. A new generation of CE instrumentation and methods (including the silicon chip-based Bioanalyzer™, and the imaged capillary isoelectric focusing system described above) improve the quantitative capabilities of this technology, making it suitable for both detailed characterization and routine use (where applicable). Improvements in HPLC column technologies allow for highresolution separations of the complex peptide mixtures derived from protease digestions of mAbs and the ADCs manufactured from them.
The combination of these improvements in analytical technology, along with appropriate bioanalytical methods such as ELISAs and cell-based potency assays, is leading to a better understanding of how ADC structure affects clinical outcomes such as efficacy and safety. This is allowing more informed decisions about which ADC candidates to advance into the clinic, and which methods should be used for routine GMP testing and stability monitoring once production of clinical material begins.
The recent Quality by Design (QbD) joint initiative between the US Food and Drug Administration and the pharmaceutical industry concerning therapeutic biotechnology products recommends that manufacturers build quality into a therapeutic drug throughout its development process. The physicochemical properties that influence the safety and efficacy profile of biotechnology products are their critical quality attributes (CQAs). For a mAb as a therapeutic, there is an increasing body of literature devoted to understanding what attributes are critical.Citation58,Citation59 For an ADC, it is clear that there will be a distinct set of CQAs, some of which may overlap with those of the mAb intermediate. As described above, some of the potential CQAs for ADCs are size variants (aggregates and fragments), drug distribution and average DAR, free drug level. Other CQAs will likely be identified on a case-by-case basis and may include charged variants associated with the antibody, other process impurities such as those related to the drug and the conjugation, and product isoforms.
These CQAs (some of which may appear on the control system and others that are used for characterization only) can be used during ADC development to demonstrate that the process can reproducibly meet the defined product criteria. The process can also be characterized using statistically-based experimental design techniques (design of experiment studies) to understand how process conditions affect these critical attributes and then suitable limits on critical process parameters can be established. Through this combination of analytical and process knowledge, ADCs with consistent quality, safety, and efficacy can be produced for clinical and commercial use.
Abbreviations
| ADC | = | antibody drug conjugate |
| ADM | = | Adriamycin |
| AEVB | = | 5-benzoylvaleric acid-auristatin E ester |
| CBI | = | 1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one |
| CEA | = | carcinoembryonic antigen |
| CD | = | circular dichroism |
| CDR | = | complementarity-determining region |
| CE-SDS | = | capillary electrophoresis in sodium dodecyl sulfate |
| cIEF | = | capillary isoelectric focusing |
| CLC | = | capillary liquid chromatography |
| CQA | = | critical quality attribute |
| Da | = | dalton |
| DAR | = | drug to antibody ratio |
| DAVLB | = | 4-desacetylvinblastine |
| DM1 | = | drug maytansinoid 1 |
| DM4 | = | drug maytansinoid 4 |
| DOX | = | doxorubicin |
| DOX-HDZ | = | doxorubicin-hydrazide |
| DTPA | = | diethylene triamine pentaacetic acid |
| DTT | = | dithiothreitol |
| EGFR | = | epidermal growth factor receptor |
| ELISA | = | enzyme-linked immunosorbent assay |
| ESI-MS | = | electrospray ionization mass spectrometry |
| GMP | = | good manufacturing practices |
| HIC | = | hydrophobic interaction chromatography |
| IEC | = | ion-exchange chromatography |
| IEF | = | isoelectric focusing |
| LIF | = | laser-induced fluorescence |
| MALDI | = | matrix-assisted laser desorption and ionization |
| MALDI-TOF MS | = | matrix-assisted laser desorption and ionization time-of-flight mass spectrometry |
| MMAE | = | monomethylauristatin E |
| MS | = | mass spectrometry |
| MTX | = | methotrexate |
| QbD | = | quality by design |
| RP-HPLC | = | reverse-phase high performance liquid chromatography |
| SPP | = | (N)-succinimidyl-4-(2-pyridylthio) pentanoate |
| vc-MMAE | = | maleimidocaproyl-valine-citrulline-p-aminobenzoxycarbonyl-MMAE |
Figures and Tables
Figure 1 Chemical structures of mAb-drug conjugates.Citation6 The linkers used for each drug are indicated in parentheses, and the labile bonds leading to drug release are shaded. For the examples shown, hydrazones release drug under acidic conditions within the lysozomes of target cells, disulfides undergo intracellular reduction, and the peptides are enzymatically hydrolyzed by lysozomal proteases. Adapted by permission from Macmillan Publishers Ltd. Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates.Citation6
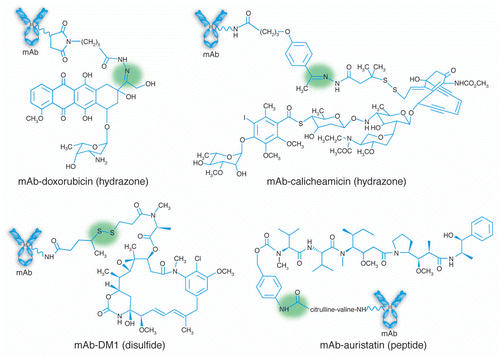
Figure 2 Hydrophobic interaction chromatography (HIC) analysis of a mAb-vc-MMAE on a TOSOH Biosciences Butyl-NPR column yields five predominant peaks that correspond to mAb containing zero, two, four, six and eight drugs. Inset: an overlay of the UV spectra of the starting mAb and the HIC peaks, normalized to the 280 nm absorbance, showing the increase in the 248 nm absorbance as the level of conjugated drug-linker increases. Method is similar to that described in Hamblett, et al.Citation23
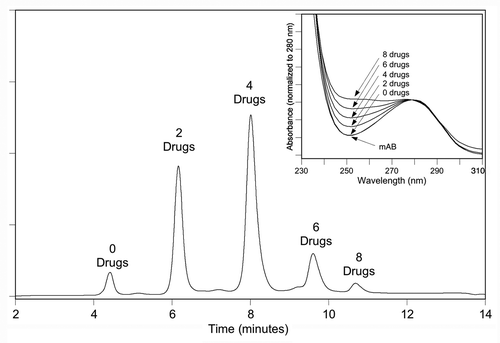
Figure 3 SEC/ESI-MS analysis of deglycosylated huC242-DM4 showing the deconvoluted mass spectrum. The label on each peak (e.g., D2) refers to the number of bound drug-linkers. Spectrum is obtained on a Waters LCT time of flight instrument as described by Lazar et al.Citation33 Inset: The total ion current in the SEC elution region of the protein. Adapted by permission from John Wiley & Sons: Lazar AC, Wang L, Blattler WA, Amphlett G, Lambert JM, Zhang W. Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry.Citation33
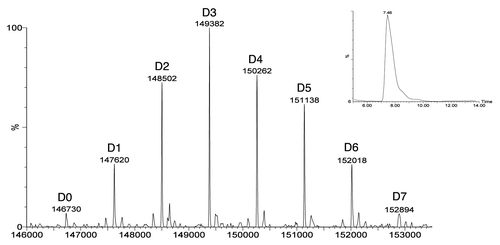
Figure 4 (A–D) Reversed-phase HPLC analysis of DTT-reduced conjugates produced using different reduction/reoxidation protocols. (E–H) Analysis of the same conjugate samples in (A–D), under non-reducing conditions, using the Agilent Bioanalyzer™, a silicon chip based system for capillary electrophoresis in the presence of SDS (CE-SDS).Citation12 Adapted with permission from Sun MM, Beam KS, Cerveny CG, Hamblett KJ, Blackmore RS, Torgov MY, et al. Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides.Citation12
Acknowledgements
The authors would like to thank Godfrey Amphlett, ImmunoGen, Inc., Michael Sun, Seattle Genetics, Inc. and Matt Kalo, Genentech, Inc. for providing original versions of figures.
References
- Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol 2009; 13:235 - 244
- Ranson M, Sliwkowski MX. Perspectives on anti-HER monoclonal antibodies. Oncology 2002; 63:17 - 24
- Morris PG, Fornier MN. Novel anti-tubulin cytotoxic agents for breast cancer. Expert Rev Anticancer Ther 2009; 9:175 - 185
- DiJoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia 2007; 21:2240 - 2245
- Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010; 363:1812 - 1821
- Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 2005; 23:1137 - 1146
- Chari RV. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res 2008; 41:98 - 107
- Chari RV. Targeted delivery of chemotherapeutics: tumor-activated prodrug therapy. Adv Drug Deliv Rev 1998; 31:89 - 104
- Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol 2005; 5:543 - 549
- Payne G. Progress in immunoconjugate cancer therapeutics. Cancer Cell 2003; 3:207 - 212
- Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 2006; 66:4426 - 4433
- Sun MM, Beam KS, Cerveny CG, Hamblett KJ, Blackmore RS, Torgov MY, et al. Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides. Bioconjug Chem 2005; 16:1282 - 1290
- McDonagh CF, Turcott E, Westendorf L, Webster JB, Alley SC, Kim K, et al. Engineered antibody-drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng Des Sel 2006; 19:299 - 307
- Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 2008; 26:925 - 932
- Laguzza BC, Nichols CL, Briggs SL, Cullinan GJ, Johnson DA, Starling JJ, et al. New antitumor monoclonal antibody-vinca conjugates LY203725 and related compounds: design, preparation and representative in vivo activity. J Med Chem 1989; 32:548 - 555
- Chari RV, Martell BA, Gross JL, Cook SB, Shah SA, Blattler WA, et al. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res 1992; 52:127 - 131
- Hudecz F, Garnett MC, Khan T, Baldwin RW. The influence of synthetic conditions on the stability of methotrexate-monoclonal antibody conjugates determined by reversed phase high performance liquid chromatography. Biomed Chromatogr 1992; 6:128 - 132
- Chari RV, Jackel KA, Bourret LA, Derr SM, Tadayoni BM, Mattocks KM, et al. Enhancement of the selectivity and antitumor efficacy of a CC-1065 analogue through immunoconjugate formation. Cancer Res 1995; 55:4079 - 4084
- Greenfield RS, Kaneko T, Daues A, Edson MA, Fitzgerald KA, Olech LJ, et al. Evaluation in vitro of adriamycin immunoconjugates synthesized using an acid-sensitive hydrazone linker. Cancer Res 1990; 50:6600 - 6607
- Willner D, Trail PA, Hofstead SJ, King HD, Lasch SJ, Braslawsky GR, et al. (6-Maleimidocaproyl)hydrazone of doxorubicin—a new derivative for the preparation of immunoconjugates of doxorubicin. Bioconjug Chem 1993; 4:521 - 527
- Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res 1993; 53:3336 - 3342
- Moran J. Presentation at 224th American Chemical Society National Meeting August 18–22, 2002 Boston, MA
- Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 2004; 10:7063 - 7070
- Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA, et al. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug Chem 2002; 13:40 - 46
- Siegel MM, Hollander IJ, Hamann PR, James JP, Hinman L, Smith BJ, et al. Matrix-assisted UV-laser desorption/ionization mass spectrometric analysis of monoclonal antibodies for the determination of carbohydrate, conjugated chelator and conjugated drug content. Anal Chem 1991; 63:2470 - 2481
- Quiles S, Raisch KP, Sanford LL, Bonner JA, Safavy A. Synthesis and preliminary biological evaluation of high-drug-load paclitaxel-antibody conjugates for tumor-targeted chemotherapy. J Med Chem 2010; 53:586 - 594
- Safavy A, Bonner JA, Waksal HW, Buchsbaum DJ, Gillespie GY, Khazaeli MB, et al. Synthesis and biological evaluation of paclitaxel-C225 conjugate as a model for targeted drug delivery. Bioconjug Chem 2003; 14:302 - 310
- Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res 2005; 11:843 - 852
- Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003; 21:778 - 784
- Siegel MM, Tabei K, Kunz A, Hollander IJ, Hamann RR, Bell DH, et al. Calicheamicin derivatives conjugated to monoclonal antibodies: determination of loading values and distributions by infrared and UV matrix-assisted laser desorption/ionization mass spectrometry and electrospray ionization mass spectrometry. Anal Chem 1997; 69:2716 - 2726
- Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, et al. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res 2010; 16:4769 - 4778
- Wang L, Amphlett G, Blattler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci 2005; 14:2436 - 2446
- Lazar AC, Wang L, Blattler WA, Amphlett G, Lambert JM, Zhang W. Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry. Rapid Commun Mass Spectrom 2005; 19:1806 - 1814
- Lazar AC, Krishnamurthy R. Evaluation of the effect of sample composition and sample load on the mass spectrometric analysis of immunoconjugates, TPY403 Proceedings of the 55th American Society for Mass Spectrometry Annual Conference on Mass Spectrometry and Allied Topics June 3–7, 2007 Indianapolis, Indiana
- Stephan JP, Chan P, Lee C, Nelson C, Elliott JM, Bechtel C, et al. Anti-CD22-MCC-DM1 and MC-MMAF conjugates: impact of assay format on pharmacokinetic parameters determination. Bioconjug Chem 2008; 19:1673 - 1683
- Kunz A. Calicheamycin derivative carrier conjugates United States Patent Application Publication, No. US 2004/01929200 A1 2004;
- Stan AC, Radu DL, Casares S, Bona CA, Brumeanu TD. Antineoplastic efficacy of doxorubicin enzymatically assembled on galactose residues of a monoclonal antibody specific for the carcinoembryonic antigen. Cancer Res 1999; 59:115 - 121
- Rosenberg AS, Worobec A. A risk-based approach to immunogenicity concerns of therapeutic protein products, Part 1: Considering consequences of the immune response to a protein. Biopharm Intl 2004; 19:22 - 26
- Rosenberg AS, Worobec A. A risk-based approach to immunogenicity concerns of therapeutic protein products, Part 2: Considering host-specific and product-specific factors impacting immunogenicity. Biopharm Intl 2004; 19:34 - 42
- Hollander I, Kunz A, Hamann PR. Selection of reaction additives used in the preparation of monomeric antibody-calicheamicin conjugates. Bioconjug Chem 2008; 19:358 - 361
- King HD, Dubowchik GM, Mastalerz H, Willner D, Hofstead SJ, Firestone RA, et al. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: inhibition of aggregation by methoxytri-ethyleneglycol chains. J Med Chem 2002; 45:4336 - 4343
- Cordoba AJ, Shyong BJ, Breen D, Harris RJ. Nonenzymatic hinge region fragmentation of antibodies in solution. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 818:115 - 121
- Hunt G, Nashabeh W. Capillary electrophoresis sodium dodecyl sulfate nongel sieving analysis of a therapeutic recombinant monoclonal antibody: a biotechnology perspective. Anal Chem 1999; 71:2390 - 2397
- Liu J, Abid S, Lee MS. Analysis of monoclonal antibody chimeric BR96-doxorubicin immunoconjugate by sodium dodecyl sulfate-capillary gel electrophoresis with ultraviolet and laser-induced fluorescence detection. Anal Biochem 1995; 229:221 - 228
- Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, et al. Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. mAbs 2010; 2
- Harris RJ, Kabakoff B, Macchi FD, Shen FJ, Kwong M, Andya JD, et al. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J Chromatogr B Biomed Sci Appl 2001; 752:233 - 245
- Vlasak J, Ionescu R. Heterogeneity of monoclonal antibodies revealed by charge-sensitive methods. Curr Pharm Biotechnol 2008; 9:468 - 481
- Maeda E, Urakami K, Shimura K, Kinoshita M, Kakehi K. Charge heterogeneity of a therapeutic monoclonal antibody conjugated with a cytotoxic anti-tumor antibiotic, calicheamicin. J Chromatogr A 2010; 1217:7164 - 7171
- Liu J, Zhao H, Volk KJ, Klohr SE, Kerns EH, Lee MS. Analysis of monoclonal antibody and immunoconjugate digests by capillary electrophoresis and capillary liquid chromatography. J Chromatogr A 1996; 735:357 - 366
- Sosic Z, Houde D, Blum A, Carlage T, Lyubarskaya Y. Application of imaging capillary IEF for characterization and quantitative analysis of recombinant protein charge heterogeneity. Electrophoresis 2008; 29:4368 - 4376
- Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res 2006; 66:3214 - 3221
- Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 2003; 102:1458 - 1465
- Fleming MS, Zhang W, Lambert JM, Amphlett G. A reversed-phase high-performance liquid chromatography method for analysis of monoclonal antibody-maytansinoid immunoconjugates. Anal Biochem 2005; 340:272 - 278
- Hurwitz E, Levy R, Maron R, Wilchek M, Arnon R, Sela M. The covalent binding of daunomycin and adriamycin to antibodies, with retention of both drug and antibody activities. Cancer Res 1975; 35:1175 - 1181
- Law CL. Anti-CD70 antibody-drug conjugates and their use for the treatment of cancer and immune disorders US patent no 7,662,387 B2 2010;
- Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem 2006; 17:114 - 124
- Wakankar AA, Feeney MB, Rivera J, Chen Y, Kim M, Sharma VK, et al. Physicochemical stability of the antibody-drug conjugate trastuzumab-DM1: changes due to modification and conjugation processes. Bioconjug Chem 2010; 21:1588 - 1595
- Rathore AS, Mhatre R. Quality by design for biopharmaceuticals: Principles and case studies 2009; Hoboken, NJ John Wiley & Sons, Inc
- Goetze AM, Schenauer MR, Flynn GC. Assessing monoclonal antibody product quality attribute criticality through clinical studies. mAbs 2:500 - 507