Abstract
Therapeutic antibodies need international patent protection as their markets expand to include industrialized and emerging countries. Because international intellectual property strategies are frequently complex and costly, applicants require sound information as a basis for decisions regarding the countries in which to pursue patents. While the most important factor is the size of a given market, other factors should also be considered.
Introduction
Patents and other intellectual property rights have territorial effect only. In order to monopolize more than one market for a product, patent protection with effect to each of these markets is required. International intellectual property (IP) strategies are thus necessary, especially in the therapeutic antibody industry because it has served a global market since its formation in the 1980s and is now facing considerable challenge by competitors. These strategies are frequently complex because of the many factors that must be considered, and the considerable resources, including labor and money, that need to be invested. Issues that are important for development of an international IP strategy that may provide meaningful IP protection at a reasonable cost-value ratio are discussed in this review.
Reasonable Use of Resources
Suitable IP protection comes at a substantial cost, but this protection is critical to gain access to revenues that can be considerable, particularly in the therapeutic antibody industry. While IP costs are a manageable issue for large pharmaceutical companies, they can become challenging for small firms and universities. For these organizations, each dollar spent on patents is one that cannot be spent for scientific staff, laboratory equipment, scientific apparatus or consumables. In all cases, a thorough cost-benefit analysis should be the basis for a filing strategy. Given their limited resources, small firms and universities must accept that blanket patent coverage will in most cases overstretch all available resources budgets, and thus restrict themselves to the strategically most important countries so called “braving the gap”-approach. Further, the decision regarding the countries in which to pursue patents should always be founded on knowledge of relevant facts.
General Filing Strategies
A general filing strategy usually involves a first patent application (“first filing”) that is filed in one member state of the Paris Convention of 1883, which is one of the first international treaties under which signing states undertake to grant juristic and natural persons from other signing states all the advantages that their respective laws grant to nationals. Most industrialized countries are member to the Paris Convention. One of the key advantages of the Paris Convention is that within a year after submitting a first filing in one member state, applicants gain the right of priority for second filings with effect to other member states, i.e., these second filings receive the priority date of the first filing.
Applicants can thus postpone costs for the patent prosecution in other countries by a year. Further, applicants can use the priority year to expand the informational basis on which further decisions with respect to the filing strategy can be based. For example, applicants may contact potential investors and test, or further develop, the subject matter of the respective invention, without creating prior art that may affect the patentability of the second filings.
In case the first filing has been made at, e.g., the European Patent Office (EPO), the German Patent and Trademark Office (GPTO) or the UK Intellectual Property Office (UKIPO), applicants will furthermore receive a search report within the priority year that provides preliminary information on questions of patentability. For first filings submitted as provisional applications before the US Patent and Trademark Office (USPTO), no such search report is issued.
However, due to formal provisions under US law known as the Hilmer doctrine,Citation1 it may also make sense to submit a first filing before the USPTO. Under the current law, a US patent application, which claims the priority of a foreign first filing only, will become prior art either 12 months or 30 months, depending on the circumstances, later than in case the priority of a US first filing has been claimed. This may result in a situation where the subject matter of the application will not be considered as prior art against a US patent application for similar, or even the same, subject matter filed by a third party in the meantime. In the latter case, the USPTO will institute interference proceedings and, as a result, the patent can get lost because the office came to the conclusion that the third party had invented first. With the recent enactment of the US patent law reform known as the “Leahy-Smith America Invents Act,” the Hilmer doctrine will become history for applications filed after March 13, 2013 and which do not claim priority of an application filed earlier. A first filing before the USPTO can however still make sense in order to take benefit from the grace period granted under US law for public disclosures of the invention made by the inventor within a year prior to the filing date.
Under these parameters, one potential strategy is to file two first filings on the same day, namely a provisional application before the USPTO, to avoid the Hilmer issue and/or take benefit from the grace period, and a non-provisional application, e.g., before the EPO, to obtain a meaningful search report in the priority year.
The Paris Convention system of priorities finds its advancement in a system known as the Patent Cooperation Treaty (PCT), which provides a unitary filing and searching system with effect to most industrialized and emerging markets. One of the largest benefits of the PCT system is that, from the date of first filing, the applicant has about 30 months to decide in which countries the patent application may be pursued. PCT applications can either be first or second filings. Similar to the Paris Convention system of priorities, the costs of filing national patent applications can thus be postponed for 30 months (or, in case the PCT application is a second filing, which claims the priority of an earlier patent application, by 18 months). This is a considerable benefit, particularly for small firms and universities that may want to maintain as many options as possible for as long as possible without spending too much money.
Different Standards for Patentability
The author has previously discussed in reference Citation2, that patent authorities in the US and Europe are in a process of harmonizing their interpretation in view of the novelty requirement and the inventive step requirement, at least with respect to drug compound patents. However, other requirements for patentability are still subject to large differences and uncertainties, particularly in the biotechnology field.
Member states of the World Trade Organization (WTO), including most European nations, the US and Japan, have signed the Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement, which aims at the harmonization of patentability standards and mutual acceptance of IP rights. However, Art. 27 (2) of TRIPS provides the option that member states may exclude from patentability inventions the commercial exploitation of which may affect issues of ordre public or morality.
Some countries have already established exclusions from patentability with respect to particular fields of biotechnology, or are about to do so. For example, Argentinia has excluded genetically engineered plants,Citation3 and the member states of the European Union (EU) have excluded human embryonic stem cells from patent protection.Citation4
For biopharmaceuticals, a recent case pending before the US Court of Appeals for the Federal Circuit (CAFC) had put in question the patentability of therapeutic proteins isolated from nature. In the Myriad Genetics case,Citation5 the first instance court of the Southern District of New York (SDNY) had ruled that patent claims related to genes taken from man, animals or plants are invalid because they relate to products of nature.
The decision was appealed before the CAFC, who on July 29, 2011 ruled that claims related to isolated DNA are patentable because the claimed molecules as such do not exist in nature. The Court found that claims reciting isolated DNA relate to molecules that have a distinctive chemical identity from molecules that exist in nature, because claimed isolated DNA molecules do not exist as in nature within a physical mixture to be purified, as they have to be chemically cleaved from their chemical combination with other genetic materials to which they are covalently bonded.
These arguments can be extrapolated to therapeutic antibodies directly isolated from man, at least if the latter undergo some kind of chemical modification, as well as to therapeutic antibodies which have been isolated by screening a naïve antibody library and therapeutic antibodies which are the product of a genetic engineering process. The recent decision has thus dispelled concerns about the non-patentability of therapeutic antibodies in the United States, although on the day of the editorial deadline of this volume of mAbs a request for a rehearing en banc at the CAFC, or even an appeal before the US Supreme Court could still be filed.
The case has fueled fears that therapeutic antibodies isolated from man would no longer be patentable, should the CAFC confirm the first instance decision.Citation6 The rationale applied by the first instance court, as well as the arguments set forth by the US Department of Justice in an amicus curiae brief submitted to the CAFC, could be considered to apply to other naturally-occurring substances, e.g., antibodies.
While the patentability of therapeutic antibodies as such is not an issue in Europe, the pending Myriad Genetics case created some uncertainty among the therapeutic antibody community in the United States. Should the first instance decision eventually be confirmed, one would have to analyze whether or not this also affects therapeutic antibodies as such, and whether or not differences can be made between, e.g., therapeutic antibodies directly isolated from individuals, therapeutic antibodies which have been isolated by screening a naïve antibody library, and therapeutic antibodies which are the product of a genetic engineering process.
The pending decision in mind it can however be ascertained that, to date, no major economy has definitely excluded therapeutic antibodies from patent protection.
Gradual Factors Affecting the Decision in which Country a Patent is to be Pursued
The decision process regarding the countries in which to pursue a patent includes a variety of inputs. One approach comprises a weighted analysis of market size, enforceability of IP rights, costs for patent prosecution and costs for IP litigation. These factors, which are referred to as “gradual factors” herein are summarized in and B.
Market Size
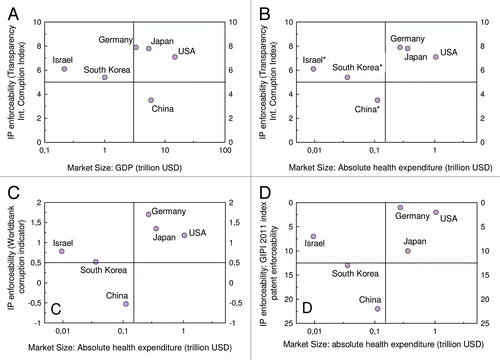
One of the most important reasons to pursue, or not, a patent in a given country is the size of its market. The bigger the latter is, the more sense it makes to pursue patent protection for that country to monopolize that market. A good measure for the size of a given market is gross domestic product (GDP), e.g., as published annually by the International Monetary Fund.Citation7 Particularly for patents relating to biopharmaceutics, this approach can further be refined by considering, also, the share of the GDP which is spent for public health. Such type of data are, for example, published by the United Nations Development Programme (UNDP).Citation8 See for an illustration of such approach. Note that the market size data refer to 2010, whereas the healthcare expenditure data refer to 2007, because the latter are the most actual data provided by the UNDP.
Enforceability of IP Rights
Another aspect that should be considered is the enforceability of IP rights in a given country. Large differences exist with respect to patent enforcement in different countries. Many of these differences have their origin in history. Predominantly in the US and the UK, common law systems were established, where case law is the predominant source of law, aspects of equity play a role and decisions are often taken by juries. In France and Germany the Napoleonic Code forms the basis of the law system, where judges have a more active role. The Japanese law system is essentially a hybrid of both.
Further, many important countries still have a considerable backlog demand when it comes to patent enforcement because courts have little legal or technical expertise with respect to patent or technical issues, or the legal system is inefficient, affected by corruption, or paralyzed by lengthy lawsuits. Further, in some countries local jurisprudence may be influenced by protectionism with respect to the domestic industry. While some countries have a notoriously bad reputation in this regard, most respective opinions rely on hearsay or personal experiences only. Regrettably, very often decisions in which enforceability of IP rights play a role are mainly based on such information, probably because reproducible data supporting these opinions are scarce.
One useful resource for information related to the enforceability of IP rights is the Special 301 Report published annually by the Office of the United States Trade Representative (USTR).Citation9 This report provides an annual review of the global state of intellectual property rights protection and enforcement in 77 countries, and comprises a watchlist of countries in which IP enforcement is considered to be suboptimal. Other reasons for a country to be included in the list may be so-called “indigenous innovation” policies or internet piracy. Another resource is the Corruption Perceptions Index as published annually by Transparency International.Citation10 While this resource reflects the general level of corruption in a given country, it is not particularly focused on IP enforcement. Nevertheless, this resource may be useful to obtain an insight into the overall state of the legal system in a given country.
Another useful resource is the Worldwide Governance Indicator (WGI) project run by the Worldbank, which publishes annual reports on Corruption control in 213 states.Citation11
Yet another comprehensive resource of information is the Global Intellectual Property Index (GIPI) published by the law firm Taylor Wessing,Citation12 which ranks the major economies, among others, according to different gradual factors related with patent prosecution and enforcement effectivity. The underlying data basis consists of results from a worldwide survey of IP owners, i.e., it reflects their opinions rather than hard data with respect to the above issues.
See for an overview of the different scores reflecting the enforceability of IP rights.
Costs for Patent Prosecution
Another gradual factor to consider is overall costs for patent prosecution, which varies substantially between different countries. Factors contributing to the overall costs are (i) official fees charged by the authorities, (ii) fees for attorney services and (iii) translation costs. To date, no reproducible comparative data exist for the average costs of patent prosecution in different countries. Some authors have tried to compare patent prosecution costs before the EPO, the USPTO and the JPO,Citation13 with the result that obtaining a patent in Europe is said to be twice as expensive as in the US, and three times as expensive as in Japan. According to the author's experience these studies suffer from some insufficiencies because patent attorney costs, which are usually much higher in the US than, for example, in Germany, have not been considered. Further, neither the waiver of patent translations in Europe under the London Agreement nor the cost-intensive translation of a patent specification into Japanese have been considered in this study.
Based on his own experience the author has thus categorized costs for patent prosecution (costs from filing until grant, including patent attorney costs and translations, but excluding drafting costs) on a nation-by-nation basis into three classes (3 = >7,500 USD, 2 = 5,000–7,500 USD, 1 = <5,000 USD). Basis for said assumption is a patent specification in English with 15 claims. See for an overview.
Furthermore, web-based software solutions are now also available. These tools provide cost prognoses for typical patent prosecution scenarios including the predictable costs, i.e., fees for translation, office fees, typical attorney fees and annuity fees. Although these tools can not predict costs caused through problems during prosecution (which can be significant particularly in antibody patents), they provide useful support for budget planning purposes.
Litigation Costs
Costs for patent litigation usually play a minor role for the decision in which country a patent should be pursued because only a fraction of the patents granted are later subject of a patent infringement trial. In case a patent is actually the subject of such trial, the protected subject matter is probably quite valuable, and thus a legitimate subject of patent protection in the respective country.
To date, patent litigation can only be done on a national basis. However, although patent infringement decisions made by a court in a given country have no prejudiciary effect on courts in other countries, they may prevent an infringer from continuing the infringement in other countries, either actively (by threat of legal proceedings) or passively (by reasonable anticipation of the infringer). It is thus not always necessary to sue an infringer in all countries where the infringement took place in order to bring the infringement to an end. This effect opens up the way for forum shopping also on the international stage, at least within certain restrictions.
The costs for patent litigation vary dramatically in the major economies. In a recent report,Citation14 patent litigation costs for first and second instance were estimated for Germany (140,000–444,000 €), UK (300,000-2,500,000 €), France (90,000-350,000 €), Netherlands (100,000-350,000 €) and the US (420,000 €). These figures are subject to high variances, particularly in the US, where other authors quote patent litigation costs of between $3,000,000 and $10,000,000,Citation15 particularly in fields of high technological complexity or high case values as it is the case for therapeutic antibodies. Under consideration of these uncertainties, the costs for patent litigation should only play a minor role in the decision in which country a patent should be pursued. See for an overview.
Non-Gradual Factors Affecting the Decision in which Country a Patent is to be Pursued
In addition to the gradual factors discussed above, which can be the subject of a weighted analysis, non-gradual factors exist which also affect the decision in which country a patent is pursued. These factors will be discussed in the following.
Countries with an Active Biosimilar Industry
One such non-gradual factor affecting the decision regarding in which country a patent is pursued is whether or not the said country has an active biosimilar industry. Biosimilars, or follow-on biologics, are versions of innovator biologics following patent and exclusivity expiry of the latter. In many cases, biosimilars are brought to the market in countries without patent protection at a time when the innovator biologic is still under protection in the major markets, like Europe or the USA. By this means, biosimilar manufacturers can test market responses for their product at a very early stage, which facilitates the entry into the major markets once patent protection has expired.
In order to close this gap, innovators may want to apply for patent protection in countries with a capable biosimilar industry, even when the market size alone does not seem to justify patent protection in this country (see for an overview). Countries like Israel (Teva), Korea (Abxis, Celltrion) and Hungary (Gedeon Richter) have an active biosimilar industry combined with relatively small market size, and may therefore fall into this category. shows two examples of antibody biosimilars already on the market.
Product Protection and Protection of Enabling Methods
According to an oft-cited rule, patent protection should be pursued in countries where the markets for a product are, in case a given patent deals with a product, e.g., a therapeutic antibody. If, however, a patent deals with an enabling method, patent protection should be achieved in countries where the industry is present. While this rule had, and still has, its merits, quite a few exceptions apply today, the most important being caused by (i) privileges related to screening technologies, (ii) requirements in case a license strategy is pursued and (iii) the reimportation problem in the European Union.
Privileges Related to Screening Technologies
Most patent systems grant claims that are related to methods or processes for the production of matter. This includes, in most cases, that the products so made are protected as well by such method patent, e.g., 35 U.S.C. §271 (g), Art. 64 (2) EPC.16
In contrast thereto, a product that has been found with a patented screening method (e.g., a phage display method) is not protected by said patent, because, for screening methods, no such provision as set forth by 35 U.S.C. §271 (g) or Art. 64 (2) EPC for methods of production exists. While owners of screening techniques often try to incorporate so-called “reach-through claims” (i.e., claims that seek to protect embodiments that have not yet been discovered by the applicant, but which might be discovered in the future by third parties using the patented method), such claims are usually deemed unpatentable at least in Europe,Citation17 and probably in the United States as well.Citation18 This is mainly due to the fact that a screening method is not a method of production in the above sense. Hence, in the case where a third party imports an antibody that was discovered with a protected screening method, this will, at least in the US, be considered as a mere “import of information” only, so that the respective import ban provision set forth in 35 U.S.C. §271 (g) is held not infringed.Citation19 gives an overview of the cases discussed above. However, other constellations with different outcome might exist as well.
The fact that patents relating to methods of screening do not extend their protection on antibodies found with such methods has encouraged the use of antibody screening methods in countries without patent protection. For example, it seems that many patents related to phage display techniques have not been put into force in Norway, which signed the European patent convention only on January 1, 2008 (). This is one of the reasons that Norway-based company Affitech claims that they have full freedom to operate to make use of third party phage display techniques protected elsewhere.Citation20
Requirements in Case of a License Strategy
Another non-gradual factor that may affect the decision in which country a patent is pursued is the strategy according to which the patentee wants to generate income. In a license strategy, royalties may be the only source of income. Anti-trust laws that apply in the major markets demand that a patent must exist in order to grant licenses to third parties; therefore a patent is a must in each country where the patentee wants to generate income. In a production strategy, i.e., when the patentee wants to produce or sell a patent-protected antibody by himself, the patentee can also obtain income in those countries where no patent protection exists because a patent is not absolutely necessary for the mere production and sale of the antibody.
Notably, a patent strategy that neglects countries with small markets, or poor IP enforcement, but where income shall be generated nonetheless, can make sense because in many cases it does not make sense for competitors to develop and market their own antibody for these small markets only.
The Reimportation Problem in the EU
According to the principle of exhaustion, products that have been sold with consent of the patentee in a given market are no longer the subject of patent protection. Europe has maneuvered itself into a situation in which the territories in which patent protection is enforced are not congruent to the market in which the principle of exhaustion applies. This has generated a situation that is the reason for problems of reimportation.
While European patents are granted under a centralized system, they immediately disassemble, after being granted, into national patents that must be validated before the national authorities. Although validation is a merely formal act, it may require the provision of a translation into the national language, and is thus associated with considerable costs. Patentees may thus consider validating patents only in countries where either the market or the industry is present, and ignore smaller EP member states.
However, the internal market that is decisive for the principle of exhaustion is the European Economic Area (EEA),21 which encompasses all member states of the European Union plus Norway and Iceland.
This means that, if a therapeutic antibody is, for example, protected by a European patent that has been validated in Germany, France and the United Kingdom, but not in Italy, a therapeutic antibody sold by the patentee in Italy can be reimported, e.g., to Germany, under the privilege of exhaustion because it has already been sold once with consent of the patentee within the borders of the internal market of the EEA. Such reimportation makes sense in the case a therapeutic antibody is sold in one EEA member state at a lower price, or in the case of re-packaging a therapeutic antibody from a bulk pack sold at a volume discount. Reimportation has been a common phenomenon with small molecular drugs, and it seems likely that it will also become an issue with therapeutic antibodies. Patent protection in all EEA member states does not circumvent reimportation (which is politically desired by the European Commission), but it can at least ensure that patentees can sell their drugs at profitable prices in all EEA member states.
The “production strategy” set forth above, according to which a patentee may ignore countries with small markets, or poor IP enforcement, and relies on the super-territorial effect of patents in neighboring countries has thus only limited use in Europe, at least in the pharmaceutical industry. However, due to recent developments in Europe under the London Agreement, which came into force in May 2008 and under which the signing states agreed to no longer require translations of European Patents, or to require only a translation of the claims (), the costs for validating European patents are decreasing. Therefore, blanket patent coverage in all member states of the EEA has become a realistic and affordable option even for small and medium sized enterprises. Future developments, including the enhanced cooperation pathway, and the community patent, will further reduce costs to obtain blanket patent coverage in Europe.
Two- or Higher Dimensional Weighted Analysis
The above mentioned gradual factors can be subjected to a weighted analysis to decide if a patent is pursued in a given country or not. In a classical example of a two-dimensional weighted analysis, the market size of the countries of interest is plotted against IP enforceability, as for example shown in . The resulting graph has four quadrants, in which the upper right quadrant comprises countries where IP protection is mandative, while the lower left quadrant comprises countries where IP protection is not absolutely necessary. The lower right quadrant comprises countries where IP protection should be considered in case of a license strategy, while the upper left quadrant comprises countries where IP protection should be considered when the receptive market is, despite relatively small size, a key market.
In , the market size of some exemplary countries was plotted against IP enforceability. In , the 2010 GDP of China, Germany, Israel, Japan, South Korea and the USA according to a survey provided by the International Monetary FundCitation7 has been plotted against the Corruption Index provided by Transparency International.Citation10 shows a similar plot, with the exception that the GDP data have been replaced by total health expenditure, as calculated by the GDP multiplied by the percentage of GDP for health expenditure. Please note that for this plot, GDP data from 2010 have been multiplied with health expenditure data from 2007, because the latter are the most actual data provided by the UNDP.Citation8 Although technically incorrect, this approach is sufficient for practical purposes.
further comprises asterisks for those countries which are not in the upper right quadrant, i.e., which, according to the above mentioned model, do not qualify as mandatory for patent protection due to small market size and/or poor IP enforceability. These asterisks indicate specific non-gradual factors that further affect said decision. Israel and Korea have, despite their relatively small markets, an active biosimilar industry. China is currently undergoing an impressive process of increasing the enforceability of IP rights. In a strategy that takes future developments within the patent lifetime of a patent to be filed into account, China, will probably obtain a better score with respect to IP enforceability in the near future.
In , IP enforceability has been expressed by means of the Worldbank corruption indicator,Citation11 whereas in , the GIPI 2011 indexCitation12 has been chosen as a measure for IP enforceability.
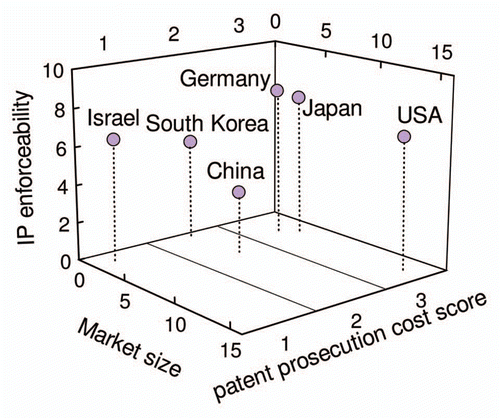
shows a three-dimensional plot in which, next to market size and IP enforceability as shown in , patent prosecution costs according to author's costs score have been added. Interestingly, the countries for which patent protection has been considered mandatory according to the weighted analysis as shown in are those in which patent prosecution is most expensive (Japan, USA and Germany, see ), while the remaining countries (Israel, South Korea, China) are less expensive.
Future Developments
Applicants should be aware that patents have a lifetime of 20 years in most countries—a period in which many things can happen. The market size of a given country may substantially shrink, or grow, or a country may sign an international contract that affects IP enforceability, or integrate said country into a free trade area where a common principle of exhaustion applies. Therefore, any considerations should not only reflect the status quo, but also anticipate the probable developments in the next 20 years.
Practical Examples
International IP strategies for different antibodies or antibody mimetics manufactured by either big pharmaceutical companies or biotechnology companies are shown in . Data have been taken from the FamPat database as of June 1, 2011. Some of these biotechnology companies have entered into agreements with pharmaceutical companies. It is clearly visible, from the table, that patents protecting antibodies or antibody mimetics that are backed by a pharmaceutical company are usually prosecuted in a relatively large number of countries (e.g, Infliximab, Johnson & Johnson), while patents protecting antibodies or antibody mimetics being marketed by biotechnology companies are usually prosecuted in a smaller number of countries. As can be seen, the minimum goal encompasses the US, Europe, Canada, Australia, Japan and (with one exception) China, while particularly biotechnology companies tend to treat other countries as optional, probably due to restricted resources, and let the actual budget decide.
Conclusion
The factors discussed here may help to provide a sound informational basis on which the decision where a patent protecting a given antibody should be pursued can be based. While the most important factor is the size of a given market, other factors should also be considered. At the same time, given their limited resources, small firms and universities should be prepared to apply some kind of “braving the gap” approach because blanket patent coverage could potentially exhaust their financial resources.
Disclaimer
The information provided herein reflect the personal views and considerations of the author. They do not represent legal counsel and should not be attributed to Michalski-Hüttermann & Partner Patent Attorneys or to any of its clients. Patent numbers and patent lifetimes have been verified with utmost care, but no liability is taken for their correctness.
Figures and Tables
Figure 1 Market size plotted vs. IP enforceability as a model for a two-dimensional weighted analysis.
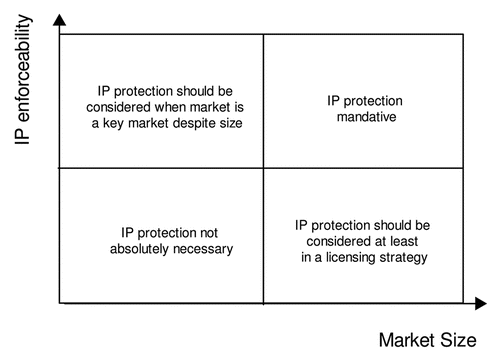
Table 1A Country scores with respect to market size and IP enforceability
Table 1B Other factors affecting the decision where to pursue a patent application
Table 2 Two examples of already existing antibody biosimilars
Table 3 Different infringement-upon-import constellations
Table 4 Key patents protecting phage display which have not been put into force in Norway
Table 5 EP member states which have waived translation requirements under the London agreement
Table 6 international IP strategies for different antibodies, or antibody mimetics data taken from the FamPat database, data of June 1, 2011
References
- In re Hilmer 359 F.2d 859 (CCPA 1966)
- Storz U. Intellectual property protection: Strategies for antibody inventions. mAbs 2011; 3:310 - 317
- Arts. 6 + 7 of the Argentine Patent Act and Argentine Guidelines for Examination of Patent application, Part C, Chapter IV, 2.1.7
- Decision of the European Court of Justice in case C-34/10 of March 10, 2011 Available from: http://curia.europa.eu
- Association for Molecular Pathology v. United States PTO, 2010 US Dist. LEXIS 35,418 (S.D.N.Y. Apr 2, 2010)
- Russo A, Vira C. The fate of biological patents: a Myriad analysis. Intellectual Property Magazine 2011; 58 - 60
- International Monetary Fund, World Economic Outlook Database, April 2011 Available from: http://www.imf.org/external/ns/cs.aspx?id=29
- UNDP database of international human development indicators, Expenditure on public health Available from: http://hdrstats.undp.org/en/tables/default.html
- Special 301 Report published annually by the Office of the United States Trade Representative Available from: www.ustr.gov/webfm_send/2841
- Corruption Perceptions Index as published annually by Transparency International Available from: www.transparency.org/policy_research/surveys_indices/cpi
- Worldwide Governance Indicators (WGI) project run by the Worldbank Available from: http://info.worldbank.org/governance/wgi/mc_countries.asp
- Global Intellectual Property Index—The Third Report Published 2011. Available from: www.taylorwessing.com/ipindex/index.php?getfile=global_ip_index_3
- van Pottelsberghe de la Potterie B, François B. The cost factor in patent systems, Working Papers CEB (2006) No 06-002.RS, Universite Libre de Bruxelles (http://ideas.repec.org/p/sol/wpaper/06-002.html)
- Mejer Malwina, Van Pottelsberghe de la Potterie B. Economic Incongruities in the European Patent System (January 2009) CEPR Discussion Paper No. DP7142. Available from: http://ssrn.com/abstract=1345668
- Hsieh S. More patent cases are being taken on contingency fee basis. Lawyers USA August 14, 2006
- U.S.C. §271 (g). Whoever without authority imports into the United States or offers to sell, sells or uses within the United States a product which is made by a process patented in the United States shall be liable as an infringer, if the importation, offer to sell, sale, or use of the product occurs during the term of such process patent […]. Art 64 (2) EPC: If the subject-matter of the European patent is a process, the protection conferred by the patent shall extend to the products directly obtained by such process
- EPO Technical Board decision EPO decision T669/04, 2005, EPO Board of appeal decisions database No T669/04
- Univ. of Rochester v. G.D. Searle & Co. 358 F.3d 916 2004 US App. LEXIS. 2458 (Fed. Cir., 2004)
- Bayer AG. Housey Pharmaceuticals, Inc. 2003 US App. Lexis 17453 (Fed. Cir. Aug. 22, 2003)
- Aldridge S. Affitech Exploits Antibody Discovery Platforms. Genetic Engineering & Biotechnology News 2008; 28