Abstract
This review covers highlights of the author’s experience becoming and working as a plasmid biologist. The account chronicles a progression from studies of ColE1 DNA in Escherichia coli to Gram-positive bacteria with an emphasis on conjugation in enterococci. It deals with gene amplification, conjugative transposons and sex pheromones in the context of bacterial antibiotic resistance.
When I was invited to write a review relating to mobile genetic elements based on the work of my own research group over the years, it struck me that I must be getting close to the end of the “program”. In any case, the opportunity to write a first person account covering much of my professional career sounded like something I should do, as it would not only be a chance to look back and tie things together but also give credit to some key associates. Thus, it goes as follows.
After completing my graduate research in biochemistry at Indiana University (the Medical Center in Indianapolis), working with a mouse leukemia model under the mentorship of Ed Hodes, I was anxious to get some experience in the new area being referred to as “molecular biology”. In those days (the mid 1960s) the term molecular biology related mainly to work with DNA and gene expression, and most of the research was being conducted with bacteria, especially Escherichia coli and their viruses. Although having no experience working with microorganisms, it was clear that the ability to do experiments with cells that divided every 30 minutes or so meant that enormous amounts of data could be gathered in short periods of time. In as much as I was working with an animal model that relied on an assay that took 6 months, the ability to conduct experiments that generated data within a few days was appealing, to say the least. Through a fortuitous series of events I became aware of some interesting papers dealing with the characterization of certain newly identified genetic elements called “plasmids”. The fact that plasmids could move from one bacterial cell to another and also play key roles in the emergence and spread of antibiotic resistance was quite intriguing. I was excited to gain acceptance into a postdoctoral position with Donald Helinski at the University of California, San Diego supported by an NIH National Cancer Institute fellowship. Don was one of only a few individuals studying the molecular nature of bacterial plasmids, and the opportunity to join his lab at that particular time in the history of plasmid biology was something for which I have always felt very lucky. Today a huge number of plasmid biologists around the globe got their start or have spent time in Don's lab as students, postdocs or visiting scientists—or are among multiple generations of “descendants” of those individuals. For an informative review of some important history of plasmid biology by Don Helinski see reference Citation1.
La Jolla
When I arrived in La Jolla in July of 1967, the activity in the Helinski lab centered heavily around the recently identified colicinogenic factor ColE1, a 6.2 kb circular plasmid in E. coli present at about 24 copies per cell.Citation2,Citation3 ColE1 was not conjugative but could be readily mobilized in trans to appropriate recipient bacteria by co-resident conjugative elements such as the sex factor F. Plasmid isolation was a relatively laborious process then and included the use of cesium chloride (CsCl) buoyant density equilibrium centrifugation. Physical resolution and plasmid enrichment made use of a Proteus mirabilis host from which ColE1 could be distinguished from the bacterial chromosome on the basis of buoyant density.Citation4 It was around that time (1967) that a report out of Jerry Vinograd's lab at Cal Tech introduced a simplified method for separating covalently closed (supercoiled) circular DNA of mitochondria and the animal virus SV40 from linear (chromosomal) DNA by including ethidium bromide, an intercalating dye, in CsCl centrifugations.Citation5 The method was readily applicable to the isolation of plasmid DNA directly from E. coli lysates.Citation2 The DNA could then be analyzed by sucrose density gradient sedimentation or electron microscopy.
My initial research efforts were aimed at trying to isolate ColE1 DNA-protein complexes with the notion that bound protein(s) might reflect a metabolically significant interaction. The first challenge was to come up with a lysis technique that would preserve both DNA and protein structure and allow detection of plasmid-protein complexes in the midst of an expected large amount of chromosome-protein-membranous material. After many unsuccessful efforts I ended up making use of a technique that had recently been reported by Godson and SinsheimerCitation6 that utilized a detergent mixture of Brij 58 (a neutral detergent) and deoxycholate for lysis of phage-infected E. coli. During a relatively low-speed centrifugation step, the vast majority of chromosomal DNA pelleted leaving the supernatant greatly enriched in plasmid DNA. Sucrose gradient analyses of these “cleared lysates” revealed a ColE1-protein complex sedimenting slightly faster (24S) than the 23S protein-free supercoiled DNA. Surprisingly, when the 24S complex was exposed to conditions expected to affect protein structure such as proteases, strong ionic detergents or heat, the 24S complex converted to a 17S relaxed (open circular) configuration. The relaxation event involved the introduction of a strand-specific nick in the supercoiled plasmid; we therefore called the 24S entity a “relaxation complex”.Citation7,Citation8 The percentage of plasmid DNA that was present in the complexed state varied from less than 30%, when glucose was in the growth medium, to more than 80% when glucose was absent.Citation9
At the time, the significance of relaxation complexes was not clear, although we had been thinking in terms of a possible connection with replication (e.g., a requirement for nicking in order to allow separation of two newly replicated daughter molecules). I was able to demonstrate that other plasmids such as ColE2, ColE3 and the colicinogenic sex factor ColIb-P9 [a much larger (93 kb) element] could also be isolated as relaxation complexes.Citation10,Citation11 Later the Helinski groupCitation12 was able to identify three ColE1-associated proteins, one of which (at 60,000 daltons) ended up covalently associated with the 5′ end of the specifically cleaved strand; and genetic analyses in the laboratories of Joe InselbergCitation13 and David SherrattCitation14 demonstrated that the nicking event related to mobilization. Analogous DNA-protein interactions were eventually found in many plasmid systems, although it was not always possible to observe “relaxable” complexes in vitro like those involving ColE1. Circular, conjugative (or mobilizable) plasmids in general are now believed to exist in vivo as membrane-bound “relaxosomes”, with a specific nicking event being the first step in the transfer of a single strand of DNA from donor to recipient cells.Citation15,Citation16 Little did I know at the time that the phenomenon of conjugation would become a primary theme in my future research career.
During my investigation of the ColE1 relaxation complex I was curious about effects that might occur if the cells were grown for a period of time in the presence of chloramphenicol (CM) (an inhibitor of protein synthesis). Interestingly, plasmid DNA continued to replicate for several hours after synthesis of chromosomal DNA had come to a halt. Indeed, newly synthesized non-complexed ColE1 DNA accumulated at an increased rate for a number of hours, whereas the amount of relaxation complex remained constant. The protein of the complex appeared stable while randomly separating from and re-associating with plasmid DNA.Citation9 There was no apparent need for specific replication proteins to be further synthesized in order for plasmid DNA to continue to replicate—a view consistent with other data in the Helinski lab in both E. coliCitation17 and P. mirabilisCitation18 hosts; however, the accelerated rate of appearance of new plasmid DNA was a major surprise.
Ann Arbor
In 1970 I was happy to be offered a faculty position at the University of Michigan with a primary appointment in the School of Dentistry and a joint appointment in Microbiology in the Medical School. The Microbiology Department (now Microbiology and Immunology) provided me with purpose and incentive to learn some general microbiology via an involvement in various teaching commitments; it also provided opportunities to recruit graduate students. My association with the School of Dentistry (Oral Biology Department as well as a newly established “Dental Research Institute” at that time) proved to be particularly valuable as it caused me to think about bacteria in the oral cavity and dental plaque and ended up playing a significant role in my future research.
As to my initial research efforts, Don Helinski had graciously allowed me to take to Michigan the project relating to chloramphenicol replication of ColE1. I was interested in the possibilities it offered as a system for investigating inhibitors of DNA replication. The intent was to first optimize the system and then use it to screen the effects of a variety of chemical compounds. It turned out that some types of media were much better than others. One particular medium (“Casamino Acids” and glucose in a phosphate buffer) resulted in ColE1 replication (in chloramphenicol) accelerating to 8 times its normal rate with copy number increasing to approximately 3,000 per cell within 15 hours—a 125 fold increase over the normal 24 copies per cell.Citation19 Another protein synthesis inhibitor, puromycin, resulted in essentially identical data; whereas tetracycline and streptomycin exhibited significant but lesser amounts of extended plasmid replication.Citation20
Upon screening of a variety of compounds for their effects on ColE1 replication after exposure to CM for several hours, we were surprised to see that very low levels of rifampicin (0.1 µg/ml) caused a dramatic inhibition.Citation21 It was unexpected because rifampicin was known to block the initiation of RNA synthesis and was not known to directly affect DNA replication. Further analyses showed that RNA polymerase was directly involved.Citation21 Other inhibitors of RNA synthesis such as streptolydigin and actinomycin D also exhibited inhibitory effects although to a much lesser extent.Citation20 We hypothesized that RNA might be utilized as a primer for DNA synthesis, a view supported by the following. First, ColE1 had recently been shown by Kingsbury and HelinskiCitation22 to be dependent on the host DNA polymerase I, an enzyme requiring a primer with a 3′ OH group; and second, a recent report by Verma et al.Citation23 had shown that short RNA fragments could serve as primers for DNA polymerase in vitro. I had maintained a close and productive interaction with the Helinski lab regarding our progress, and members of Don's group were able to show that a significant percentage of ColE1 molecules that had accumulated during extensive exposure to CM contained a small segment of RNA in one strand of the covalently closed molecules. This was based on the fact that RNase or alkali would “open” one of the two DNA strands; and the accumulation of such sensitive molecules was blocked by rifampicin.Citation24 It appeared that during synthesis of plasmid DNA for many hours in the presence of CM, the ability to remove primer RNA by normal repair processes became inefficient—perhaps related to instability of the 3′ exonuclease activity of DNA polymerase I.
Evidence for the involvement of RNA in the priming of DNA synthesis was also beginning to come out of Arthur Kornberg's laboratory about the same time with regard to the E. coli bacteriophages M13 and φX174.Citation25,Citation26 There were also reports related to replication of lambda phage DNACitation27 as well as the E. coli chromosome.Citation28 Soon the involvement of RNA in priming DNA synthesis was evident in many biological systems. Details of its role in the case of ColE1 were subsequently characterized (see ref. Citation29 and Citation30).
New Territory
Although my first few years at Michigan had gone well, it was becoming apparent that ColE1 was generating significant interest in a number of other laboratories. Its small size and the ease with which it could be isolated and analyzed had made it an attractive system for study. Since my own research group was quite small, I was becoming concerned about how long we could remain competitive. I was happy with what we were accomplishing, and two of my papers even became widely cited for methodology relating to plasmid isolationCitation7 and plasmid amplificationCitation19 in the context of the new and rapid development of recombinant DNA technology using ColE1-related vectors. However, it seemed prudent at that time to broaden our research interests, and I began to think about what other types of bacteria might have to offer with regard to their extrachromosomal content.
Extensive conversations with a clinical microbiologist in the Dental School, Walter Loesche, had familiarized me with organisms in the oral cavity such as Streptococcus mutans, which were being identified as primary culprits in dental caries, and Streptococcus sanguis, a commensal organism associated with dental plaque. Since nothing was known about the plasmid content of these as well as most other Gram-positive bacteria (exceptions were Staphylococcus aureus and Streptomyces) we decided to have a look. I had recently hired an undergraduate student, Gary Dunny, as a part-time glassware washer. Gary soon became interested in what was going on in the lab and got involved in a search for plasmid DNA in S. mutans. After screening numerous strains, he was able to identify a small cryptic plasmid in a strain known as LM7.Citation31 Another undergraduate student, Art Franke identified an erythromycin resistance plasmid in Streptococcus pyogenes, an important human pathogen.Citation32 And we eventually found plasmids also in Streptococcus faecalisCitation33 and S. sanguis.Citation34 As discussed below there was much to get excited about regarding where some of these new findings would take us, and within a few years our work with ColE1 came to an end. We were off and running, and streptococci appeared to be our game.
S. faecalis (later renamed Enterococcus faecalis) turned out to have some particularly attractive properties. Although somewhat fastidious, it had a number of features similar to E. coli: (1) it could divide almost as fast; (2) it inhabited the human gut at similar levels; (3) it was commonly involved in nosocomial infections; (4) multiple antibiotic resistance was common; and (5) it was loaded with plasmids. S. faecalis could often be identified in the oral cavity as well, and a few strains had even been reported to cause tooth decay in rats.Citation35 They were occasionally associated with acute periodontal disease and even with root canal infections (reviewed in ref. Citation36). We began to obtain a number of these strains and found plasmids in most of them. One limiting aspect at first was the absence of an efficient approach to doing genetic studies; however, this changed dramatically with reports by Tomura et al.Citation37 in Japan and Jacob and HobbsCitation38 in the UK demonstrating conjugation. This was somewhat unexpected, due to a common belief in those days that conjugation was essentially a Gram-negative behavior (Streptomyces being the exception). We were quickly able to confirm the phenomenon, and it soon became evident that plasmid-related conjugation was widespread in S. faecalis.Citation39 Indeed, by the late 1970s conjugative plasmids in a variety of streptococcal species were being reported; and today the phenomenon is known to be widespread among Gram-positive bacteria.Citation40,Citation41
S. faecalis DS5 and the Enterococcal Plunge
S. faecalis DS5 was a clinical isolate we obtained from the American Type Culture Collection and renamed; it was originally identified in a hospital in Miami, Florida. We found that it contained several plasmids: a small multicopy plasmid pAMα1 (9.7 kb) encoding tetracycline (Tc) resistance; a 28 kb plasmid pAMβ1 encoding erythromycin (Em) resistance; and a relatively large plasmid pAMγ1 (60 kb) encoding hemolysin and bacteriocin production.Citation33,Citation39 In broth matings pAMγ1 was observed to be conjugative and was able to efficiently mobilize in trans the otherwise non-conjugative pAMα1. The pAMβ1 plasmid also turned out to be conjugative and to have quite a broad host-range among Gram-positive bacteria; although we did not initially observe its transfer from DS5 due to a specific inhibition by the co-resident pAMγ1.Citation40–Citation42 We were eventually able to demonstrate that DS5 harbored two additional plasmids, pAMγ2 and pAMγ3, which earlier had been “masked” in sucrose-density gradient analyses due to their similarity in size to pAMγ1.Citation42 It was around this time (mid to late 70s) that plasmid research was becoming greatly aided by the high-resolution power offered by the newly applied use of agarose gel electrophoresis together with recently discovered restriction endonucleases.
pAMα1 and Amplification of Tc Resistance
The tetracycline resistance of pAMα1 identified in the DS5 strain exhibited an interesting behavior in that cells that had been cultured in the presence of Tc for extended periods of times (e.g., several subcultures) gave rise to enlarged circular DNA forms, a phenomenon that was accompanied by an increase in the level of Tc-resistance.Citation43 If such cells were subsequently grown in the absence of Tc there was a return to the original state. The behavior suggested a gene amplification and resembled some work being reported from Bob Rownd's laboratory at the University of Wisconsin dealing with a relatively large and more complex multiple resistance plasmid NR1 in Proteus mirabilis.Citation44,Citation45 Because of its small size, the pAMα1 system offered significant technical advantages for studying such a phenomenon. Yoshihiko Yagi, my first graduate student, ended up doing his Ph.D. dissertation research on characterizing the amplification process. Yoshi was able to demonstrate that growth in the presence of Tc resulted in the generation of multiple tandem repeats of a 4.1-kb segment of the 9.7 kb plasmid.Citation46 The amplification involved a series of recombination events between directly repeated sequences designated RS1 and RS2 (∼380 bp) that flanked the amplifiable DNA segment (). Identification of the direct repeats was based on a DNA-heteroduplex technique using electron microscopy, as we were not in a position to do any DNA sequencing in those days.Citation47 The amplification phenomenon was significantly reduced if the host was deficient in homologous recombination; and models involving three different recombination patterns, not mutually exclusive, were offered to explain the generation of the tandem repeats.Citation47,Citation48
Perkins and Youngman at Harvard later showed that pAMα1 was actually a cointegrate of two plasmids, which they designated pAMα1Δ1 (4.6 kb) and pAMα1Δ2 (5.15 kb).Citation49 pAMα1Δ1, believed not to replicate independently in S. faecalis, replicated efficiently in Bacillus subtilis; indeed it closely resembled pBC16 and other Bacillus plasmids, as well as pUB110 in S. aureus. It was apparent that the formation of the composite making up pAMα1 involved a recombination event between homologous sequences on the two plasmids and that these sequences corresponded to the same ones involved in the amplification mechanism (). Years later when we obtained the nucleotide sequence of pAMα1 Vicky Francia, a very talented postdoc in the lab, was able to show that the RS1 and RS2 sequences were actually only 57% identical and corresponded to functional transfer origins (oriT sites) that could be acted upon by putative relaxases representing either MobB encoded on one member (pAMα1Δ2) of the cointegrate or MobE encoded on the other.Citation50 Vicky showed that the rate-limiting step in the amplification process involved a site-specific recombination between RS1 and RS2 facilitated by MobB or MobE. The recombination could involve two separate plasmid copies or could occur within a partially replicated plasmid. Alternatively, it could involve a relaxase-catalyzed excision of a segment (pAMα1Δ1) containing the tet determinant, which is then inserted into another pAMα1 copy (although not required, the latter step could easily involve homologous recombination).Citation47,Citation48 Subsequent steps in the amplification process would not require the site-specific event, since the added homology arising from the first tandem duplication would allow them to occur easily via homologous recombination.
pAMα1 was one of the first systems to really launch us into streptococcal research in the sense that we were able to begin doing some real hypothesis-driven investigations. In addition to the amplification studies, postdoc Daphna Oliver showed that pAMα1 was useful in allowing us to identify, and to some degree characterize, a number of conjugative plasmids based on their ability to mobilize the Tc-resistance trait.Citation51 This was particularly valuable in cases where a bacterial host harbored multiple conjugative plasmids.Citation42,Citation51,Citation52
S. faecalis DS16 and a Wealth of Interesting Genetic Elements
A strain that we obtained from St. Joseph's Mercy Hospital in Ann Arbor proved to become important to our research efforts at Michigan for years to come. It was one of many strains that we had been screening for the presence of plasmid DNA. S. faecalis DS16 was of interest initially because it was both hemolytic and resistant to four antibiotics: erythromycin (Em), streptomycin (Sm), kanamycin (Km) and Tc. It harbored two low-copy number plasmids: pAD1, a 60 kb highly conjugative element encoding both hemolysin and bacteriocin activity; and pAD2, a 28 kb nonconjugative plasmid encoding resistance to Em, Sm and Km.Citation53,Citation54 The Em-resistance determinant (erm) of pAD2 was located on a transposon that we designated Tn917. The Tc-resistance determinant (tet) resided on the bacterial chromosome where it was present on a transposon we subsequently called Tn916. Transposition was detected via mating experiments using DS16 as a donor and selecting for Em- or Tc-resistant transconjugants. The latter harbored pAD1 derivatives containing insertions of Tn917 or Tn916, respectively.Citation55,Citation56 Insertions appeared at many locations on the plasmid, and analyses using restriction enzymes and agarose gel electrophoresis showed that Tn917 had a size of 5 kb and Tn916 was 18 kb. The two transposons were a welcomed find, as they became useful tools at a time when the ability to do genetic analyses in S. faecalis was quite limited. Tn917 exhibited an interesting behavior whereby postdoc Paul Tomich showed that exposure to low levels of Em upregulated transposition by an order of magnitude.Citation56 As shown later by graduate student Jay Shaw, this appeared related to transcriptional read-though of an inducible erm determinant into adjacent down-stream transposition genes.Citation57 Sequence determination showed that Tn917 encoded a transposase and resolvase characteristic of the Tn3-family of transposons of Gram-negative bacteria.Citation57,Citation58 As for Tn916, this element had some rather unique properties, which will be discussed later.
With regard to pAD1 it is important to note that hemolysin-production was not unusual among strains of S. faecalis. Its expression was often associated with a bacteriocin activity, and there had been reports that the two activities were related.Citation59,Citation60 This was the case for the pAD1 hemolysin/bacteriocin as well, and the dual activity is now referred to as cytolysin. pAD1 per se proved to be a member of a widely disseminated family of conjugative, cytolysin-encoding plasmids, especially those associated with opportunistic enterococcal infections in humans.Citation42,Citation61–Citation64 Interestingly, pAD1 was essentially indistinguishable from the pAMγ1 plasmid of strain DS5 discussed earlier in reference Citation42. The cytolysin activity of pAD1 was soon found to enhance pathogenicity in animal infection models, and S. faecalis strains associated with human parenteral infections were found more likely to be hemolytic than fecal isolates.Citation65–Citation72 Mike Gilmore, who spent several months in my laboratory in the mid 1980s, went on to beautifully characterize the pAD1 cytolysin-related genes and their regulation when he took a position at the University of Oklahoma.Citation73
Enterococcus faecalis
For many years S. faecalis along with Streptococcus faecium had been loosely referred to as enterococci because of their common location in the intestines.Citation74 In 1984 Schleifer and Kilpper-Balz formally proposed the genus Enterococcus, based on significant genomic differences from streptococci.Citation75 At the time this was a little disheartening, since we were just getting used to thinking of ourselves as a “Streptococcus lab” and Don LeBlanc and I had recently organized and conducted an American Society for Microbiology (ASM) International Conference on Streptococcal Genetics in Sarasota, Florida. It had been nice being associated with a group of organisms believed to be involved with more different human illnesses than any other genus, a point we liked to think went over well with granting agencies. It took us a while to get used to the new name, but the notoriety that was being acquired by enterococci due to their increasing involvement in nosocomial infections and multiple antibiotic resistance (especially to vancomycin) helped us continue to make a good case for funding. Future ASM conferences (held every 4–5 years) were kind in allowing us to meet with them under the topic “Genetics of Streptococci, Enterococci and Lactococci.” The Lactococcus people were in the same boat, having also recently been separated from Streptococcus. In 2000 the first ASM international conference strictly on Enterococci took place in Banff, Canada. Organized by Mike Gilmore, it was highly successful and brought together many Enterococcus researchers who had never previously interacted on a personal level. For the remainder of this presentation I will use the genus name Enterococcus: S. faecalis DS16 and S. faecalis DS5 will, respectively, be E. faecalis DS16 and E. faecalis DS5, etc.
The Tn916 Surprise
Our initial studies on E. faecalis DS16 relating to the Tc-resistance element Tn916 had revealed some unexpected results. Experiments using DS16 as a donor had shown that the chromosome-borne Tc-resistance trait could be transferred in matings on solid surfaces (filter membranes); and although insertions onto pAD1 were prevalent among transconjugants, some bore the resistance trait on the recipient chromosome. Art Franke, now a graduate student, had found earlier that chromosomal mutational markers could be mobilized at low frequency if certain conjugative plasmids such as pAD1 were present.Citation55 However when a derivative of DS16 cured of plasmid DNA was used as a donor, transfer of the Tc-resistance trait was still observed.Citation76 The frequency was low (10−9 to 10−8 in filter matings) and efforts to reveal extrachromosomal DNA in transconjugants proved negative. Transfer occurred in the presence of DNase (ruling out transformation) as well as to a mutant strain, UV202, defective in homologous recombination. In addition, culture filtrates were devoid of any transducing potential. When transconjugants were subsequently used as donors, the Tc-resistance trait transferred again; and if pAD1, pAMγ1 or pOB1 were introduced first, transposition of an intact Tn916 to plasmid DNA could be shown to occur. We were left to conclude that the transposon was able to move intercellularly in the absence of plasmid DNA by a process that required cell-to-cell contact; and the fact that other chromosomal markers were not mobilized under similar conditions suggested that Tn916 might encode its own fertility.Citation76 Such a notion was inconsistent with the current view of conjugation in those days, and we of course encountered our share of skeptics.
Further analyses by Cynthia Gawron-Burke, a new postdoc in the lab, showed that transfer of Tn916 from plasmid-free donors resulted in insertions at many different sites on the recipient chromosome.Citation77 In fact, a significant number of transconjugants (as many as 50%) acquired insertions at more than one location. Some had as many as six or more copies located at different sites. When different transconjugants were used as donors, they exhibited different frequencies of transfer, ranging from less than 10−8 to more than 10−6 per donor. Later experiments found up to 10−4 per donor.Citation78 Importantly, if a plasmid such as pAD1 was introduced into these different donors, Cynthia found that the frequencies of transposition of Tn916 to pAD1 correlated with the frequency of independent transfer in the absence of plasmid; that is, plasmid-free donors that transferred Tn916 at higher frequencies also showed transposition to pAD1 at higher frequencies.Citation77 She also found that when Tn916 was located on a plasmid and transferred passively to a recipient under the control of the plasmid, a significant percentage of the time (as high as 50%) the transposon excised from the plasmid and either inserted into the chromosome or was lost. Thus transposition functions were activated upon entrance into a new host. Tn916 could also be cloned on a multicopy vector in E. coli, however, it was unstable and tended to excise giving rise to insertions onto the chromosome.Citation79,Citation80 Conjugation functions did not appear operative in E. coli, but transposition from the chromosome to a resident plasmid was able to occur.Citation81 Considering the data all together we hypothesized () that movement of Tn916 began with a spontaneous rate-limiting excision event giving rise to a plasmid-like, but non-replicative, circular element with the potential to: (1) re-insert into the chromosome or a resident plasmid; or (2) transfer conjugatively by a plasmid-like process into a recipient cell (i.e., single stranded transfer with the complementary strand being synthesized in the recipient) where it assumed a circular configuration able to express appropriate transposition functions enabling it to insert into recipient DNA.Citation77 The term “conjugative transposon” became the operative term for what we were dealing with.
Around the same time a few other laboratories, namely those of Thea Horaud (formerly Horodniceanu) at the Pasteur Institute and Walter Guild at Duke University were beginning to report that resistance determinants from clinical isolates of Streptococcus pneumoniae, Streptococcus pyogenes and Streptococcus agalactiae could be observed to transfer conjugatively in the apparent absence of plasmid DNA (see review by Clewell and Gawron-BurkeCitation79 for a historical perspective on this). Although the involvement of transposons was not evident at that time, one thing was becoming clear: the transfer of antibiotic resistance among a number of important streptococcal pathogens appeared more likely to occur in the absence than in the presence of plasmid DNA. Indeed, plasmids seemed relatively uncommon in organisms such as S. pneumoniae and S. pyogenes. A transposon, Tn1545, identified on the chromosome of a strain of S. pneumoniae eventually proved to be a conjugative multiple-resistance element very similar to Tn916.Citation82 We were also discovering other elements similar to Tn916, namely Tn918 from E. faecalis RC73 and Tn919 in a strain of S. sanguis.Citation83,Citation84 Tn916 and closely related elements were found to be particularly promiscuous, with evidence for dissemination throughout more than 23 different bacterial genera.Citation85 The transposons were generally found on chromosomal DNA, although in some cases on low copy-number plasmids, an example of which was Tn925 on the conjugative plasmid pCF10.Citation86
A genetic characterization of Tn916 was conducted over a number of years and involved the efforts of numerous of graduate students and postdocs, namely Elisabeth Senghas, Mitsuyo Yamamoto, Joanne Jones, Gerald Fitzgerald, Yan Su, Lois Zitzow, Deborah Jaworski, Sasha Showsh and Michael McIlwraith. A major degree of credit goes to long-time research associate Susan Flannagan, who was a key factor in many aspects of these investigations. Because of space considerations, I will not provide a detailed discussion of these studies but list below some of the key properties of Tn916 determined by us and some other labs. For more detail, I refer the reader to earlier reviews in references Citation79, Citation85, Citation87 and Citation88. Reviews from other investigators, including those related to different types of conjugative transposons, are also noted.Citation89–Citation93
Tn916 Properties
Sequencing analyses showed that Tn916 has a size of close to 18 kb and corresponds to 38.8% G + C.Citation94 It contains 24 open reading frames (ORF) and its termini contain imperfect inverted repeats with identity at 20 of 26 nucleotides. Insertion does not result in the duplication of target sequences.
Tetracycline resistance is of the tet(M) variety and is inducible. Regulation of its expression appears to involve a transcription-attenuation mechanism.Citation95
Near the left end of Tn916 are determinants for an integrase and excisase, as well as related controlling genes.Citation96–Citation98 In the right portion of the transposon are genes related to conjugation, including an origin of transfer oriT ().Citation96,Citation99
Tn916 prefers target sites consisting of 20–30 nucleotides that are A-rich on one side and T-rich on the other.Citation87,Citation100
Inserted transposons are flanked by hexanucleotide “coupling” sequences that are not necessarily identical. Excision is believed to involve a recombinational event involving a staggered cleavage across the two coupling sequences leading to a circular intermediate structure.Citation85,Citation101 If the coupling sequences are not identical, the intermediate contains a heteroduplex at the “joint”. Insertion involves a reversal of this process and involves a recombination between the hexanucleotide joint of the intermediate and a joint-like hexanucleotide sequence centrally located within the AT-rich target.
Circular intermediates of Tn916 exhibit a closed circular configuration based on identification of such structures in E. coli by Scott et al. (1988) at Emory UniversityCitation101 (also see Clewell et al.Citation85).
The frequency of transfer of Tn916 is highly influenced by the nucleotide content of the coupling sequences.Citation78
If there is more than one copy of Tn916 within a single cell, the spontaneous excision of one activates the other(s) in trans.Citation102
Tn916 and related transposons commonly insert into a hot-spot, target site on the cytolysin plasmid pAD1 causing a hyperhemolytic expression.Citation103
Celli and Triu-Cuot have reported that exposure to tetracycline results in transcription that extends beyond tet and can indirectly enhance transcription of xis and int.Citation104 If excision has occurred, transcription can proceed across the junction sequence into the similarly oriented conjugation determinants thereby upregulating conjugative transfer.
Sex Pheromones
During the mid to late 1970s, when we and other laboratories were examining various strains of E. faecalis for plasmid content and the ability to transfer DNA, it became evident that while some plasmids transferred at relatively high frequencies in broth matings (e.g., 10−4 to 10−2 per donor in 4 hours), the movement of others was detectable only if the matings were conducted on solid surfaces. In the latter case matings were done by first collecting mixtures of donors and recipients onto a filter membrane and then placing the membrane on a nutrient agar surface. After an overnight incubation, the cells were removed and plated for transconjugants, which generally appeared at frequencies on the order of 10−4 or higher per donor. Plasmids like pAD1, pAMγ1 and some other cytolysin-encoding plasmids were among those that transferred at high frequency in broth (on filter membranes they transferred at greater than 10−1). Whereas plasmids such as pAMβ1, and some similarly sized Em-resistance-encoding plasmids generally required solid surface matings for efficient detection.Citation40,Citation105 Interestingly, those plasmids transferring at high frequencies in broth had a relatively narrow host range—unlike the others (requiring solid surfaces), which usually exhibited a broad host-range.
During mating experiments using donors that transferred plasmid DNA at high frequencies in broth, we began to notice an interesting clumping phenomenon. One multiple-plasmid-bearing E. faecalis strain (39-5) that had originally been isolated from the gums (subgingival crevices) of a patient with acute periodontal diseaseCitation106 was particularly dramatic in its clumping during matings with the plasmid-free strain JH2-2. The related conjugative plasmid was the bacteriocin-encoding pPD1.Citation107 In an effort to understand better what might be causing such an effect Gary Dunny, who by now was a Microbiology graduate student, did an experiment whereby a culture filtrate of recipient cells was added to donors, and in a separate experiment a filtrate of donors was added to recipients, for a period of time prior to mating to see what influence this might have on the phenomena. We were wondering if the clumping might occur more rapidly than the usual 3–4 hours normally required to observe it (i.e., when the cells were mixed), due to an induction caused by the secretion of a substance by the donor or recipient causing the other to respond in a way that influenced aggregation and/or conjugation. Quite surprisingly, Gary found that the filtrate of the JH2-2 recipients caused a dramatic clumping of the 39-5 donor cells without the need to even mix them with the recipients. Similar results were obtained using donors carrying pAD1, pAMγ1 or pOB1.Citation108,Citation109 We also found that when donors were exposed to a recipient-filtrate for a couple of hours prior to mixing with recipient cells, conjugative transfer of plasmid DNA occurred efficiently in short (e.g., 10 min) matings, in contrast to the 90 min period generally needed in conventional mating experiments before transfer became optimal. Without prior exposure of donors to recipient filtrate, little if any transfer was observed in 10 min. Thus it was clear that recipient cells secreted something that induced the conjugative system of donors and that part of the induction involved the synthesis of a surface component that facilitated aggregation; and the induced cells aggregated with themselves as well as recipients. The inducing factor, which we initially called “clumping inducing agent” or CIA, appeared to be a peptide because it was heat stable and protease-sensitive. In the context of an experiment where we looked for induction of transfer (i.e., in 10 min matings) caused by pre-exposure to a recipient filtrate, we referred to the agent (in lab jargon) as a “fertility boosting ingredient” or FBI. The CIA and FBI activities were thought to be one and the same and had all the characteristics of a sex pheromone, something that had not previously been reported in a bacterial system. Extracellular signaling agents effecting gene transfer had been known in the case of transformation (i.e., competence factors), but these did not fit the basic notion of a pheromone. That is, they were essentially auto-inducing (quorum sensing) agents, and not substances secreted by one organism to specifically stimulate a genetically distinguishable member of the same species.
Induction of aggregation required biosynthesis of a proteinaceous material we referred to as “aggregation substance” (AS), and the surface component to which AS was assumed to bind was referred to as “binding substance” (later called enterococcal binding substance [EBS]).Citation109 The newly synthesized AS could be visualized on the donor surface by immunoelectron microscopy, initially in the case of cells carrying pPD1.Citation107 The presence of both phosphate and divalent cations such as Mg++ or Ca++ was needed in order for induced cells to clump or aggregate, and purified E. faecalis lipoteichoic acid (LTA) inhibited aggregation at concentrations of 0.1 to 1.0 µg/ml.Citation110 LTA was assumed to represent at least some component of EBS. The AS encoded by several different pheromone-responding plasmids exhibited significant immunological cross-reactivity. Interestingly, we were able to show that donor cells alone, when induced to clump by a culture filtrate of recipients, actually exchanged plasmid DNA although at a lower frequency than when interacting with plasmid-free recipients.Citation111
We were soon able to show that when a particular plasmid was acquired by conjugation, it “shut down” the production of the related sex pheromone; however the transconjugant continued to produce different pheromones that were specific for donors with unrelated conjugative plasmids.Citation109 It became clear that plasmid-free bacteria produced multiple pheromones, each specific for a different family of conjugative plasmids. We named the various pheromones by relating them to the particular plasmid that was induced; for example cPD1 was the pheromone to which cells harboring pPD1 would respond. Similarly we had cAD1, cAMγ1 and cOB1 (relating to pAD1, pAMγ1 and pOB1, respectively). Ron Craig, a postdoc, developed a simple microtiter dilution assay that became our standard method for quantifying CIA activity.Citation109
Within a few years it became evident that donor cells encoding a pheromone response secreted a peptide inhibitor of the corresponding sex pheromone.Citation112 At first we thought the activity represented a modified form of endogenously produced pheromone, but subsequent studies showed that it was a completely different ribosomally synthesized peptide that was plasmid-encoded and acted as a competitive inhibitor of the pheromone.Citation113,Citation114 The production of inhibitor therefore was part of the shut-down mechanism in the sense that it masked any donor pheromone activity that continued to be secreted. In addition it probably prevented a mating response to recipients that were too distant (i.e., pheromone concentration too low) for a collision with donors that would lead to formation of a mating pair. As for nomenclature, the inhibitor of the pheromone cPD1 was referred to as iPD1. Similarly, iAD1 inhibited cAD1, iAMγ1 inhibited cAMγ1, etc.
We were very fortunate to engage in a collaboration with Akinori Suzuki, a peptide chemist at the University of Tokyo at that time, who was interested in purifying and characterizing some of our enterococcal peptides. The first to be purified and sequenced was cPD1, which was soon followed by cAD1.Citation115,Citation116 Both were hydrophobic, linear octapeptides that proved to be active at submicromolar concentrations. The related inhibitors iPD1,Citation117 and iAD1,Citation118 also proved to be highly active, hydrophobic octapeptides. Revealing the various structures enabled preparation of synthetic forms, which in turn greatly facilitated subsequent studies of the pheromone response. The preparation of synthetic hybrids of cPD1 and cAD1 enabled the Japanese group to show that the amino terminal half of the pheromone was important to specificity.Citation119
The screening of more than 100 clinical isolates showed that the sex pheromones were extremely common in E. faecalis, and strains that were resistant to multiple antibiotics were significantly more likely than drug-sensitive organisms to be responders to CIA (i.e., culture filtrate of a plasmid-free strain) or producers of an activity that induced clumping by a given responder.Citation109 Some strains carried as many as three different pheromone-responding plasmids simultaneously, each encoding a response to a different pheromone.Citation42,Citation52 If a strain harboring more than one pheromone-responding plasmid was exposed to only one specific pheromone, conjugative transfer was limited specifically to the corresponding plasmid.Citation110
Culture filtrates from a number of different bacterial genera tested had no CIA activity in that a battery of plasmid-containing E. faecalis strains that responded readily to E. faecalis filtrates exhibited no clumping response. An exception was E. faecalis RC73, which responded to filtrates of S. aureus and Streptococcus gordonii—a species commonly associated with normal dental plaque and sometimes bacterial endocarditis.Citation83 The relevant conjugative plasmid in RC73 was identified and designated pAM373 with no known selectable or identifiable traits; although it could be easily marked with a transposon like Tn916 or Tn917. There was no evidence that S. aureus or S. gordonii utilized the related “cAM373” activities as sex pheromones within their own species; however we found much later that they could contribute to the uptake of pAM373 from E. faecalis.Citation120,Citation121
Studies with plasmid-free strains showed that pheromones were secreted at concentrations proportional to cell density during growth in broth and leveled out during entry into stationary phase.Citation109 Some years later postdoc Keith Weaver found that cAD1 expressed at much higher levels (about 16-fold) if the cells were grown anaerobically compared to cells grown aerobically, unlike the case for other pheromones (e.g., cPD1, cAM373) examined.Citation122 In the case of the plasmid-free E. faecalis OG1, which produces gelatinase (a protease) at high levels upon entering stationary phase, degradation of pheromone activity occurred within 1–2 hours. Because OG1 produced significantly higher levels of the pheromone cAD1 than some other strains and was therefore a desirable organism to work with, we eliminated the protease problem by generating the gelatinase-negative mutant OG1X.Citation112 It's noteworthy that OG1 (formerly called 2SaR) had been previously isolated in Boston from the oral cavity of a dental patient with a high level of caries and had been shown to be cariogenic in a rat model.Citation123 Thus, two oral strains of dental significance, 39-5 and OG1, were key participants in the discovery and early studies of bacterial sex pheromones, which seemed fitting for research going on in a dental school. Clearly we were now immersed in “molecular dentistry.” Also of note is that OG1 later became the primary strain from which gelatinase was originally isolated and characterizedCitation124 and was used by graduate student Yan Su in the identification and sequencing of the related gene.Citation125 Gelatinase was subsequently found by others to play a significant role in virulence in certain enterococcal infections.Citation73
Pheromone Precursors and Processing
In the early to mid 1980s exhaustive attempts to clone a pheromone gene were unsuccessful, to the disappointment of several postdocs who were keen to succeed in such efforts. [These individuals (Bryan White, Mike Smith, and Reinhard Wirth) made significant contributions, however, toward the development of new technology that became valuable in future studies.] At the time there was no sequence information available for the E. faecalis genome, so we were dependent on shotgun cloning and screening of plasmid recombinants from thousands of E. coli transformants for production of activity. It wasn't until more than ten years later when enough sequencing data for the enterococcal chromosome became available that sequences relating to pheromones could be recognized in newly appearing databases. It was intriguing to find that pheromone sequences appeared within the carboxyl termini of signal sequences of lipoprotein precursors.Citation126 With a focus on the cAD1 precursor we were able to show that a mutant defective in pheromone production did not result in any noticeable effect on growth; and although the cells were poor recipients, the ability of pAD1-containing mutants to respond to exogenous cAD1 and transfer plasmid DNA was not affected.Citation127 The function of the cAD1-related lipoprotein remains unknown, as is the case for the lipoproteins associated with the precursors of cPD1, cOB1, cCF10 and cAM373; although homologues are apparent in the data base.Citation126
We were able to identify a membranous zinc metallopeptidase we designated Eep that was necessary for cleavage of the cAD1 precursor at a site that gives rise to the amino-terminus of the peptide.Citation128 A more general host lipoprotein signal peptidase cleaved at the site that generates the carboxyl terminus. Eep was found to also process the precursors of cPD1, cOB1 and cCF10, but not that of cAM373. The precursors of the various plasmid-encoded inhibitors all corresponded to what appeared to be “unattached” signal sequences; and there was evidence that Eep was involved in processing these as well, with the exception of iAM373.Citation126,Citation128 Eep turned out to represent what was being identified at the time as a new group of proteins in animals and bacteria with a metallopeptidase activity able to function within the cell membrane.Citation129
Our Bread and Butter Plasmid, pAD1
In the early 1980s we began a genetic investigation of pAD1. As a representative of a family of highly transmissible, cytolysin-producing plasmids with a global presence, pAD1 seemed to be a good choice for study. We thought it would be relatively straightforward to generate mutational insertions using the transposon Tn917, which by virtue of its erm determinant also allowed pAD1::Tn917 derivatives to be readily followed in mating experiments.
Yasuyoshi Ike, who had recently joined our group as a postdoctoral fellow from Japan, initiated the analyses. Based on our observations that exposure of plasmid-containing cells to pheromone induced a clumping response, we had hypothesized that regulation of expression involved a negative control system. Thus, Yasu set out to generate plasmid mutants that constitutively expressed conjugation functions including clumping. In addition we proposed that constitutively clumping variants should also arise from mutations causing an inability to shut down the production of endogenous pheromone—thus facilitating self-induction. Yasu was able to obtain and map both types of mutations, revealing determinants that were designated traA and traB, respectively.Citation130 This marked the beginning of extensive analyses of pAD1 over the next 20 years with key contributors being: graduate students Elizabeth Ehrenfeld and Linda Pontius; postdoctoral fellows Keith Weaver, David Heath, Koichi Tanimoto, Shuhei Fujimoto, Maria Bastos, Keping Wu, Haruyoshi Tomita and Vicky Francia; and my long time highly valued research associate Florence An. It is not my intention to provide here a comprehensive discussion of the pAD1 system. For that I refer the reader to a review published relatively recently in reference Citation131. However, a series of highlights covering most of the interesting and, to some extent, unique features of pAD1 are listed below. I'd like to note that the work included significant use of a Tn917lac transcription reporter derivative together with related delivery vectors made available by Phil Youngman, who had constructed such elements primarily for genetic studies in Bacillus subtilis.Citation132
General properties and structure.
pAD1 (see map in ) was found to contain 59,264 bp with an overall G + C content of 34.0%—a bit lower than the 37.5% G + C of the E. faecalis genome determined by Paulsen et al.Citation133,Citation134 A 9.2 kb region associated with cytolysin-related determinants was about 28% G + C, implying it was likely acquired from a different species. Over half the plasmid is represented by determinants associated with conjugation, including approximately 4 kb dealing with regulation. The replicative origin (oriV) represents a small segment (less than 170 bp) centrally located within repA, which encodes an initiator of plasmid replication.Citation135 repA also contains within it a minor origin of transfer (oriT1) that functions with much less efficiency (1,000-fold) than the primary origin of transfer (oriT2) located about 180° away on the circular element.Citation120,Citation136
Replication and maintenance.
pAD1 is present in cells to the extent of 1–4 copies per chromosome; and based on repA homologies in the database, some of which also contain an internal oriV, the plasmid is believed to utilize a theta-type replication mechanism (see ref. Citation135). An operon consisting of repB and repC (see and ) encodes a partitioning systemCitation137 with RepB representing a member of the ParA superfamily of ATPases.Citation138 A series of iterons (12- and 13-octanucleotide diverging repeats [TAGTARRR] separated by an AT-rich, 75-nucleotide “spacer”) is located between the diverging repA and repB. RepC binds co-operatively to the iterons, whereas RepB interacts with the same region in the presence of ATP—but only if RepC is bound.Citation137 The iteron region probably represents a “centromere-like” structure during partitioning by associating with a host segregation apparatus during cell division.Citation139 The presence of isolated iteron sequences near the promoters of repA and repB suggests that RepC may contribute to regulating expression of these determinants. A plausible model involving a “folding” of the DNA () in such a way that the two sets of iterons interact via complexing of RepB and/or RepC has been suggested.Citation131
When a segment of DNA containing repA, repBC and the iterons was spliced into a vector otherwise unable to replicate in E. faecalis, not surprisingly replication was observed with plasmid DNA appearing at a copy number similar to that of pAD1.Citation140 Interestingly, when repA was present alone and under control of an artificial promoter it facilitated replication and resulted in a high plasmid copy number.Citation135 The importance of the artificial promoter would suggest that expression from its native (weaker?) promoter may not occur at a high enough level to enable efficient maintenance in the absence of a partitioning system.
Entry and exit of cAD1 and iAD1.
As noted above, TraB is involved in shutdown of endogenous cAD1. However, while mutants defective in TraB produce cAD1, the level is relatively low, which is why clumping occurs only when the cells are at high density. In this context, the pheromone resembles a quorum-sensing agent. When cloned on a multi-copy vector, traB resulted in a 32-fold reduction in exogenous cAD1.Citation141 TraB is a membrane protein believed to interact with a host export system, which may also involve interaction with the host-encoded peptide processing enzyme Eep (see below and ref. Citation131).
TraC is a surface lipoprotein that represents the specific receptor for exogenous cAD1,Citation142 and is believed to be associated with a membranous host-encoded peptide uptake system.Citation143 The plasmid-encoded inhibitor peptide iAD1 is believed to act competitively with cAD1 at the receptor to de-sensitize donor cells to endogenously produced pheromone. TraC mutants are greatly reduced in sensitivity to pheromone and also result in a 4–8 fold increase in iAD1 detectable in filtrates due to a failure to bind to the cell surface (i.e., to TraC).
Regulation of the cAD1 response.
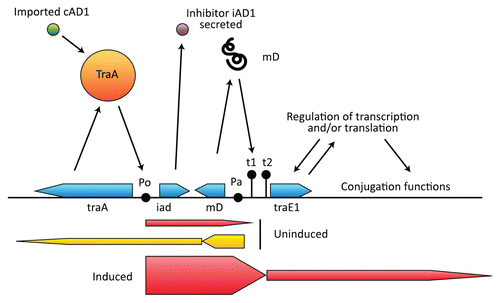
There are three primary regulatory elements that govern expression of the pAD1 conjugation system: the proteins TraA and TraE1, and an RNA designated mD. As illustrated in , TraA negatively regulates expression from the P0 promoter for iad, the determinant for the inhibitor iAD1. A low level of transcription from P0 occurs in the absence of induction to allow expression of iAD1 and continues beyond iad but no further than the terminator t1. Induction occurs as a result of incoming cAD1 binding directly to TraA causing its dissociation from P0 allowing upregulation of expression from P0. This in turn leads to transcription through the terminators t1 and t2 and into traE1.Citation144,Citation145 TraE1 further upregulates itself (at PE) as well as downstream structural genes associated with conjugation functions. The mD RNA (∼200 nt) is transcribed from Pa and opposes the transcription from P0.Citation146–Citation148 Expression from Pa occurs at a relatively high level when cells are in the uninduced state and is terminated at a point shortly downstream giving rise to the mD product; however a small amount of transcription extends beyond P0 and into traA. Thus Pa is also the promoter for traA expression. mD RNA is a potent enhancer of transcription termination at t1, and during induction, mD RNA synthesis is strongly downregulated.Citation148 Overall, regulation results from the interplay of expression from the two opposing promoters P0 and Pa (collisions of RNA polymerases) with the important purpose of tightly regulating transcription termination at t1 and t2. In the absence of exogenous cAD1 (i.e., in the absence of recipient cells), even a very low level of read-through would result in an undesirable induction of conjugation functions (i.e., because TraE1 upregulates itself).
Aggregation and entry exclusion.
Downstream of traE1 are about 37 open reading frames encoding a variety of mostly unknown conjugation functions. Relatively close to traE1 are two determinants, sea1 and asa1 (), which encode a 95.6 kDa entry exclusion protein (Sea1) and the 137 kDa aggregation substance (Asa1), respectively. Characterization of these determinants and their products, including aspects of their regulation, has been conducted in the laboratory of Reinhard Wirth, a former postdoc now located at the University of Regensburg in Germany.Citation149–Citation152 Both proteins are located on the cell surface with their C-termini connected with the cell membrane. Mutations in sea1 result in an increased uptake of plasmid DNA from other pAD1-containing donors exposed to cAD1. Mutations in asa1, which prevent clumping, are poor donors in broth, although matings on solid surfaces appear normal. This implies that the primary role of AS is for binding of donors to recipients when in liquid suspension. Immunoelectron microscopy studies showed Asa1 appearing as “hair-like” structures and present as “patches” on the bacterial surface.Citation149,Citation153
The origin of transfer and related functions.
A significant distance farther downstream of asa1 is the primary origin of transfer oriT2, located between two determinants designated traW and traX ().Citation120 TraW is a member of a family of “coupling proteins” associated with Type IV secretion systems.Citation154,Citation155 TraX encodes a relaxase that nicks at a specific site within the origin, thereby initiating the transport of a single strand of DNA from donor to recipient. An in-frame deletion within traW or traX eliminated the ability to conjugate or mobilize in trans a vector containing oriT2. TraX was also shown to facilitate a site-specific recombination between two oriT2 sequences (e.g., forming a cointegrate with a vector also containing oriT2).Citation120
The oriT2 locus consists of a large inverted repeat sequence (∼140 nt) adjacent to a series of five 6-bp direct repeats separated by 5-bp spacings; and TraX nicks at a site within one of the inverted repeats. The small direct repeats confer specificity for TraX. The pheromone-responding pAM373 contains a transfer origin with an inverted repeat sequence essentially identical to that of the oriT2 of pAD1 but with small adjacent direct repeats that are different and specific for its own relaxase.Citation120 TraX belongs to a unique class of relaxases also represented by that of the mobilizable E. coli plasmid CloDF13.Citation156
An alternative mechanism for switching on conjugation functions.
Conjugation functions of pAD1 were found to undergo a phase variation event relating to a reversible switching on and off at frequencies of 10−4 to 10−3 per cell.Citation157 Surprisingly, this involved an increase/decrease in the number of iterons present between repA and repB ().Citation158 For example, switched-on variants contained a 31- or 32 bp increase in one or the other of the two iteron-containing arms separated by the 75 bp “spacer.” Although further characterization is needed, we believe that the change in the number of iterons (e.g., from 13 to 17 in the case of the repA-proximal arm) may affect the relative positioning of the opposing repA and repBC promoters (each of which contains an isolated iteron able to bind to RepC) in “folded DNA” (). Conceivably, this results in an increased expression of RepC, which might bind to a few iteron sequences that have also been identified within traA.Citation158 The result could be a reduction in TraA synthesis, allowing a derepression of conjugation functions.
Induction of Asa1 expression by subinhibitory concentrations of certain protein synthesis inhibitors.
Subinhibitory concentrations of chloramphenicol, erythromycin or tetracycline were able to induce clumping of cells carrying pAD1 or related derivatives.Citation159 In contrast, induction of conjugative plasmid transfer did not occur. The minimum inducing concentrations were about 20% that of the minimum inhibitory concentrations of each drug. Drug exposure did not have a similar effect on pPD1, pCF10 or pAM373. Transposon insertions in asa1 or traE1 blocked the drug-inducing clumping response. The basis of this novel phenomenon remains unknown but appears to involve events (presumably translational) relating to the control of expression of traE1 (reviewed in ref. Citation131 and Citation159). It is of interest from the perspective that Asa1 is known to contribute to virulence (see below); and thus, under certain conditions, the clinical use of specific drugs (e.g., at concentrations too low) might do more harm than good.
pAM373 and Intergeneric Transfer
Our identification of an E. faecalis plasmid conferring a response to an activity produced also by S. aureus and S. gordoniiCitation83 prompted an interest in characterizing the related enterococcal plasmid pAM373. The plasmid carried no selectable markers and represented one of five plasmids in the clinical isolate RC73. Its size of 37 kb was significantly smaller than any of the other pheromone-responding plasmids we had worked with, most of which were well over 60 kb. Analyses by graduate student Erika DeBoever revealed significant homologies with conjugation-related determinants of the other pheromone-responding plasmids; and there were similarities in overall organization.Citation160 The determinant for aggregation substance, however, was somewhat unique and only half the size of AS determinants encoded by other plasmids; and a determinant/function for entry exclusion was absent.Citation161 A traA (pAD1) homologue was present, but there appeared to be no traB equivalent. A postdoc Yoshiyuki Ozawa did some interesting analyses implying that during induction the negatively regulating TraA maintained an association with the transcription complex via an interaction with the RpoB subunit of the host RNA polymerase.Citation162 It is conceivable that this might relate to a downstream regulatory (antitermination?) effect at t1 (similar to the t1 of pAD1).
The “cAM373” determinants of E. faecalis, S. aureus and S. gordonii have all been identified; all were present within precursors (signal sequences) of completely different lipoproteins.Citation121,Citation163 The peptides were not identical but had a close resemblance, and synthetic forms exhibited activity. cAM373 activity induced intergeneric transfer from E. faecalis to S. aureus and to S. gordonii.Citation120,Citation121
As mentioned earlier, S. gordonii is a member of the human commensal oral flora and a component of “normal” dental plaque; it is also a frequent culprit in infective endocarditis. Although incidental to the “cAM373” connection, it is noteworthy that some members of our group (postdocs Meg Vickerman and Ginette Tardif, and graduate student Mark Sulavik), spent significant time studying an unrelated aspect of S. gordonii, namely its synthesis of glucosyltransferase (GTF). GTF utilizes sucrose in catalyzing production of a glucan polymer, which facilitates the accumulation of cells on saliva-coated hydroxyapatite (HA) beads intended to mimic dental enamel. Meg Vickerman originally characterized the HA binding/accumulation phenomenon as a graduate student in Garth Jones' lab in the Microbiology Department before joining our group as a postdoc.Citation164 We eventually identified the GTF gene (gtfG) as well as an adjacent upstream gene encoding a novel positive transcriptional regulator Rgg.Citation165–Citation167 The Rgg find turned out to be quite significant as homologues are now widely found among most Gram-positive bacteria, some of which regulate virulence; and it has been suggested recently by Mashburn-Warren et al.Citation168 that Rgg-like proteins in general relate to regulation of activities that reflect population densities and peptide production. Indeed, they appear to be part of a superfamily of proteins that bind cytoplasmic signals, which, amazingly, includes the pheromone-binding PrgX protein (a pAD1 TraA homologue) related to the E. faecalis pCF10 system.Citation169
Antibiotic Resistance and Pathogenicity
Over the years it was interesting to follow how some of our work intertwined with clinical aspects of enterococcal behavior. Enterococci were gaining notoriety for being one of the three most frequent organisms associated with nosocomial infections; and multiple antibiotic resistance was becoming an increasingly more serious issue. Resistance to vancomycin (Vm), a so-called “last-resort” antibiotic, emerged in the late 1980s and soon became common among enterococcal isolates.Citation170 It was evident early on that E. faecalis sex pheromones and related plasmids were contributing to the uptake and spread of multiple resistance traits, and a postdoc, Sasha Showsh, was eventually able to identify a pheromone-responding plasmid (pAM368) carrying resistance to a high level of Vm.Citation109,Citation171 This particular plasmid conferred a response to cAM373, also produced by S. aureus, enhancing concern about the potential entry of Vm-resistance into this important human pathogen. Although the pheromone-responding plasmids seemed to have a narrow host range with little evidence for their ability to replicate in other genera, we had been able to show that their transfer functions indeed enabled movement to S. aureus.Citation120 Plasmid DNA might therefore survive by integration into the recipient genome, and/or transposons should at least be “deliverable”. Establishment might also occur if the plasmid first cointegrated with a resident donor plasmid with broader replication potential. Of course the mobilization and establishment of certain non-conjugative, but mobilizable, plasmids under the influence of pheromone-responding plasmids would be expected as well.
The first high-level Vm-resistance strain of S. aureus was identified in 2002 in Michigan and appeared to have acquired its resistance from a Vm-resistant E. faecalis via their interaction within the foot ulcer of a diabetic patient.Citation172,Citation173 However, there was no evidence that a pheromone-responding plasmid was involved in this case.Citation174 Interestingly though, an essentially identical Vm-resistance transposon (Tn1546) was present in both strains. It was also interesting that the Vm-resistant S. aureus strain, as well as an essentially identical, but Vm-sensitive, staphylococcal isolate from the nasal cavity of the same patient, carried a plasmid representative of the pSK41 family. pSK41 carries a conjugation-related determinant (traH) for a lipoprotein precursor reported to give rise to a secretable cAD1-like peptide, which in fact was observed in the case of the Michigan strains.Citation174,Citation175 Although pheromones were probably not involved in the staphylococcal acquisition of Vm-resistance here, the production of two pheromone activities able to facilitate uptake of both pAD1-like as well as pAM373-like plasmids raises further concern about future interactions with resistant strains of E. faecalis.
A connection with bacterial virulence was also becoming evident. Joe Chow and associates reported in reference Citation66, that aggregation substance of pAD1 contributed to an increase in size of heart-valve vegetations in a rabbit endocarditis model, a phenomenon also reported by Schlievert et al.Citation176 in the case of the AS encoded by pCF10. AS also contributes to phenomena such as bacterial invasion of certain epithelial cellsCitation177,Citation178 as well as survival in phagocytes.Citation179–Citation181 In addition, a component in serum appears to induce expression of AS and therefore might also influence the course of bacterial infection.Citation182,Citation183 Finally, it is interesting that certain pheromone/inhibitor peptides induce neutrophil chemotaxis and conceivably could have immunomodulatory effects on infected hosts.Citation184,Citation185
Concluding Remarks
Although our entry into work with E. faecalis was somewhat fortuitous, the fact that these organisms were easy to work with and had a multitude of plasmids and transposons made them attractive organisms right from the beginning. Sex pheromones and conjugative transposons were novelties waiting to be discovered and provided a career's worth of research opportunities. I've tried here to provide some of the highlights of our journey, with some emphasis on how we got involved working with specific systems.
Reflecting on how things evolved over the years has made me greatly appreciate what microorganisms can tell us about genetic potential and biological diversity. The fact that relaxases that trigger conjugative transfer can also participate in plasmid co-integration (e.g., in generating pAMα1) via specific recombination events between certain oriT sites as well as facilitate a recombination-driven gene amplification resulting in increased resistance to an antibiotic provides an example of triple-purpose function. I would not have anticipated that an element like Tn916 would end up being a forerunner of sorts to what now appears to be a vast array of complex integrative conjugative elements (ICE) with both medical and environmental significance (see Wozniak and WaldorCitation93). And the thought that small segments of signal sequences of lipoprotein precursors could end up being utilized as mating signals raises the question of what else these bacteria are doing with such peptides.
In looking back, my early decision to get involved with plasmids in the context of the newly developing field of molecular biology proved to be a good choice. Much has happened since my La Jolla years in the late 1960s when Don Helinski began seeding the planet with plasmid biologists. When I first visited Michigan for my job recruitment seminar and interacted with numerous faculty members, I had to go to some length to explain what a plasmid was. In the 1970s and 80s, plasmids became valuable tools in facilitating an understanding of gene structure and expression in all kinds of biological systems. New biotech companies were sprouting up giving rise to a whole new industry. I would not have guessed in the late 1960s that ColE1-related vectors would play such a significant role in this development—including their use in the eventual sequencing of the human genome.
My roughly 40 years as a plasmid biologist have included much fun and excitement. I am particularly fortunate to have had the opportunity to work with so many enthusiastic and hardworking students, postdocs and research associates, and to have had such great support from the University of Michigan Dental and Medical Schools. A long-term connection with a number of Japanese colleagues from Gunma University Medical School (the group of Yasuyoshi Ike) had a significant impact on our progress. Our interaction with Akinori Suzuki and his research group at the University of Tokyo was also extremely valuable. In addition I have always had a productive and interactive relationship with former student and good friend Gary Dunny who for many years has been a successful investigator at the University of Minnesota and who continues to do highly significant work related to the enterococcal pCF10 sex pheromone system. I can't say enough about the hard-working and loyal support of Florence An (now deceased), who was with me for over 25 years, and Susan Flannagan who was with me for over 20 years. These individuals as well as a number of others played important roles in the overall activity of our lab.
Now “retired” and no longer on the University payroll, I still enjoy interacting with colleagues and continue to participate at a reduced level in some writing, consulting (unpaid) and attending conferences. Some of this has involved work with Christine Sedgley and Susan Flannagan on an intriguing new “siblicide” phenomenon relating to bacteriocin-production. Their discovery involved a pheromone-responding, antibiotic-resistance plasmid identified in the Sedgley laboratory in a strain of E. faecalis originally associated with a monkey root canal infection.Citation186 The behavior may be relevant to the way certain bacteria colonize specific surfaces. In any case enterococci with a dental connection appear to contribute again.
Figures and Tables
Figure 1 pAMα1 and amplification of tetracycline resistance. (A) pAMα1 is shown as being originally generated by the formation of a cointegrate structure through a specific and reversible recombination mechanism involving relaxase operating on the oriT sites of two smaller plasmids pAMα1Δ1 and pAMα1Δ2. (B) Amplification generating multiple copies of the tet determinant via recombination between the two “recombination sequences” RS1 and RS2. The first event involves relaxase and could, for example, bring about excision of the segment containing tet and its reinsertion into an intact pAMα1 copy. Other mechanisms, not mutually exclusive, are possible (see text. Subsequent steps can involve host-directed homologous recombination.
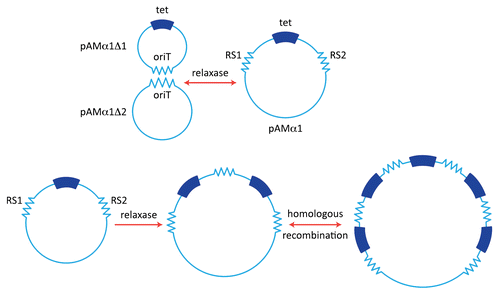
Figure 2 Tn916 behavior and map. (A) The transposon is shown as excising and generating a circular, plasmid-like but nonreplicative intermediate, which can then insert into another site in the genome (on chromosome or a plasmid) or transfer by a plasmid-like process to a recipient cell. (B) A simplified view of the transposon showing the general locations of determinants involved in excision/insertion, tetracycline resistance and conjugation.
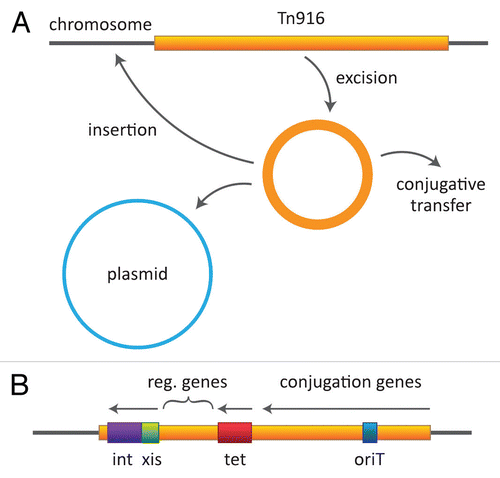
Figure 3 Map of pAD1. The related functions encoded on the plasmid are noted in different colors with the various determinants indicated. A detailed description of the specific regions can be found in reference Citation131 (redrawn from ref. Citation131).
Figure 4 Map of the replication and partitioning region of pAD1. Two clusters of iterons (TAGTARRR) are shown between repA and repBC. Isolated iteron sequences are also located within the promoters of repA and repBC. RepC binds cooperatively to iteron sequences; in the presence of ATP, RepB also binds. RepC binding to the isolated iterons probably affects expression of repBC and repA. A “folded” conformation may be stabilized by the binding and effect interaction between the opposing promoters. Phase variation resulting from changes in the number of iterons could affect this interaction and modulate expression of RepBC and RepA (redrawn from ref. Citation131).
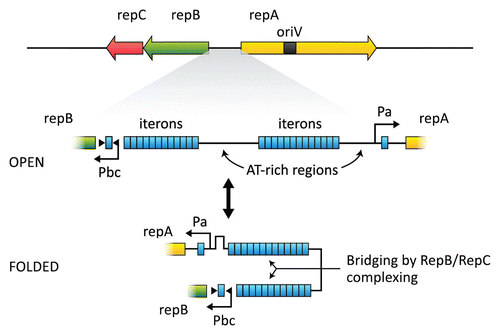
Figure 5 pAD1 region that regulates conjugation. The negative regulator TraA binds to the promoter P0 and dissociates when bound to the pheromone cAD1. A limited amount of transcription occurs from P0 to the terminator t1 in the uninduced state, allowing expression of the inhibitor iAD1. Pa is the promoter for mD, an RNA that enhances transcription termination at t1. Pa is also the promoter for traA. Induction results in transcription through t1 and t2 and into traE1, which encodes a positive regulator for conjugation-related determinants as well as itself.
Acknowledgements
I thank all those individuals who have spent time in my laboratory over the years and have directly or indirectly been involved in the above story. I also thank Susan Flannagan for her careful reading of the manuscript and valuable comments. Major support over the years came from the National Institutes of Health via grants GM33956, AI10318 and DE02731.
References
- Helinski DR. Funnell BE, Phillips GJ. Introduction to plasmids: a selective view of their history. Plasmid Biology 2004; Washington DC ASM Press 1 - 20
- Bazaral M, Helinski DR. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol 1968; 36:185 - 194
- Roth TF, Helinski DR. Evidence for circular DNA forms of a bacterial plasmid. Proc Natl Acad Sci USA 1967; 58:650 - 657
- DeWitt W, Helinski DR. Characterization of colicinogenic factor E1 from a non-induced and mitomycin C-induced Proteus strain. J Mol Biol 1965; 13:692 - 703
- Radloff R, Bauer W, Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplexed DNA in HeLa cells. Proc Natl Acad Sci USA 1967; 57:1514 - 1521
- Godson GN, Sinsheimer RL. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta 1967; 149:476 - 488
- Clewell DB, Helinski DR. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an open circular DNA form. Proc Natl Acad Sci USA 1969; 62:1159 - 1166
- Clewell DB, Helinski DR. Properties of a deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry 1970; 9:4428 - 4440
- Clewell DB, Helinksi DR. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol 1972; 110:1135 - 1146
- Clewell DB, Helinski DR. Evidence for the existence of the colicinogenic factors E2 and E3 as supercoiled circular DNA-protein relaxation complexes. Biochem Biophys Res Comm 1970; 40:608 - 613
- Clewell DB, Helinski DR. Evidence for the colicinogenic factor-sex factor ColIb-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Comm 1970; 41:150 - 156
- Lovett MA, Helinksi DR. Relaxation complexes of plasmid DNA and protein. II. Characterization of the proteins associated with the unrelaxed and relaxed complexes of plasmid ColE1. J Biol Chem 1975; 250:8790 - 8795
- Inselburg J. Studies of colicin E1 plasmid functions by analysis of deletions and TnA insertions of the plasmid. J Bacteriol 1977; 132:332 - 340
- Warren GJ, Twigg AG, Sherratt DJ. ColE1 plasmid mobility and relaxation complex. Nature 1978; 274:259 - 261
- Lanka E, Wilkins BM. DNA processing reactions in bacterial conjugation. Annu Rev Biochem 1995; 64:141 - 169
- Llosa M, Gomis-Ruth F, Coll M, de la Cruz F. Bacterial conjugation: a two-step mechanism for DNA transport. Mol Microbiol 2002; 45:1 - 8
- Bazaral M, Helinski DR. Replication of a bacterial plasmid and episome in Escherichia coli. Biochemistry 1970; 9:399 - 406
- Goebel W, Helinski DR. Generation of higher multiple circular forms in bacteria. Proc Natl Acad Sci USA 1968; 61:1406 - 1413
- Clewell DB. Nature of ColE1 plasmid replication in Escherichia coli in the presence of chloramphenicol. J Bacteriol 1972; 110:667 - 676
- Clewell DB, Evenchik BG. Effects of rifampicin, streptolydigin and actinomycin D on the replication of ColE1 plasmid DNA in Escherichia coli. J Mol Biol 1973; 75:503 - 513
- Clewell DB, Evenchik B, Cranston JW. Direct inhibition of ColE1 plasmid DNA replication in Escherichia coli by rifampicin. Nature New Biol 1972; 237:29 - 31
- Kingsbury DT, Helinski DR. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor ColE1. Biochem Biophys Res Comm 1970; 41:1538 - 1544
- Verma IM, Meuth NL, Bromfeld E, Manly KF, Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nature New Biol 1971; 233:131 - 134
- Blair DG, Sherratt DJ, Clewell DB, Helinski DR. Isolation of supercoiled colicinogenic factor E1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci USA 1972; 69:2518 - 2522
- Wickner W, Brutlag D, Schekman R, Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci USA 1972; 69:965 - 969
- Sheckman R, Wickner W, Westergaard O, Brutlag D, Geider K, Bertsch LL, Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA sythesis resistant to rifampicin. Proc Natl Acad Sci USA 1972; 69:2691 - 2695
- Inokuchi H, Dove WF. Physical studies of RNA involvement in bacteriophage lambda DNA replication and prophage excision. J Mol Biol 1973; 74:721 - 727
- Lark KG. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol 1972; 28:47 - 60
- Kues U, Stahl U. Replication of plasmids in gram-negative bacteria. Microbiol Rev 1989; 53:491 - 516
- del Solar G, Giraldo R, Ruiz-Echevarria MJ, Espinosa M, Diaz-Orejas R. Replication and control of circular bacterial plasmids. Microbiol Mol Biol Rev 1998; 62:434 - 464
- Dunny GM, Birch N, Hascall G, Clewell DB. Isolation and characterization of plasmid DNA from Streptococcus mutans. J Bacteriol 1973; 114:1362 - 1364
- Clewell DB, Franke A. Characterization of a plasmid determining resistance to erythromycin, lincomycin and vernamycin B in a strain of Streptococcus pyogenes. Antimicrob Agents Chemother 1974; 5:534 - 537
- Clewell DB, Yagi Y, Dunny GM, Schultz S. Characterization of three plasmid DNA molecules in a strain Streptococcus faecalis: Identification of a plasmid determining erythromycin resistance. J Bacteriol 1974; 117:283 - 289
- Yagi Y, McLellan T, Frez W, Clewell DB. Characterization of a small plasmid determining resistance to erythromycin, lincomycin and vernamycin B in a strain of Streptococcus sanguis isolated from dental plaque. Antimicrob Agents Chemother 1978; 13:884 - 887
- Orland FJ, Blayney R, Harrison RW, Reyniers JA, Trexler PG, Ervin RF, et al. Experimental caries in germ-free rats inoculated with enterococci. J Am Dent Assoc 1955; 50:259 - 272
- Sedgley CM, Clewell DB. Bacterial plasmids in the oral cavity and endodontic microflora. Endodont Topics 2005; 9:37 - 51
- Tomura T, Hirano T, Ito T, Yoshioka M. Transmission of bacteriocinogenicity by conjugation in group-D streptococci. Jpn J Microbiol 1973; 17:445 - 452
- Jacob AE, Hobbs SJ. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol 1974; 117:360 - 372
- Dunny GM, Clewell DB. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a non-infectious drug resistance plasmid. J Bacteriol 1975; 124:784 - 790
- Clewell DB. Plasmids, drug resistance and gene transfer in the genus Streptococcus. Microbiol Rev 1981; 45:409 - 436
- Clewell DB, Francia MV. Funnell B, Phillips JG. Conjugation in Gram-positive bacteria. Plasmid Biology 2004; Washington, DC Am Soc Microbiol 227 - 256
- Clewell DB, Yagi Y, Ike Y, Craig RA, Brown BL, An FY. Schlessinger D. Sex pheromones in Streptococcus faecalis. Multiple pheromone systems in strain DS5, similarities of pAD1 and pAMγ1, and mutants of pAD1 altered in conjugative properties 1982; Washington, DC Am Soc Microbiol 97 - 100
- Clewell DB, Yagi Y, Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: Evidence for gene amplification during growth in the presence of tetracycline. Proc Natl Acad Sci USA 1975; 72:1720 - 1724
- Rownd R, Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nature New Biol 1971; 234:40 - 43
- Morris CF, Rownd R. Transition of the R factor R12 in Proteus mirabilis. J Bacteriol 1974; 118:867 - 879
- Yagi Y, Clewell DB. Plasmid-determined tetracycline resistance in Streptococcus faecalis: tandemly repeated resistance determinants in amplified forms of pAMα1. J Mol Biol 1976; 102:583 - 600
- Yagi Y, Clewell DB. Identification and characterization of a small sequence located at two sites on the amplifiable tetracycline-resistance plasmid pAMα1 in Streptococcus faecalis. J Bacteriol 1977; 129:400 - 406
- Yagi Y, Clewell DB. Amplification of the tetracycline resistance determinant of plasmid pAMα1 in Streptococcus faecalis: Dependence on host recombination machinery. J Bacteriol 1980; 143:1070 - 1072
- Perkins JB, Youngman P. Streptococcus plasmid pAMα1 is a composite of two separable replicons, one of which is closely related to Bacillus plasmid pBC16. J Bacteriol 1983; 155:607 - 615
- Francia MV, Clewell DB. Amplification of the tetracycline-resistance determinant of pAMα1 in Enterococcus faecalis requires a site specific recombination event involving relaxase. J Bacteriol 2002; 184:5187 - 5193
- Oliver D, Brown B, Clewell DB. Characterization of plasmid deoxyribonucleic acid in a cariogenic strain of Streptococcus faecalis: an approach to identifying genetic determinants on cryptic plasmids. J Bacteriol 1977; 130:759 - 765
- Murray BE, An FY, Clewell DB. Plasmids and pheromone response of the β-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother 1988; 32:547 - 551
- Clewell DB, Tomich P, Gawron-Burke C, Franke A, Yagi Y, An F. Mapping of the Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to the transposition of Tn917. J Bacteriol 1982; 152:1220 - 1230
- Tomich P, An F, Damle S, Clewell DB. Plasmid related transmissibility and multiple drug resistance in Streptococcus faecalis subspecies zymogenes strain DS16. Antimicrob Agents Chemother 1979; 15:828 - 830
- Franke A, Dunny GM, Brown B, An F, Oliver D, Damle S, Clewell DB. Schlessinger D. Gene transfer in Streptococcus faecalis: evidence for the mobilization of chromosomal determinants by transmissible plasmids 1978; Washington, DC Am Soc Microbiol 45 - 47
- Tomich P, An F, Clewell DB. Properties of an erythromycin inducible transposon (Tn917) in Streptococcus faecalis. J Bacteriol 1980; 141:1366 - 1374
- Shaw J, Clewell DB. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol 1985; 164:782 - 796
- An FY, Clewell DB. Tn917 transposase. Sequence correction reveals open reading frame corresponding to the tnpA determinant of Tn3-family elements. Plasmid 1991; 25:121 - 124
- Brock TD, Davie JM. Probable identity of a group D hemolysin with a bacteriocine. J Bacteriol 1963; 86:708 - 712
- Granato PA, Jackson RW. Bicomponent nature of lysin from Streptococcus zymogenes. J Bacteriol 1969; 100:865 - 868
- Borderon E, Bieth G, Horodniceanu T. Genetic and physical studies of Streptococcus faecalis hemolysin plasmids. FEMS Lett 1982; 14:51 - 55
- Colmar I, Horaud T. Enterococcus faecalis hemolysin-bacteriocin plasmids belong to the same incompatibility group. Appl Environ Microbiol 1987; 53:567 - 570
- Ike Y, Clewell DB. Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J Bacteriol 1992; 174:8172 - 8177
- LeBlanc DJ, Lee L, Clewell DB, Behnke D. Broad geographical distribution of a cytotoxin gene mediating β-hemolysis and bacteriocin activity among strains of Streptococcus faecalis. Infect Immun 1983; 49:1015 - 1022
- Ike Y, Hashimoto H, Clewell DB. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun 1984; 45:528 - 530
- Chow JW, Thal LA, Perri MB, Vazquez JA, Donabedian SM, Clewell DB, et al. Plasmid-associated hemolysin and aggregation substance production contributes to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother 1993; 37:2474 - 2477
- Jett BD, Jensen HG, Nordquist RE, Gilmore MS. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun 1992; 60:2445 - 2452
- Stevens SX, Jensen HG, Jett BD, Gilmore MS. A hemolysin-encoding plasmid contributes to bacterial virulence in experimenetal Enterococcus faecalis endophthalmitis. Invest Ophthalmol Vis Sci 1992; 33:1650 - 1656
- Ike Y, Hashimoto H, Clewell DB. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol 1987; 25:1524 - 1528
- Huycke MM, Spiegel CA, Gilmore MS. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 1991; 35:1626 - 1634
- Huycke MM, Gilmore MS. Frequency of aggregation substance and cytolysin genes among enterococcal endocarditis isolates. Plasmid 1995; 34:152 - 156
- Coque TM, Patterson JE, Steckelberg JM, Murray BE. Incidence of hemolysin, gelatinase and aggregation substance among enterococci isolates from patients with endocarditis and other infections, and from feces of hospitalized and community based individuals. J Infect Dis 1995; 171:1223 - 1229
- Gilmore MS, Coburn PS, Nallapareddy SR, Murray BE. Gilmore MS, et al. Enterococcal virulence 2002. The Enterococci: Pathogenesis, Molecular Biology and Antibiotic Resistance 2002; Washington, DC ASM Press 301 - 354
- Sherman JM. The enterococci and related streptococci. J Bacteriol 1938; 35:81 - 93
- Schleifer KH, Kilpper-Balz R. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom. Rev. as Enterococcus faecalis comb. nov and Enterococcus faecium comb. nov. Internat J System Bacteriol 1984; 34:31 - 34
- Franke AE, Clewell DB. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of “conjugal” transfer in the absence of a conjugative plasmid. J Bacteriol 1981; 145:494 - 502
- Gawron-Burke C, Clewell DB. A transposon in Streptococcus faecalis with fertility properties. Nature 1982; 300:281 - 284
- Jaworski DD, Clewell DB. Evidence that coupling sequences play a frequency-determining role in conjugative transposition of Tn916 in Enterococcus faecalis. J Bacteriol 1994; 176:3328 - 3335
- Clewell DB, Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol 1986; 40:635 - 659
- Gawron-Burke C, Clewell DB. Regeneration of insertionally inactivated streptococcal DNA fragments following excision of Tn916 in Escherichia coli. A strategy for targeting and cloning genes from gram-positive bacteria. J Bacteriol 1984; 159:214 - 221
- Clewell DB, Jaworski DD, Flannagan SE, Zitzow LA, Su YA. Ferretti JJ, Gilmore MS, Klaenhammer TR, Brown F. The conjugative transposon Tn916 of Enterococcus faecalis: Structural analysis and some key factors involved in movement. Genetics of Streptococci, Enterococci and Lactococci 1995; 85:Basel Karger 11 - 15
- Courvalin P, Carlier C. Tn1545: a conjugative shuttle transposon. Mol Gen Genet 1987; 206:259 - 264
- Clewell DB, An F, White B, Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol 1985; 162:1212 - 1220
- Fitzgerald G, Clewell DB. Identification and characterization of a conjugative transposon (Tn919) in Streptococcus sanguis. Infect Immun 1985; 47:415 - 420
- Clewell DB, Flannagan SE, Jaworski DD. Unconstrained bacterial promiscuity: the Tn916-Tn1545 family of conjugative transposons. Trends Microbiol 1995; 3:229 - 236
- Christie PJ, Korman RZ, Zahler SA, Adsit JC, Dunny GM. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtili. J Bacteriol 1987; 169:2529 - 2536
- Clewell DB, Flannagan SE. Clewell DB. The conjugative transposons of gram-positive bacteria. Bacterial Conjugation 1993; New York Plenum Press 369 - 393
- Clewell DB. deBruijn FJ, Lupski JR, Weinstock GM. Conjugative transposons. Bacterial Genomes; Physical Structure and Analysis 1998; New York Chapman and Hall 130 - 139
- Roberts AP, Mullany P. A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol 2009; 17:251 - 258
- Rice LB. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob Agents Chemother 1998; 42:1871 - 1877
- Salyers AA, Shoemaker NB, Stevens AM, Li LY. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol Rev 1995; 59:579 - 590
- Scott JR, Churchward GG. Conjugative transposition. Annu Rev Microbiol 1995; 49:367 - 397
- Wozniak RA, Waldor MK. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol 2010; 8:552 - 563
- Flannagan SE, Zitzow LA, Su YA, Clewell DB. Nucleotide sequence of the 18 kb conjugative transposon Tn916 from Enterococcus faecalis. Plasmid 1994; 32:350 - 354
- Su YA, He P, Clewell DB. Characterization of the tet(M) determinant of Tn916: evidence for regulation by transcription attenuation. Antimicrob Agents Chemother 1992; 36:769 - 778
- Senghas E, Jones JM, Yamamoto M, Gawron-Burke C, Clewell DB. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol 1988; 170:245 - 249
- Su YA, Clewell DB. Characterization of the left four kilobases of conjugative transposon Tn916. Determinants involved in excision. Plasmid 1993; 30:234 - 250
- Jaworski DD, Flannagan SE, Clewell DB. Analyses of traA, int-Tn and xis-Tn mutations in the conjugative transposon Tn916 in Enterococcus faecalis. Plasmid 1996; 36:201 - 208
- Jaworski DD, Clewell DB. A functional origin of transfer (oriT) on the conjugative transposon Tn916. J Bacteriol 1995; 177:6644 - 6651
- Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. Sequence requirements for target activity in site-specific recombination mediated by the Int protein of transposon Tn1545. Mol Microbiol 1993; 8:179 - 185
- Scott JR, Kirchman PA, Caparon MG. An intermediate in the transposition of the conjugative transposon Tn916. Proc Natl Acad Sci USA 1988; 85:4809 - 4813
- Flannagan SE, Clewell DB. Conjugative transfer of Tn916 in Enterococcus faecalis: trans activation of homologous transposons. J Bacteriol 1991; 173:7136 - 7141
- Ike Y, Flannagan SE, Clewell DB. Hyperhemolytic phenomena associated with insertions of Tn916 into the hemolysin determinant of Enterococcus faecalis plasmid pAD1. J Bacteriol 1992; 174:1801 - 1809
- Celli J, Trieu-Cuot P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol 1998; 28:103 - 117
- Clewell DB. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Microbiol Infect Dis 1990; 9:90 - 102
- Rosan B, Williams N. Hyaluronidase production by oral enterococci. Arch Oral Biol 1964; 9:291 - 298
- Yagi Y, Kessler R, Shaw J, Lopatin D, An F, Clewell DB. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol 1983; 128:1207 - 1215
- Dunny GM, Brown BL, Clewell DB. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Nat Acad Sci USA 1978; 75:347 - 349
- Dunny GM, Craig RA, Carron RI, Clewell DB. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 1979; 2:454 - 465
- Ehrenfeld EE, Kessler RE, Clewell DB. Identification of pheromone induced surface proteins in Streptococcus faecalis and evidence of a role for lipoteichoic acid in formation of mating aggregates. J Bacteriol 1986; 168:6 - 12
- Clewell DB, Brown BL. Sex pheromone cAD1 in Streptococcus faecalis: Induction of a function related to plasmid transfer. J Bacteriol 1980; 143:1063 - 1065
- Ike Y, Craig RA, White B, Yagi Y, Clewell DB. Modification of Streptococcus faecalis sex pheromone after acquisition of plasmid DNA. Proc Natl Acad Sci USA 1983; 80:5369 - 5373
- Clewell DB, An F, Mori M, Ike Y, Suzuki A. Streptococcus faecalis sex pheromone (cAD1) response: evidence that the peptide inhibitor excreted by pAD1-containing cells may be plasmid determined. Plasmid 1987; 17:65 - 68
- Pontius LT, Clewell DB. Conjugative transfer of Enterococcus faecalis plasmid pAD1: nucleotide sequence and transcriptional fusion analysis of a region involved in positive regulation. J Bacteriol 1992; 174:3152 - 3160
- Suzuki A, Mori M, Sakagami Y, Isogai A, Funjino M, Kitada C, et al. Isolation and structure of baterial sex pheromone cPD1. Science 1984; 226:849 - 850
- Mori M, Sakagami Y, Narita M, Isogai A, Fujino M, Kitada C, et al. Isolation and structure of the bacterial sex pheromone, cAD1, that induces plasmid transfer in Streptococcus faecalis. FEBS Lett 1984; 178:97 - 100
- Mori M, Tanaka H, Sakagami Y, Isogai A, Fugino M, Kitada C, et al. Isolation and structure of the sex pheromone inhibitor iPD1, excreted by Streptococcus faecalis donor strains harboring plasmid pPD1. J Bacteriol 1987; 169:1747 - 1749
- Mori M, Isogai A, Sakagami Y, Funjino M, Kitada C, Clewell DB, et al. Isolation and structure of Streptococcus faecalis sex pheromone inhibitor, iAD1, that is excreted by donor strains harboring plasmid pAD1. Agric Biol Chem 1986; 50:539 - 541
- Kitada C, Fujino M, Mori M, Sakagami Y, Isogai A, Suzuki A, et al. Izumiya N. Synthesis and structure-activity relationships of Streptococcus faecalis sex pheromones, cPD1 and cAD1. Peptide Chemistry 1984 1985; Osaka Protein Research Foundation 44 - 48
- Francia MV, Clewell DB. Transfer origins in the conjugative plasmids pAD1 and pAM373. Identification of the pAD1 nic site, a specific relaxase and a possible TraG-like protein. Mol Microbiol 2002; 45:375 - 395
- Vickerman MM, Flannagan SE, Jesionowski AM, Brossard KA, Clewell DB, Sedgley CM. A genetic determinant in Streptococcus gordonii Challis encodes a peptide with activity similar to enterococcal sex pheromone cAM373, which facilitates intergeneric DNA transfer. J Bacteriol 2010; 192:2535 - 2545
- Weaver KE, Clewell DB. Control of Enterococcus faecalis sex pheromone cAD1 elaboration: effects of culture aeration and pAD1 plasmid-encoded determinants. Plasmid 1991; 25:177 - 189
- Gold OG, Jordan HV, van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol 1975; 20:473 - 477
- Makinen P, Clewell DB, An F, Makinen KK. Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase (“gelatinase”) from Streptococcus faecalis (strain OG1-10). J Biol Chem 1989; 264:3325 - 3334
- Su YA, Sulavik MC, He P, Makinen KK, Makinen P, Fiedler S, et al. Nucleotide sequence of the gelatinase gene (gelE) from Enterococcus faecalis var. liquifaciens. Infect Immun 1991; 59:415 - 420
- Clewell DB, An FY, Flannagan SE, Antiporta M, Dunny GM. Enterococcal sex pheromone precursors are part of signal sequences for surface lipoproteins. Mol Microbiol 2000; 35:246 - 247
- An FY, Clewell DB. Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis. J Bacteriol 2002; 184:1880 - 1887
- An FY, Sulavik MC, Clewell DB. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J Bacteriol 1999; 181:5915 - 5921
- Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis; a control mechanism conserved from bacteria to humans. Cell 2000; 100:381 - 398
- Ike Y, Clewell DB. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis using transposon Tn917 as an insertional mutagen. J Bacteriol 1984; 158:777 - 783
- Clewell DB. Properties of Enterococcus faecalis plasmid pAD1, a member of a widely disseminated family of pheromone-responding, conjugative, virulence elements encoding cytolysin. Plasmid 2007; 58:205 - 227
- Youngman P. Hardy KG. Plasmid vectors for recovering and exploiting Tn917 transpositions in Bacillus and other gram-positive bacteria. Plasmids: A Practical Approach 1987; Oxford IRL Press Ltd. 79 - 104
- Francia MV, Haas W, Wirth R, Samberger E, Muscholl-Silberhorn A, Gilmore MS, et al. Completion of the nucleotide sequence of the Enterococcus faecalis conjugative virulence plasmid pAD1 and identification of a second transfer origin. Plasmid 2001; 46:117 - 127
- Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 2003; 28:2071 - 2074
- Francia MV, Fujimoto S, Tille P, Weaver KE, Clewell DB. Replication of Enterococcus faecalis pheromone-responding plasmid pAD1: location of the minimal replicon and oriV site and RepA involvement in initiation of replication. J Bacteriol 2004; 186:5003 - 5016
- An FY, Clewell DB. The origin of transfer (oriT) of the enterococcal pheromone-responding, cytolysin plasmid pAD1 is located within the repA determinant. Plasmid 1997; 37:87 - 94
- Francia MV, Weaver KE, Goicoechea P, Tille P, Clewell DB. Characterization of an active partitioning system for the Enterococcus faecalis pheromone-responding plasmid pAD1. J Bacteriol 2007; 189:8546 - 8555
- Motallebi-Veshareh M, Rouch DA, Thomas CM. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol 1990; 4:1455 - 1463
- Funnell BE, Slavcev RA. Funnell BE, Phillips GJ. Partition systems of bacterial plasmids. Plasmid Biology 2004; Washington, DC ASM Press 81 - 104
- Weaver KE, Clewell DB, An FY. Identification, characterization and nucleotide sequence of a region of Enterococcus faecalis pheromone-responsive plasmid pAD1 capable of autonomous replication. J Bacterol 1993; 175:1900 - 1909
- An FY, Clewell DB. Characterization of the determinant (traB) encoding sex pheromone shutdown by the hemolysin/bacteriocin plasmid pAD1 in Enterococcus faecalis. Plasmid 1994; 31:215 - 221
- Tanimoto K, An FY, Clewell DB. Characterization of the traC determinant of the Enterococcus faecalis hemolysin/bacteriocin plasmid pAD1. Binding of sex pheromone. J Bacteriol 1993; 175:5260 - 5264
- Leonard BAB, Podbielski A, Hedberg PJ, Dunny GM. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc Natl Acad Sci USA 1996; 93:260 - 264
- Fujimoto S, Clewell DB. Regulation of the pAD1 sex pheromone response of Enterococcus faecalis by direct interaction between the cAD1 peptide mating signal and the negatively regulating, DNA-binding TraA protein. Proc Natl Acad Sci USA 1998; 95:6430 - 6435
- Tanimoto K, Clewell DB. Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: expression of the positive regulator TraE1. J Bacteriol 1993; 175:1008 - 1018
- Bastos MCF, Tanimoto K, Clewell DB. Regulation of transfer of the Enterococcus faecalis pheromone-responding plasmid pAD1: temperature-sensitive transfer mutants and identification of a new regulatory determinant traD. J Bacteriol 1997; 179:3250 - 3259
- Bastos MCF, Tomita H, Tanimoto K, Clewell DB. Regulation of the Enterococcus faecalis pAD1-related sex pheromone response: analyses of traD expression and its role in controlling conjugation. Mol Microbiol 1998; 30:381 - 392
- Tomita H, Clewell DB. A pAD1-encoded small RNA molecule, mD, negatively regulates Enterococcus faecalis pheromone response by enhancing transcription termination. J Bacteriol 2000; 182:1062 - 1073
- Galli D, Wirth R, Wanner G. Identification of aggregation substances of Enterococcus faecalis after induction by sex pheromone. An immunological and ultrastructural investigation. Arch Microbiol 1989; 151:486 - 490
- Galli D, Lottspeich F, Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol 1990; 4:895 - 904
- Galli D, Friesenegger A, Wirth R. Transcriptional control of sex-pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structural gene for (pPD1-encoded) aggregation substance. Mol Gen Genet 1992; 233:161 - 168
- Weidlich G, Wirth, Gall D. Sex pheromone plasmid pAD1-encoded surface exclusion protein of Enterococcus faecalis. Mol Gen Genet 1992; 233:161 - 168
- Wanner G, Formanek H, Galli D, Wirth R. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immuno labeling, transmission and high resolution scanning electron microscopic techniques. Arch Microbiol 1989; 151:491 - 497
- Cabezon E, Sastre JI, de la Cruz F. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol Gen Genet 1997; 254:400 - 406
- Schroder G, Krause S, Zechner EL, Traxler B, Yeo HJ, Lurz R, et al. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates?. J Bacteriol 2002; 184:2767 - 2779
- Nunez B, de la Cruz F. Two atypical mobilization proteins are involved in plasmid CloDF13 relaxation. Mol Microbiol 2001; 39:1088 - 1099
- Pontius LT, Clewell DB. A phase variation event that activates conjugation functions encoded by the Enterococcus faecalis plasmid pAD1. Plasmid 1991; 26:172 - 175
- Heath DG, An FY, Weaver KE, Clewell DB. Phase variation of Enterococcus faecalis pAD1 conjugation functions relates to changes in iteron sequence region. J Bacteriol 1995; 177:5453 - 5459
- Wu K, An FY, Clewell DB. Enterococcus faecalis pheromone-responding plasmid pAD1 gives rise to an aggregation (clumping) response when cells are exposed to subinhibitory concentrations of chloramphenicol, erythromycin or tetracycline. Plasmid 1999; 41:82 - 88
- DeBoever EH, Clewell DB, Fraser CM. Enterococcus faecalis conjugative plasmid pAM373; complete nucleotide sequence and genetic analysis of sex pheromone response. Mol Microbiol 2000; 37:1327 - 1341
- DeBoever EH, Clewell DB. The Enterococcus faecalis pheromone-responding plasmid pAM373 does not encode an entry exclusion function. Plasmid 2001; 45:47 - 60
- Ozawa Y, DeBoever EH, Clewell DB. Enterococcus faecalis sex pheromone plasmid pAM373: analyses of TraA and evidence for its interaction with RpoB. Plasmid 2005; 54:57 - 69
- Flannagan SF, Clewell DB. Identification and characterization of genes encoding sex pheromone cAM373 activity in Enterococcus faecalis and Staphylococcus aureus. Mol Microbiol 2002; 44:803 - 817
- Vickerman MM, Clewell DB, Jones GW. Sucrose-promoted accumulation of growing glucosyltransferase variants of Streptococcus gordonii on hydroxyapatite surfaces. Infect Immun 1991; 59:3523 - 3530
- Sulavik MC, Tardif G, Clewell DB. Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the Spp phenotype of Streptococcus gordonii Challis. J Bacteriol 1992; 174:3577 - 3586
- Sulavik MC, Clewell DB. Rgg is a positive transcriptional regulator of the Streptococcus gordonii gtfG gene. J Bacteriol 1996; 178:5826 - 5830
- Vickerman MM, Sulavik MM, Nowak JD, Gardner NM, Jones GW, Clewell DB. Nucleotide sequence analysis of the Streptococcus gordonii glucosyltransferase gene, gtfG. DNA Seq 1997; 7:83 - 95
- Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. Via an Rgg regulator. Mol Microbiol 2010; 78:589 - 606
- Declerck N, Bouillaut L, Chaix D, Rugani N, Slamti L, Hoh F, et al. Structure of PlcR: insights into virulence regulation and evolution of quorum sensing in Gram-positive bacteria. Proc Natl Acad Sci USA 2007; 104:18490 - 18495
- Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev 2000; 13:686 - 707
- Showsh SA, DeBoever EH, Clewell DB. A vancomycin-resistance plasmid in Enterococcus faecalis that encodes sensitivity to a sex pheromone also produced by Staphylococcus aureus. Antimicrob Agents Chemother 2001; 45:2177 - 2178
- Sievert DM, Boulton MI, Stoltman G, Johnson D, Stobierski MG, Downes FP, et al. Staphylococcus aureus resistant to vancomycin—United States. Morb Mortal Wkly Rep 2002; 51:565 - 567
- Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, et al. Molecular analysis of a clinical isolate of Staphylococcus aureus with high-level resistance to vancomycin. Science 2003; 302:1569 - 1571
- Flannagan SE, Chow JW, Donabedian SM, Brown WJ, Perri MB, Zervos MJ, et al. Characterization of the plasmid content of an Enterococcus faecalis VRE isolated from a patient also colonized by a Staphylococcus aureus with a VanA phenotype. Antimicrob Agents Chemother 2003; 47:3954 - 3959
- Firth NP, Fink D, Johnson L, Skurray RA. A lipoprotein signal peptide encoded by the staphylococcal conjugative plasmid pSK41 exhibits an activity resembling that of Enterococcus faecalis pheromone cAD1. J Bacteriol 1994; 176:5871 - 5873
- Schlievert PM, Gahr PJ, Assimacopoulos AP, Dinges MM, Stoehr JA, Harmala JW, et al. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun 1998; 66:218 - 223
- Isenmann R, Schwarz M, Rozdzinski E, Marre R, Beger HG. Aggregation substance promotes colonic mucosal invasion of Enterococcus faecalis in an ex vivo model. J Surg Res 2000; 89:132 - 138
- Olmsted SB, Dunny GM, Erlandsen SL, Wells CL. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured epithelial cells. J Infect Dis 1994; 170:1549 - 1556
- Sussmuth SD, Muscholl-Silberhorn A, Wirth R, Susa M, Marre R, Rozdzinski E. Aggregation substance promotes adherence, phagocytosis and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect Immun 2000; 68:4900 - 4906
- Rakita RM, Vanek NN, Jacques-Palaz K, Mee M, Mariscalco MM, Dunny GM, et al. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect Immun 1999; 67:6067 - 6075
- Vanek NN, Simon SI, Jacques-Palaz K, Mariscalco MM, Dunny GM, Rakita RM. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol Med Microbiol 1999; 26:49 - 60
- Hirt H, Schlievert PM, Dunny GM. In vivo induction of virulence and antibiotic resistance transfer mediated by the sex pheromone-sensing system of pCF10. Infect Immun 2002; 70:716 - 723
- Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun 1992; 60:25 - 30
- Ember JA, Hugli TE. Characterization of the human neutrophil response to sex pheromones from Streptococcus faecalis. Am J Pathol 1989; 134:797 - 805
- Sannomiya P, Craig RA, Clewell DB, Suzuki A, Fujino M, Till GO, et al. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc Natl Acad Sci USA 1990; 87:66 - 70
- Sedgley CM, Clewell DB, Flannagan SE. Plasmid pAMS1-encoded bacteriocin-related “siblicide” in Enterococcus faecalis. J Bacteriol 2009; 191:3183 - 3188