Abstract
Integrative and conjugative elements (ICEs) are particularly interesting model systems for horizontal gene transfer, because they normally reside in an integrated state in the host chromosome but can excise and self-transfer under particular conditions, typically requiring exquisite regulatory cascades. Despite important advances in our understanding of the transfer mechanisms of a number of ICE, many essential details are lacking. Recently we reported that ICEclc, a 103-kb ICE of Pseudomonas knackmussii B13, has two active origins of transfer (oriTs), which is very much unlike conjugative plasmids that usually employ a single oriT. We discuss here how this dual oriT system could function and how it actually could have presented an evolutionary advantage for ICEclc distribution.
ICEclc is a 103 kb integrative and conjugative element (ICE) in Pseudomonas knackmussii B13, which contains genes for aromatic compound degradation. It is integrated in one or two copies at the 3′-end of genes for tRNAGly but can excise and circularize primarily in stationary phase cells that have been grown on 3-chlorobenzoate (). The circularized form of ICEclc is supposed to transfer to a recipient cell through its own conjugation apparatus, and can subsequently integrate into the recipient chromosome at one of the tRNAGly copies.Citation1 Recently we reported a new discovery on the ICEclc transfer system that sets it considerably apart from others.Citation2 The most notable finding of the study was that ICEclc has two separate and functional origins of transfer (oriTs), with different sequence context but containing a similar repeat motif. One of them (oriT1), contains part of the coding sequence of the relaxase gene (orf50240) as well as its promoter region. The other (named oriT2) is positioned within the inrR gene, which encodes a positive regulator for the expression of the integrase gene (intB13), that is essential for ICEclc excision and integration. Notably, both oriT regions are conserved among several ICEs (or suspected ICEs) related to ICEclc. Compared to most plasmid conjugation systems in which a single oriT is used for transfer, this dual oriT system of ICEclc is strikingly unique and immediately leads to two fundamental questions: (1) why ICEclc evolved with two functional oriTs and (2) how mechanistically a circular molecule can be processed during transfer when two oriTs are employed.
For the first question, experimental results suggested that the dual oriT system actually facilitates more efficient transfer of ICEclc than a single one would. This was tested in conjugation experiments with enhanced Green Fluorescent Protein (eGFP)-labeled ICEclc variants lacking one or the other or both oriTs, which showed that both oriTs are used for transfer with indistinguishable efficiencies.Citation2 Frequency calculations on single and double oriT mutants compared to wild-type transfer suggested that having two oriTs leads to a fourfold more efficient ICEclc transfer compared to having a single oriT. This difference may have been sufficiently big to confer an evolutionary advantage for the selection of an element with two oriTs, when assuming that a higher transfer rate would translate into a more efficient distribution in a microbial community.
As for the second mechanistic question, it is still difficult to explain a mechanism of processing two oriTs by the current model of plasmid conjugation. Conjugative plasmid processing basically starts with the formation of nucleoprotein complex called the relaxosome, which contains a relaxase protein, several accessory proteins and a cognate oriT.Citation3 Supported by the accessory proteins that optimize and stabilize the relaxosome, the relaxase catalyzes site- and strand-specific cleavage at the nic site in the oriT and covalently binds it to the 5′-end of the cleaved single-strand DNA (ssDNA). The complete plasmid ssDNA is subsequently unwound in a 5′-3′ direction through DNA helicase activity. Simultaneously, the uncleaved strand serves as a template for synthesis of new complementary strand via rolling-circle replication. The ssDNA-relaxase complex is finally transferred to a recipient cell through a specialized apparatus, such as the type IV secretion system in Gram-negative bacteria. We could identify the gene encoding the relaxase, which was essential for ICEclc transfer and for processing both oriTs.Citation2 However, an in vivo strand exchange assay in E. coli demonstrated that the relaxase alone can catalyze the nicking-rejoining reaction on the oriT1 sequence, but not on oriT2. This suggested that other ICEclc factors in conjunction to the relaxase may be necessary to process oriT2 (). The mechanistic problem in simultaneous processing of a single circular DNA molecule at two oriT sites would be that two nicked sites would lead to stalling relaxosomes. Possible solutions for this paradox may be the following. First of all, there could be a mechanism to regulate which of the two oriT sites to nick in a single cell. This hypothesis is supported by work by Avila et al. showing that plasmid R6K contains two functional oriTs on different strands with identical nic sites, and at least 35% of R6K molecules in vivo are nicked at both oriTs simultaneously.Citation4 Secondly, cells may stochastically behave differently leading to variation of used nicking sites across the population. In case of ICEclc transfer only a few percent of cells in a population become ‘transfer competent' during stationary phase conditions.Citation5 Finally, cells could perhaps fire ‘twice’ and reuse a circularized unwound ICEclc that is nicked and reconstituted a second time at the other oriT. Although the complete transfer apparatus and mechanism of ICEclc is still mostly unknown, since ICEclc and its relatives show little sequence homology to known plasmid conjugation systems, the dual oriT system is an indication that we may find more surprises on the possible modes of conjugation when studying ICE as compared to what is already known from conjugative plasmids.
Figures and Tables
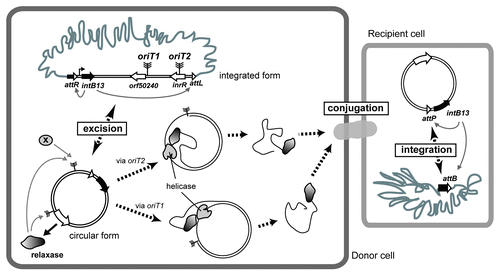
Figure 1 Schematic representation of ICEclc transfer. In donor cells, IntB13 catalyzes the excision of the ICEclc to a circular form in stationary phase. Relaxase recognizes both oriTs, but further DNA processing would be proceeded at either one of them. For nicking-rejoining oriT2, other factors (shown in X) would be required. The ssDNA-relaxase complex is then supposed to be transferred into recipient cells through a type IV secretion system. In the recipient, the incoming DNA is recircularized and used as a template to reconstruct the second strand. Then IntB13 mediates the chromosomal integration of ICEclc into the integration site attB.
Acknowledgements
Research on ICEclc was supported by grants from the Swiss National Science Foundation (31003A-124711). R.M. was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science for Research Abroad.
Addendum to:
References
- Sentchilo V, Czechowska K, Pradervand N, Minoia M, Miyazaki R, van der Meer JR. Intracellular excision and reintegration dynamics of the ICEclc genomic island of Pseudomonas knackmussii sp. strain B13. Mol Microbiol 2009; 72:1293 - 1306
- Miyazaki R, van der Meer JR. A dual functional origin of transfer in the ICEclc genomic island of Pseudomonas knackmussii B13. Mol Microbiol 2011; 79:743 - 758
- de la Cruz F, Frost LS, Meyer RJ, Zechner EL. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol Rev 2010; 34:18 - 40
- Avila P, Nunez B, de la Cruz F. Plasmid R6K contains two functional oriTs which can assemble simultaneously in relaxosomes in vivo. J Mol Biol 1996; 261:135 - 143
- Sentchilo V, Ravatn R, Werlen C, Zehnder AJ, van der Meer JR. Unusual integrase gene expression on the clc genomic island in Pseudomonas sp. strain B13. J Bacteriol 2003; 185:4530 - 4538