Abstract
Streptomycetes, Gram-positive soil bacteria well known for the production of antibiotics feature a unique conjugative DNA transfer system. In contrast to classical conjugation which is characterized by the secretion of a pilot protein covalently linked to a single-stranded DNA molecule, in Streptomyces a double-stranded DNA molecule is translocated during conjugative transfer. This transfer involves a single plasmid encoded protein, TraB. A detailed biochemical and biophysical characterization of TraB, revealed a close relationship to FtsK, mediating chromosome segregation during bacterial cell division. TraB translocates plasmid DNA by recognizing 8-bp direct repeats located in a specific plasmid region clt. Similar sequences accidentally also occur on chromosomes and have been shown to be bound by TraB. We suggest that TraB mobilizes chromosomal genes by the interaction with these chromosomal clt-like sequences not relying on the integration of the conjugative plasmid into the chromosome. Elucidation of the molecular function of TraB highlights how nature is able to develop new machineries from other cellular processes. Whereas DNA transfer by classical conjugation is done by specialized type IV protein secretion systems, for the conjugative DNA transfer in Streptomyces a chromosome segregation system was rebuild.
Elucidation of the molecular function of TraB highlights how nature is able to develop new machineries from other cellular processes. Whereas DNA transfer by classical conjugation is done by specialized type IV protein secretion systems, for the conjugative DNA transfer in Streptomyces a chromosome segregation system was rebuild.
Bacteria evolved distinct machineries to translocate DNA across membranes. Conjugation, the transfer of DNA between two distinct cells, involves a sophisticated protein secretion system that translocates a pilot protein with a covalently attached single-stranded DNA molecule.Citation1–Citation3 In contrast, segregation of chromosomal DNA during bacterial cell division is characterized by the translocation of doublestranded DNA.Citation4
Chromosome Segregation During Cell Division and Sporulation by FtsK
Chromosome segregation is mediated by the septal DNA translocator FtsK, a component of the divisome in nearly all bacteria. FtsK is a multi-domain protein which has several distinct functions during cell division.Citation5 It is a HerA-like ATPase translocating double-stranded chromosomal DNA through the closing septum to avoid guillotining of the chromosomes.Citation4 By interaction with the XerCD recombinase and topoisomerase IV, FtsK supports decatenation of intertwined chromosomes and the resolution of chromosomal dimers at dif.Citation6,Citation7 Polarized DNA translocation is achieved by the specific interaction of the C-terminal γ-domain of FtsK with KOPS (FtsK orienting polar sequences).Citation8,Citation9 KOPS are 8-bp sequence motifs that are unevenly distributed on bacterial chromosomes with a strong bias from the origin to the dif region. It is still a matter of debate, whether KOPS simply represent assembly sites of FtsK or whether FtsK hexamers are able to assemble on any DNA and its DNA translocation activity is modulated by the interaction with KOPS.Citation10–Citation13
In the gram positive mycelium forming soil bacterium Streptomyces FtsK has a major role during sporulation.Citation14 In contrast to most other bacteria that divide by binary fission, Streptomyces forms a multiply branching mycelium by apical tip extension which contains only few septal cross walls.Citation15 The mycelial compartments harbor multiple copies of a linear chromosome. Upon nutrient limitation Streptomyces undergoes morphological differentiation by erecting unbranched aerial hyphae. Subsequently, more than 50 septa are simultaneously formed ending up in a chain of spores. This process involves the Mre-proteins which determine rod-shaped morphology in other bacterial species.Citation16 Since Streptomyces mycelium contains multiple chromosomal copies, the main role of FtsK in Streptomyces is the segregation of the chromosomes during sporulation to generate spores that contain a single chromosome. Whereas in most other bacteria FtsK segregates a circular chromosome, Streptomyces FtsK has to translocate a linear chromosome.
Conjugative DNA-Translocation by the FtsK-Homolog TraB
Streptomyces was reported to possess unusual conjugative plasmids that require only a single plasmid encoded protein, TraB, for the transfer from the plasmid carrying donor into a plasmid free recipient.Citation17,Citation18 TraB was shown to interact with the cis acting locus of transfer clt,Citation19 a small (<200 bp) region required for conjugative plasmid transfer.Citation17 Deletion of a clt-locus dramatically reduces frequency of plasmid transfer.Citation20 Insertion of a clt locus into a non-transferable plasmid allows its mobilization if the corresponding TraB protein is provided in trans.Citation17
In adaptation to the mycelial growth, conjugative Streptomyces plasmids encode a second DNA translocation system for the subsequent spreading of the newly transferred plasmid within the recipient mycelium resulting in its rapid colonization. For these secondary DNA-translocations across the septal crosswalls of the recipient mycelium, called plasmid spreading, TraB has to interact with a multi protein complex of several Spd proteins, whose molecular functions are widely unknown.Citation18,Citation21
The TraB protein is an ATPase which resembles FtsK in many aspects. It has an identical domain architecture, with an N-terminal membrane anchor region.Citation22 This region which might be involved in proper protein localization is connected by a so called linker region to the DNA-translocase domain. The linker region was discussed to be involved in protein interaction but the exact function of this region still awaits its elucidation. The C-terminal wHTH domains of Streptomyces TraB proteins display a previously unrecognized homology to the FtsK γ-domain. A more detailed comparison of the respective domains of the closely related TraB proteins of the plasmids pSG5, pSVH1 and pFP11 revealed highest similarity within the DNA translocase region. Whereas the N-terminal 276 aa of TraBpSVH1 showed an identity of 75% and 54% to those of TraBpSG5 and TraBpFP11, respectively, the DNA-translocase domain (TraBpsVH1_277–688) had an identity of 80% and 77%. Lowest similarity was observed for the wHTH domain (TraBpSVH1_689–772) which had an identity of 58% and 49%. The variability of the TraB wHTH domains demonstrates the fast evolution of plasmid TraB proteins as an adaptation to the need of recognizing and transferring a specific plasmid molecule. In contrast, FtsK proteins of different bacteria hardly differ in their γ-domains.
Moreover, the mode of DNA recognition of FtsK and TraB proteins is very similar. Whereas the γ-domain of FtsK recognizes 8-bp KOPS, the wHTH folds of TraB protein interact with 8-bp TRS (TraB Recognition Sequence) repeats, present in the clt region of the corresponding plasmid.Citation22 Generation of chimeric TraB proteins originating from different Streptomyces plasmids located the protein region determining sequence specific DNA recognition to helix α3 of the wHTH fold. Exchange of only 13 aa of TraBpSVH1 against the 13 aa, comprising helix α3 of TraBpIJ101 generated a protein with a switched specificity, now recognizing the clt of plasmid pIJ101.Citation22 This is remarkably since recognition of the 8-bp TRS repeats does most probable not involve a monomer but three subunits of the TraB hexamer, as demonstrated for the FtsK γ-domain.Citation12 During DNA binding of FtsK, each monomer interacts with different bases of the KOPS.
Localization of the sequence specificity determining TraB domain to the wHTH motif and helix α3 explains the high variability of the C-termini of TraB proteins to meet the requirements of recognizing different plasmid clt loci.
Evolution of Plasmid-Encoded TraB Proteins from FtsK-Like Ancestor Proteins
On the one hand, the plasmid encoded TraB proteins are highly similar to FtsK, revealing a common ancestor. On the other hand, they are specifically adapted to the recognition of their cognate clt to direct transfer of a specific plasmid molecule. In contrast to FtsK which mediates DNA translocation between two cellular compartments, Streptomyces TraB proteins are able to translocate DNA between two distinct cells. Rendering a septal chromosome translocator protein into a conjugative plasmid translocator requires three distinct adaptations:
Localization.
Whereas FtsK interacts with other proteins of the divisome at the division septum, Streptomyces conjugation involves the tips, as it is suggested by the localization of a TraB-eGFP fusion protein.Citation19
Specific DNA recognition.
Whereas FtsK interacts with KOPS distributed all over the chromosome to determine polarity of DNA-translocation, TraB proteins specifically recognize the clt region of a Streptomyces plasmid.Citation22
Regulation.
The main function of Streptomyces FtsK is the generation of uninuclear spores during septation of the aerial mycelium. Expression of Streptomyces FtsK is therefore highest at the onset of sporulation.Citation23 In contrast, plasmid transfer takes only place at early time points, when Streptomyces grows as substrate mycelium. Expression of traB is in all Streptomyces plasmids under control of a GntR-type transcriptional repressor which responds to an unknown signal resulting in the expression of TraB during mating.
Transfer of Chromosomal Markers by the Interaction of TraB with clt-like Chromosomal Sequences clc
The plasmid clt-locus containing the 8-bp TRS repeats is only required for plasmid transfer. Surprisingly, the clt-locus is not involved in the transfer of chromosomal marker genes.Citation17 This is in contrast to the known mechanism of chromosome mobilization in type IV secretion, which relies on the presence of the cis-acting oriT in the chromosome to initiate DNA transfer in a polarized manner.Citation2 Identification of multiple clt-like chromosomal sequences (clc) in the S. coelicolor genome provides an explanation for the dispensability of a plasmid clt in the transfer of chromosomal marker genes, though the in vivo functional proof of clcs is still missing.
A PATSCAN analysis of the S. coelicolor genomic sequence for pSVH1 clt-like sequences (four copies of GAC CCG GA, with a spacing of up to 13 bp and allowing one miss match) identified 25 hits (). Streptomycetes are known to contain multiple plasmids integrated in their genome. S. coelicolor carries SLP1 and several copies of a pSAM2-like plasmid at different positions in the chromosome. Therefore, one could speculate that the clt-like clc sequences are part of or remnants of mobile genetic elements. However, a detailed analysis of the S. coelicolor cltpSVH1-like sequences in the genome showed that in most cases they were located within genes without disrupting their coding region. E.g., SCO6684 (ramB, probably encoding an ABC transporter) and SCO4600 (encoding the NADH dehydrogenase subunit NuoB2) carry highly repeated sequences of 150 bp (GGC CCG GAG CCG GAG CCG GAG CCG GAG CCG GAG CCG GAG CCG GAG CCG GAG TTG GGG TCG GGG CTG GAG CCA GAG CCA GAG CCA GCG TCA GAG CCA GAG TCG GGG CCG AGC ACC CGA GCG CAT CGG CGG CGG CAT TCG CGG TGC ACA CC) and 123 bp (CCG CCG GAG CCG GAG CCG GAG CCG GAG CCG GAG CCG GAG CCG GAG CCG GCC AGG GGC CGG GCC AGG GGC CGG GCT CGG GTG AGG GCA CGG ACA CGG GCT CGG ATG CGG GGC GAG GCC GAT GA) length, respectively. These insertions contain four TRS repeats (underlined) and encode amino acids GPE PEP EPE PEP EPE PEL GSG LEP EPE PAS EPE SGP STA SAS AAA FAV HT and PPE PEP EPE PEP EPE PGQ GPG QGP GSG EGT DTG SDA GRG R. These insertions are only found in SCO6684 and SCO4600 but not in the corresponding homologs of S. avermitilis [SAV7500 (amfA); SAV4882] or in those of other Streptomyces genomes.
Another example is SCO4022 encoding a glycosyl transferase. SCO4022 carries at is 3′end an extension containing five TRS repeats and which encodes PDA PPT THT PDP HLP DPH LPD PHL PDP HLP DPH RTW SDA R*. Again, this C-terminal extension is not present in the homologs of other Streptomyces species (e.g., SAV4196), clearly suggesting that it represents an extra insertion in S. coelicolor genes which does not contribute to the activity of the respective enzyme. Since no remnants of a mobile element are detected in the surrounding regions, these insertion might have been generated by an unknown mechanism without involvement of a plasmid.
Conjugative plasmids of other bacteria transfer chromosomal markers only at low frequencies, since mobilization requires preceding integration at specific chromosomal sites. This indicates a selective pressure for the plasmids to acquire genetic traits, as resistance, degradation of xenobiotics, pathogenicity or biosynthetic capabilities to provide an advantage to the host cell. With the exception of the giant linear plasmids, such traits are normally not found on Streptomyces plasmids. The ability to efficiently mobilize chromosomal markers without plasmid integration might substitute the presence of plasmid encoded genetic traits. Moreover, given the ubiquity of small plasmids in the population, one can speculate that there is an adaptive selection for the presence of clc sequences in Streptomyces genomes to benefit from the presence of a plasmid.
Model of the Streptomyces Conjugative DNA-Translocation System; Open Questions
Streptomyces plasmids do not seem to encode a system for establishing mating pairs. Therefore, conjugative DNA transfer occurs only on solid agar during growth as substrate mycelium. Formation of multiple branching hyphae by apical tip extension provides good probability that mating partners will meet.
During conjugation a plasmid has to pass membranes and peptidoglycan (PG) layers of donor and recipient. Nevertheless only very few Streptomyces plasmids encode a lytic transglycosylase.Citation24 Since Streptomyces hyphae grow by apical tip extension, the machinery for PG remodeling has to be present at the hyphal tip. Therefore, the mycelial tips are the most likely sites, where conjugation takes place. This is supported by the localization of TraB at the hyphal tips.Citation19 Presence of TraB might direct fusion of the walls of the mating partners, maybe by interaction with a chromosomally encoded lytic transglycosylase.
After fusion of the PG-layer, TraB might also govern fusion of the membranes of donor and recipient. For the FtsK homolog SpoIIIE, involved in the translocation of the chromosome into the fore spore of B. subtilis, a membrane fusing activity has been experimentally demonstrated.Citation25,Citation26 Membrane insertion of TraB hexamers that assembled at clt sequences provide a pore structure for the DNA translocation. Whereas evidence was reported that FtsK of E. coli might not require pore formation for DNA translocation,Citation27 TraB pores were detected in planar black lipid membranes by single channel recordings.Citation22
TraB was shown to bind to the clt locus non-covalently without processing the plasmid.Citation19 This raises the question, how TraB is able to translocate a circular DNA molecule. While during cell division the chromosome is already present at the closing septum, allowing FtsK to assemble at KOPS sequences at each chromosomal arm, TraB has to translocate the DNA across an intact membrane. Since it is highly unlikely that a circular molecule can be transported across the membrane, involvement of a further DNA processing enzyme, e.g., a topoisomerase has to be postulated. Such an activity was described for FtsK which stimulates topoisomerase IV activity to allow decatenation of chromosomes.Citation28 Another possibility would be the fusion of the two TraB hexamers to a single channel structure releasing the circular plasmid end into the recipient. This model was developed to explain release of the transferred chromosome during B. subtilis sporulation.Citation29
A further unsolved problem regards the mobilization of chromosomes. Streptomyces chromosomes are linear and carry covalently linked telomeric proteins (TP) at the ends. Due to the molecular size of the central channel, TraB hexamers should not allow translocation of TP-bound DNA. Therefore one has to postulate a mechanism for removal and reattachment of TP during conjugative transfer of chromosomal DNA. For the FtsK homolog SpoIIIE which segregates chromosomal DNA into the forespore during sporulation of Bacillus subtilis, a so called wire stripping activity removing all DNA bound regulatory proteins during DNA translocation has been demonstrated.Citation30
Elucidation of the TraB-mediated conjugative DNA translocation in Streptomyces demonstrates that nature developed at least two distinct plasmid transfer systems from other cellular processes. On the one side, the classical conjugation system found in nearly all gram negative bacteria and many gram positives, which is derived from a protein secretion system. On the other side, the newly characterized Streptomyces transfer system, which evolved from an intracellular chromosome segregation system.
Figures and Tables
Figure 1 Model of conjugative DNA translocation in mycelial streptomycetes. After establishing contact between donor (gray) and recipient mycelium (white, dashed lines indicate neighboring mycelial compartments) and partial fusion of the hyphal tips, TraB hexamers (blue) assemble at clt by specifically recognizing 8-bp TRS repeats. The TraB hexamers form pore structures to the recipient and direct the translocation of a double-stranded plasmid molecule. DNA transfer is energized by ATP hydrolysis. Up to now it is unclear (?) whether the circular plasmid DNA has to be processed into a linear molecule and which enzymes might be involved. For further description see text.
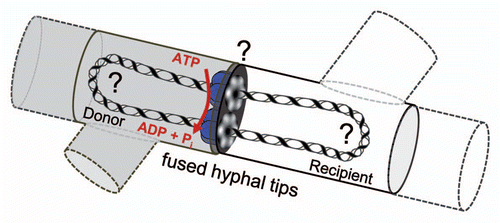
Table 1 PAT SCAN analysisTable Footnote* of the S. coelicolor genome for the presence of cltpSVH1-like chromosomal sequences (clc)Table Footnote*
Achnowledgments
The research was supported by the DFG (SFB766) and by a DAAD fellowship to E.S.
References
- Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev 2009; 73:775 - 808; PMID: 19946141; http://dx.doi.org/10.1128/MMBR.00023-09
- de la Cruz F, Frost LS, Meyer RJ, Zechner EL. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol Rev 2010; 34:18 - 40; PMID: 19919603; http://dx.doi.org/10.1111/j.1574-6976.2009.00195.x
- Schröder G, Lanka E. The mating pair formation system of conjugative plasmids—A versatile secretion machinery for transfer of proteins and DNA. Plasmid 2005; 54:1 - 25; PMID: 15907535; http://dx.doi.org/10.1016/j.plasmid.2005.02.001
- Massey TH, Mercogliano CP, Yates J, Sherratt DJ, Löwe J. Double-stranded DNA translocation: structure and mechanism of hexameric FtsK. Mol Cell 2006; 23:457 - 469; PMID: 16916635; http://dx.doi.org/10.1016/j.molcel.2006.06.019
- Dubarry N, Possoz C, Barre FX. Multiple regions along the Escherichia coli FtsK protein are implicated in cell division. Mol Microbiol 2010; 78:1088 - 1100; PMID: 21091498; http://dx.doi.org/10.1111/j.1365-2958.2010.07412.x
- Grainge I, Lesterlin C, Sherratt DJ. Activation of XerCD-dif recombination by the FtsK DNA translocase. Nucleic Acids Res 2011; 39:5140 - 5148
- Ip SC, Bregu M, Barre FX, Sherratt DJ. Decatenation of DNA circles by FtsK-dependent Xer site-specific recombination. EMBO J 2003; 22:6399 - 6407; PMID: 14633998; http://dx.doi.org/10.1093/emboj/cdg589
- Bigot S, Saleh OA, Cornet F, Allemand JF, Barre FX. Oriented loading of FtsK on KOPS. Nat Struct Mol Biol 2006; 13:1026 - 1028; PMID: 17041597; http://dx.doi.org/10.1038/nsmb1159
- Sivanathan V, Allen MD, de Bekker C, Baker R, Arciszewska LK, Freund SM, et al. The FtsK gamma domain directs oriented DNA translocation by interacting with KOPS. Nat Struct Mol Biol 2006; 13:965 - 972; PMID: 17057717; http://dx.doi.org/10.1038/nsmb1158
- Graham JE, Sherratt DJ, Szczelkun MD. Sequence-specific assembly of FtsK hexamers establishes directional translocation on DNA. Proc Natl Acad Sci USA 2010; 107:20263 - 20268; PMID: 21048089; http://dx.doi.org/10.1073/pnas.1007518107
- Sivanathan V, Emerson JE, Pages C, Cornet F, Sherratt DJ, Arciszewska LK. KOPS-guided DNA translocation by FtsK safeguards Escherichia coli chromosome segregation. Mol Microbiol 2009; 71:1031 - 1042; PMID: 19170870; http://dx.doi.org/10.1111/j.1365-2958.2008.06586.x
- Löwe J, Ellonen A, Allen MD, Atkinson C, Sherratt DJ, Grainge I. Molecular mechanism of sequence-directed DNA loading and translocation by FtsK. Mol Cell 2008; 31:498 - 509; PMID: 18722176; http://dx.doi.org/10.1016/j.molcel.2008.05.027
- Ptacin JL, Nollmann M, Bustamante C, Cozzarelli NR. Identification of the FtsK sequence-recognition domain. Nat Struct Mol Biol 2006; 13:1023 - 1025; PMID: 17041598; http://dx.doi.org/10.1038/nsmb1157
- Wang L, Yu Y, He X, Zhou X, Deng Z, Chater KF, et al. Role of an FtsK-like protein in genetic stability in Streptomyces coelicolor A3(2). J Bacteriol 2007; 189:2310 - 2318; PMID: 17209017; http://dx.doi.org/10.1128/JB.01660-06
- Flärdh K, Buttner MJ. Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 2009; 7:36 - 49; PMID: 19079351; http://dx.doi.org/10.1038/nrmicro1968
- Kleinschnitz EM, Heichlinger A, Schirner K, Winkler J, Latus A, Maldener I, et al. Proteins encoded by the mre gene cluster in Streptomyces coelicolor A3(2) cooperate in spore wall synthesis. Mol Microbiol 2011; 79:1367 - 1379; PMID: 21244527; http://dx.doi.org/10.1111/j.1365-2958.2010.07529.x
- Pettis GS, Cohen SN. Transfer of the plJ101 plasmid in Streptomyces lividans requires a cis-acting function dispensable for chromosomal gene transfer. Mol Microbiol 1994; 13:955 - 964; PMID: 7854128; http://dx.doi.org/10.1111/j.1365-2958.1994.tb00487.x
- Grohmann E, Muth G, Espinosa M. Conjugative plasmid transfer in gram-positive bacteria. Microbiol Mol Biol Rev 2003; 67:277 - 301; PMID: 12794193; http://dx.doi.org/10.1128/MMBR.67.2.277-301.2003
- Reuther J, Gekeler C, Tiffert Y, Wohlleben W, Muth G. Unique conjugation mechanism in mycelial streptomycetes: a DNA-binding ATPase translocates unprocessed plasmid DNA at the hyphal tip. Mol Microbiol 2006; 61:436 - 446; PMID: 16776656; http://dx.doi.org/10.1111/j.1365-2958.2006.05258.x
- Servín-González L. Identification and properties of a novel clt locus in the Streptomyces phaeochromogenes plasmid pJV1. J Bacteriol 1996; 178:4323 - 4326; PMID: 8763967
- Tiffert Y, Gotz B, Reuther J, Wohlleben W, Muth G. Conjugative DNA transfer in Streptomyces: SpdB2 involved in the intramycelial spreading of plasmid pSVH1 is an oligomeric integral membrane protein that binds to dsDNA. Microbiology 2007; 153:2976 - 2983; PMID: 17768240; http://dx.doi.org/10.1099/mic.0.2006/005413-0
- Vogelmann J, Ammelburg M, Finger C, Guezguez J, Linke D, Flotenmeyer M, et al. Conjugal plasmid transfer in Streptomyces resembles bacterial chromosome segregation by FtsK/SpoIIIE. EMBO J 2011; 30:2246 - 2254; PMID: 21505418; http://dx.doi.org/10.1038/emboj.2011.121
- Dedrick RM, Wildschutte H, McCormick JR. Genetic interactions of smc, ftsK and parB genes in Streptomyces coelicolor and their developmental genome segregation phenotypes. J Bacteriol 2009; 191:320 - 332; PMID: 18978061; http://dx.doi.org/10.1128/JB.00858-08
- Xu M, Zhu Y, Zhang R, Shen M, Jiang W, Zhao G, et al. Characterization of the genetic components of Streptomyces lividans linear plasmid SLP2 for replication in circular and linear modes. J Bacteriol 2006; 188:6851 - 6857; PMID: 16980488; http://dx.doi.org/10.1128/JB.00873-06
- Sharp MD, Pogliano K. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc Natl Acad Sci USA 1999; 96:14553 - 14558; PMID: 10588743; http://dx.doi.org/10.1073/pnas.96.25.14553
- Liu NJ, Dutton RJ, Pogliano K. Evidence that the SpoIIIE DNA translocase participates in membrane fusion during cytokinesis and engulfment. Mol Microbiol 2006; 59:1097 - 1113; PMID: 16430687; http://dx.doi.org/10.1111/j.1365-2958.2005.05004.x
- Dubarry N, Barre FX. Fully efficient chromosome dimer resolution in Escherichia coli cells lacking the integral membrane domain of FtsK. EMBO J 2010; 29:597 - 605; PMID: 20033058; http://dx.doi.org/10.1038/emboj.2009.381
- Bigot S, Marians KJ. DNA chirality-dependent stimulation of topoisomerase IV activity by the C-terminal AAA+ domain of FtsK. Nucleic Acids Res 2010; 38:3031 - 3040; PMID: 20081205; http://dx.doi.org/10.1093/nar/gkp1243
- Burton BM, Marquis KA, Sullivan NL, Rapoport TA, Rudner DZ. The ATPase SpoIIIE transports DNA across fused septal membranes during sporulation in Bacillus subtilis. Cell 2007; 131:1301 - 1312; PMID: 18160039; http://dx.doi.org/10.1016/j.cell.2007.11.009
- Marquis KA, Burton BM, Nollmann M, Ptacin JL, Bustamante C, Ben-Yehuda S, et al. SpoIIIE strips proteins off the DNA during chromosome translocation. Genes Dev 2008; 22:1786 - 1795; PMID: 18593879; http://dx.doi.org/10.1101/gad.1684008