Abstract
The extraordinary evolutionary success of transposable elements (TEs) invites us to question the nature of the co-evolutionary dynamics between TE and host. Although sometimes assumed to be wholly parasitic, TEs have penetrated and spread throughout eukaryotic genomes at a rate unparalleled by other parasites. This near-ubiquity, occurring despite the potentially deleterious effects of insertional mutagenesis, raises the possibility that a counterbalancing benefit exists for the host. Such a benefit may act at the population level to generate genomic diversity within a species and hence greater adaptability under new selective pressures, or at the level of primary gain for the individual. Recent studies have highlighted the occurrence of retrotransposition events in the germline and discovered a surprisingly high rate of mobilization in somatic cells. Here we examine the available evidence for somatic retrotransposition and discuss how this phenomenon may confer a selective advantage upon an individual or species.
Transposable elements are a prominent feature of our genetic heritage. In addition to providing nearly half of the human genome,Citation1,Citation2 TEs have generated numerous sequences that distinguish our DNA from that of other primates and more distant relatives.Citation3,Citation4 Whether these differences are a cause or effect of evolution, and whether TEs are parasitic or symbiotic mobile genetic elements, is the subject of long-term debate.Citation5,Citation6
Three retrotransposon families remain mobile in the human genome: L1, Alu and SVA.Citation7,Citation8 Of these, L1 is considered the main driver of retrotransposition (). Proteins translated from its two open reading frames mobilize L1 RNAs in cisCitation9 as well as Alu, SVA and other RNAs incorporating a polyA tail in transCitation10-Citation12 (). Approximately 3,000 retrotransposons (~100 L1, ~3,000 Alu, < 100 SVA) are transposition-competent per individual,Citation13 in contrast to the millions of immobile sequences produced by ancestral TEs.Citation1
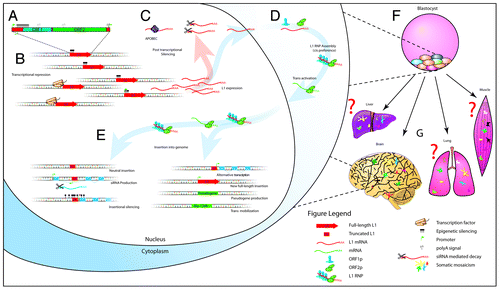
Figure 1. L1 is the main driver of retrotransposition in human cells. (A) L1 structure. ORF1 encodes an RNA-packaging protein and ORF2 encodes a protein (ORF2p) with endonuclease and reverse transcriptase domains.Citation50,Citation51 (B) Expression of L1 is limited by transcriptional repression and (C) post transcriptional regulation. (D) L1 ORF1p and ORF2p form an RNP with a marked cis preference,Citation9 but ORF2p can also mobilize other RNAs with a polyA tail in trans. (E) Diverse effects of L1 insertional mutagenesis on gene expression. (F) L1 is known to be highly active during embryogenesis, and in neural cells (G), resulting in somatic mosaicism. Somatic retrotransposition in other adult tissues may also occur.
Other than a common pattern of near-exclusion from exons,Citation14 the genomic distributions of L1, Alu and SVA are markedly different. L1 sequences are depleted in intronsCitation1 and very recent L1 insertions are more likely to be excluded from protein-coding genes than older insertions,Citation14,Citation15 suggesting that these events are strongly selected against.Citation16,Citation17 By contrast, recent Alu insertions are almost randomly distributed in the genome and SVA insertions are enriched in protein-coding genes.Citation14 As noted above, the L1 machinery mediates L1, Alu and SVA mobilization, implying that each family is inserted in a similar genomic pattern and then redacted from the genome by natural selection depending on their impact. It is also possible that insertion site preference is modulated by unknown host factor interactions specific to each family.
An obvious consequence of insertional mutagenesis is genetic disease; TEs are associated with more than 75 human disorders.Citation13,Citation18 Likewise there are numerous documented cases of alternative transcripts and chimeric genes produced by TE insertions, often leading to expression of a host gene in a new spatiotemporal contextCitation19,Citation20 (). Several L1 sequence features, including a long polyA tail and strong internal 5′ and 3′ promotersCitation19,Citation21 can also dramatically alter the expression of a host gene in cases of intronic integration,Citation17 while the epigenetic marks associated with L1 and other retrotransposonsCitation22 can modify chromatin state at integration sites and thereby drive rapid shifts in gene expression ().
Given the multiple routes by which TEs can deleteriously alter the functional landscape of a genome, it is perhaps surprising that the global human population presents such a large number of dimorphic insertions.Citation23 Recent studies using high-throughput sequencing (for reviews, see refs. Citation13, Citation24 and Citation25) have yielded a wealth of new insertion sites in healthy and diseased individuals, suggesting the full catalog of dimorphic and private insertions has been vastly underestimated and that roughly 1/20 live births harbor de novo retrotransposition events.
Most of these new insertions are thought to be neutral and are ultimately lost or fixed through genetic drift. The overall impact of the remaining insertions is likely to be overwhelmingly deleterious, raising the question of why retrotransposition is allowed to continue at an apparently high rate. More effective TE suppression would prevent harmful mutations, both in the germline and during somatic development.Citation26 A model of successful parasitism would suggest that we have simply failed; that somehow despite a clear selective advantage to the host in silencing retrotransposons, L1 has managed to evade all attempts to prevent its activity. However, suppression of L1 has been effective during our recent evolution: less than 0.002% of human L1 copies are transposition-competent, and even fewer are frequently active or “hot”Citation7,Citation27. While the current state of L1 activity is a snapshot of a dynamic system, this could nonetheless suggest that it is evolutionarily advantageous to limit retrotransposition but not to totally eradicate it. For example, the South American rat genus OryzomysCitation28,Citation29 has won this evolutionary arms race, apparently achieving L1 quiescence but, interestingly, this outcome coincides with a notable increase in karyotypic instability.Citation30
This leads us to consider the position that regulated germline retrotransposition confers a benefit upon a host population. L1 provides clues for how this system may have co-evolved with the governing transcriptional programs of the host. Paradoxically, the canonical L1 promoter has retained motifs necessary for its transcriptional suppression in the germline and throughout development (e.g., SOX2 binding sitesCitation31,Citation32) while new, usually 5′ truncated, L1 insertions are rapidly inactivated despite breaking free of the suppression inherent to the canonical L1 promoterCitation33,Citation34 (). Thus, L1 maintains its own suppression but is not entirely silenced, leading to a tolerable rate of insertional mutagenesis while maintaining increased genomic malleability and genetic diversity that may be selected on when a population is strongly pressured (e.g., in the cases of pandemic or famine). For example, an L1-mediated TRIM5-CypA gene fusionCitation35 following the divergence of Old and New World primates provides owl monkeys with HIV resistance not seen in other New World monkeys.
Nonetheless, a model founded exclusively upon observations of germline retrotransposition may be critically incomplete. We propose that L1 activity during ontogenesisCitation36-Citation38 () may serve to accelerate TE and host co-evolution. Recent reports suggest that the brain is a hotspot of somatic mosaicism caused by L1 mobilization during neurogenesis.Citation31,Citation39-Citation41 If calculations of 80 somatic L1 insertions per neuron, of which there are ~1011 present in the human body,Citation42 are even approximately accurate,Citation31 then a single human individual may have more somatic L1 insertions than the total number of private germline L1 insertions in the global population. Informing this scenario further, we recently developed a technology to map somatic L1 insertions in human cells.Citation39 Our principal conclusions were that these events preferentially impacted protein-coding genes expressed in the brain, that the hippocampus—as seen previouslyCitation31—was particularly enriched for somatic retrotransposition and that neural cells indeed presentCitation43 a remarkable degree of somatic genome mosaicism. Despite this advance, numerous questions are yet to be answered, including (1) the timing of somatic L1 mobilization throughout life; (2) how many events occur per individual, organ or cell; (3) whether certain population groups are particularly affected; (4) which transcription factors govern L1 activation in somatic cells other than neurons and (5) whether the same rules that apply in germ cells (e.g., a limited number of “hot” donor elements and familiesCitation7,Citation27) also apply to somatic cells.
Moreover, as somatic events are by definition non-heritable, it is the propensity for L1 mobilization, rather than its consequences, on which natural selection may apply. If true, this may suggest that the brain is enriched for somatic mobilization as an innocent bystander in an evolutionary arms race occurring primarily in the germline. A large percentage of genes expressed in the brain are also expressed in the testis (the “brains and balls” phenomenonCitation44), meaning that L1 transcription may be activated in somatic cells as an accident of evolution. The mutagenic effects of these insertions may then be simply tolerated by somatic cells; in addition to a reduction in impact due to heterozygosity, each mutation is expected to affect only a small sub-population of mature cells.
Another, more striking, possibility is that somatic retrotransposition confers some primary gain upon the individual host. As noted by others,Citation45 Barbara McClintock’s celebrated discovery of transposition-derived kernel variegation in maizeCitation46 was also the first description of somatic mosaicism caused by a transposable element. Singer et al.Citation43 more recently provided a compelling case for the potential action of L1 in producing somatic mosaicism in neural cells, resulting in greater genetic diversity and thus a greater variety of behavioral phenotypes in isogenic animals. As at the population level, genetic diversity may be beneficial at the cellular level. One classic example, driven by RAG proteins domesticated from an ancient transposon,Citation47,Citation48 is V(D)J recombination, where somatic rearrangements in immunoglobins and T-cell receptorsCitation49 provide genetic diversification crucial for the adaptive immune system.
The contribution of TEs to the fitness and success of species may not be limited to their well-documented effects on the genome mediated through germline retrotransposition. Their potential role in driving genetic diversity both within and between individuals adds yet another layer to the complex relationship between TEs and their hosts. Characterization of the regulation and functional impact of somatic retrotransposition is now feasible,Citation39 and may soon settle debate on whether TEs are merely globally successful parasites, or diverse genomic symbiotes.
| Abbreviations: | ||
| TE | = | transposable element |
| LINE- 1 or L1 | = | long interspersed nuclear element |
| ORF | = | open reading frame |
Acknowledgments
G.J.F. receives the support of a C.J. Martin Overseas Based Biomedical Fellowship from the Australian NHMRC (575585). J.K.B. is supported by a Wellcome Trust Clinical Fellowship (090385/Z/09/Z) through the Edinburgh Clinical Academic Track (ECAT). G.J.F. is funded by an Institute Strategic Programme Grant and a New Investigator Award from the British BBSRC (BB/H005935/1) and an EU FP7 Collaborative Project research grant (FP7-HEALTH-2010–259743).
References
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature 2001; 409:860 - 921; http://dx.doi.org/10.1038/35057062; PMID: 11237011
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science 2001; 291:1304 - 51; http://dx.doi.org/10.1126/science.1058040; PMID: 11181995
- Chimpanzee Sequencing and Analysis Consortium. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 2005; 437:69 - 87; http://dx.doi.org/10.1038/nature04072; PMID: 16136131
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002; 420:520 - 62; http://dx.doi.org/10.1038/nature01262; PMID: 12466850
- Britten RJ, Davidson EH. Gene regulation for higher cells: a theory. Science 1969; 165:349 - 57; http://dx.doi.org/10.1126/science.165.3891.349; PMID: 5789433
- Doolittle WF, Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature 1980; 284:601 - 3; http://dx.doi.org/10.1038/284601a0; PMID: 6245369
- Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, et al. Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci USA 2003; 100:5280 - 5; http://dx.doi.org/10.1073/pnas.0831042100; PMID: 12682288
- Mills RE, Bennett EA, Iskow RC, Devine SE. Which transposable elements are active in the human genome?. Trends Genet 2007; 23:183 - 91; http://dx.doi.org/10.1016/j.tig.2007.02.006; PMID: 17331616
- Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, et al. Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol 2001; 21:1429 - 39; http://dx.doi.org/10.1128/MCB.21.4.1429-1439.2001; PMID: 11158327
- Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet 2003; 35:41 - 8; http://dx.doi.org/10.1038/ng1223; PMID: 12897783
- Esnault C, Maestre J, Heidmann T. Human LINE retrotransposons generate processed pseudogenes. Nat Genet 2000; 24:363 - 7; http://dx.doi.org/10.1038/74184; PMID: 10742098
- Hancks DC, Goodier JL, Mandal PK, Cheung LE, Kazazian HH Jr. Retrotransposition of marked SVA elements by human L1s in cultured cells. Hum Mol Genet 2011; 20:3386 - 400; http://dx.doi.org/10.1093/hmg/ddr245; PMID: 21636526
- Faulkner GJ. Retrotransposons: mobile and mutagenic from conception to death. FEBS Lett 2011; 585:1589 - 94; http://dx.doi.org/10.1016/j.febslet.2011.03.061; PMID: 21477589
- Stewart C, Kural D, Stromberg MP, Walker JA, Konkel MK, Stutz AM, et al. A comprehensive map of mobile element insertion polymorphisms in humans. PLoS Genet 2011; 7:e1002236; http://dx.doi.org/10.1371/journal.pgen.1002236; PMID: 21876680
- Ewing AD, Kazazian HH Jr. High-throughput sequencing reveals extensive variation in human-specific L1 content in individual human genomes. Genome Res 2010; 20:1262 - 70; http://dx.doi.org/10.1101/gr.106419.110; PMID: 20488934
- Boissinot S, Entezam A, Furano AV. Selection against deleterious LINE-1-containing loci in the human lineage. Mol Biol Evol 2001; 18:926 - 35; PMID: 11371580
- Han JS, Szak ST, Boeke JD. Transcriptional disruption by the L1 retrotransposon and implications for mammalian transcriptomes. Nature 2004; 429:268 - 74; http://dx.doi.org/10.1038/nature02536; PMID: 15152245
- Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet 2002; 3:370 - 9; http://dx.doi.org/10.1038/nrg798; PMID: 11988762
- Faulkner GJ, Kimura Y, Daub CO, Wani S, Plessy C, Irvine KM, et al. The regulated retrotransposon transcriptome of mammalian cells. Nat Genet 2009; 41:563 - 71; http://dx.doi.org/10.1038/ng.368; PMID: 19377475
- Lamprecht B, Walter K, Kreher S, Kumar R, Hummel M, Lenze D, et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med 2010; 16:571-9, 1p following 9.
- Swergold GD. Identification, characterization, and cell specificity of a human LINE-1 promoter. Mol Cell Biol 1990; 10:6718 - 29; PMID: 1701022
- Lunyak VV, Prefontaine GG, Nunez E, Cramer T, Ju BG, Ohgi KA, et al. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science 2007; 317:248 - 51; http://dx.doi.org/10.1126/science.1140871; PMID: 17626886
- Ewing AD, Kazazian HH Jr. Whole-genome resequencing allows detection of many rare LINE-1 insertion alleles in humans. Genome Res 2011; 21:985 - 90; http://dx.doi.org/10.1101/gr.114777.110; PMID: 20980553
- Beck CR, Garcia-Perez JL, Badge RM, Moran JV. LINE-1 Elements in Structural Variation and Disease. Annu Rev Genomics Hum Genet 2011; 12:187 - 215; http://dx.doi.org/10.1146/annurev-genom-082509-141802; PMID: 21801021
- Ray DA, Batzer MA. Reading TE leaves: new approaches to the identification of transposable element insertions. Genome Res 2011; 21:813 - 20; http://dx.doi.org/10.1101/gr.110528.110; PMID: 21632748
- Miki Y, Nishisho I, Horii A, Miyoshi Y, Utsunomiya J, Kinzler KW, et al. Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res 1992; 52:643 - 5; PMID: 1310068
- Beck CR, Collier P, Macfarlane C, Malig M, Kidd JM, Eichler EE, et al. LINE-1 Retrotransposition Activity in Human Genomes. Cell 2010; 141:1159 - 70; http://dx.doi.org/10.1016/j.cell.2010.05.021; PMID: 20602998
- Casavant NC, Scott L, Cantrell MA, Wiggins LE, Baker RJ, Wichman HA. The end of the LINE?: lack of recent L1 activity in a group of South American rodents. Genetics 2000; 154:1809 - 17; PMID: 10747071
- Grahn RA, Rinehart TA, Cantrell MA, Wichman HA. Extinction of LINE-1 activity coincident with a major mammalian radiation in rodents. Cytogenet Genome Res 2005; 110:407 - 15; http://dx.doi.org/10.1159/000084973; PMID: 16093693
- Koop BF, Baker RJ, Genoways HH. Numerous chromosomal polymorphisms in a natural population of rice rats (Oryzomys, Cricetidae). Cytogenet Cell Genet 1983; 35:131 - 5; http://dx.doi.org/10.1159/000131854; PMID: 6342982
- Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, et al. L1 retrotransposition in human neural progenitor cells. Nature 2009; 460:1127 - 31; http://dx.doi.org/10.1038/nature08248; PMID: 19657334
- Tchénio T, Casella JF, Heidmann T. Members of the SRY family regulate the human LINE retrotransposons. Nucleic Acids Res 2000; 28:411 - 5; http://dx.doi.org/10.1093/nar/28.2.411; PMID: 10606637
- Athanikar JN, Badge RM, Moran JVA. YY1-binding site is required for accurate human LINE-1 transcription initiation. Nucleic Acids Res 2004; 32:3846 - 55; http://dx.doi.org/10.1093/nar/gkh698; PMID: 15272086
- Yang N, Zhang L, Zhang Y, Kazazian HH Jr. An important role for RUNX3 in human L1 transcription and retrotransposition. Nucleic Acids Res 2003; 31:4929 - 40; http://dx.doi.org/10.1093/nar/gkg663; PMID: 12907736
- Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 2004; 430:569 - 73; http://dx.doi.org/10.1038/nature02777; PMID: 15243629
- Garcia-Perez JL, Marchetto MC, Muotri AR, Coufal NG, Gage FH, O'Shea KS, et al. LINE-1 retrotransposition in human embryonic stem cells. Hum Mol Genet 2007; 16:1569 - 77; http://dx.doi.org/10.1093/hmg/ddm105; PMID: 17468180
- Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, et al. L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev 2009; 23:1303 - 12; http://dx.doi.org/10.1101/gad.1803909; PMID: 19487571
- van den Hurk JA, Meij IC, Seleme MC, Kano H, Nikopoulos K, Hoefsloot LH, et al. L1 retrotransposition can occur early in human embryonic development. Hum Mol Genet 2007; 16:1587 - 92; http://dx.doi.org/10.1093/hmg/ddm108; PMID: 17483097
- Baillie JK, Barnett MW, Upton KR, Gerhardt DJ, Richmond TA, De Sapio F, et al. Somatic retrotransposition alters the genetic landscape of the human brain. Nature 2011; 479:534 - 7; http://dx.doi.org/10.1038/nature10531; PMID: 22037309
- Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature 2005; 435:903 - 10; http://dx.doi.org/10.1038/nature03663; PMID: 15959507
- Muotri AR, Marchetto MC, Coufal NG, Oefner R, Yeo G, Nakashima K, et al. L1 retrotransposition in neurons is modulated by MeCP2. Nature 2010; 468:443 - 6; http://dx.doi.org/10.1038/nature09544; PMID: 21085180
- Mattick JS, Taft RJ, Faulkner GJ. A global view of genomic information–moving beyond the gene and the master regulator. Trends Genet 2010; 26:21 - 8; http://dx.doi.org/10.1016/j.tig.2009.11.002; PMID: 19944475
- Singer T, McConnell MJ, Marchetto MC, Coufal NG, Gage FH. LINE-1 retrotransposons: mediators of somatic variation in neuronal genomes?. Trends Neurosci 2010; 33:345 - 54; http://dx.doi.org/10.1016/j.tins.2010.04.001; PMID: 20471112
- Graves JA. Review: Sex chromosome evolution and the expression of sex-specific genes in the placenta. Placenta 2010; 31:Suppl S27 - 32; http://dx.doi.org/10.1016/j.placenta.2009.12.029; PMID: 20163856
- Levin HL, Moran JV. Dynamic interactions between transposable elements and their hosts. Nat Rev Genet 2011; 12:615 - 27; http://dx.doi.org/10.1038/nrg3030; PMID: 21850042
- McClintock B. Controlling elements and the gene. Cold Spring Harb Symp Quant Biol 1956; 21:197 - 216; PMID: 13433592
- Hiom K, Melek M, Gellert M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell 1998; 94:463 - 70; http://dx.doi.org/10.1016/S0092-8674(00)81587-1; PMID: 9727489
- Melek M, Gellert M, van Gent DC. Rejoining of DNA by the RAG1 and RAG2 proteins. Science 1998; 280:301 - 3; http://dx.doi.org/10.1126/science.280.5361.301; PMID: 9535663
- Hozumi N, Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci USA 1976; 73:3628 - 32; http://dx.doi.org/10.1073/pnas.73.10.3628; PMID: 824647
- Doucet AJ, Hulme AE, Sahinovic E, Kulpa DA, Moldovan JB, Kopera HC, et al. Characterization of LINE-1 ribonucleoprotein particles. PLoS Genet 2010; 6:e1001150; http://dx.doi.org/10.1371/journal.pgen.1001150; PMID: 20949108
- Khazina E, Truffault V, Buttner R, Schmidt S, Coles M, Weichenrieder O. Trimeric structure and flexibility of the L1ORF1 protein in human L1 retrotransposition. Nat Struct Mol Biol 2011; 18:1006 - 14; http://dx.doi.org/10.1038/nsmb.2097; PMID: 21822284