In Wagstaff et al.,Citation1 we described our findings using an adaptation of a tagged Alu system that would allow for the recovery of over 200 de novo Alu inserts in culture. This is a valuable system that provides a complementary approach to genomic analysis. For example, the features of the recovered Alu inserts were highly consistent with the observed characteristics of genomic Alu elements, validating the assay system as a useful tool to study SINEs. This was particularly surprising to us, as the tissue culture assay system requires the dramatic change of the length of the 300 bp Alu transcript through the addition a sequence tag of almost 2000 bases. Analysis of the distribution of de novo inserts also reinforced their mutagenic potential, with new Alu elements showing an insertional preference into genes and other SINEs. However, one of the most interesting observations was the consistent expansion of the A-tail of de novo inserts due to slippage of the LINE-1 ORF2 protein during reverse transcription. This property of the ORF2 protein has several implications on mobile element biology. In the manuscript, we discuss the importance of the ORF2 protein slippage in the maintenance of active SINE source elements in primate lineages. Second, the A-tail expansion of Alu elements may provide an explanation for the appearance of Alu “stealth-driver” elementsCitation2 or the Alu “backseat” driver in the Orangutan genome.Citation3 This observation reinforces an ongoing dynamic model where active Alu elements “die” via the loss of their long homogenous A-tail as new ones are reintroduced. Third, there is a resemblance of the ORF2 slippage to the reiterative synthesis used by telomerase reinforcing the previously suggested evolutionary connection between telomerase and non-LTR element proteins.Citation4 Thus, telomerase studies may lead to insights into L1 ORF2p biology and vice-versa. However, there is another potential impact of this feature of ORF2p where it may have had an important role in the genesis of SINEs, which is discussed here.
Alu elements are considered non-autonomous elements because they depend on LINE-1 factors for their own mobilization.Citation5 The dependency of non-autonomous elements on autonomous elements is supported by different evolutionary and genome analyses and results from biological assay systems that demonstrate coevolution of LINE/SINE pairs. A striking observation is the high success rate of some of these parasitic elements. For example, some plant genomes have several orders of magnitude higher copy numbers of the MITE mPing than the DNA transposon Ping.Citation6 Although less dramatic, the number of Alu copies (~1.1million) in the human genome doubles the copy number of its partner LINE-1, (~0.5 million).Citation7 The accumulation of Alu inserts may represent a post-insertion bias, where the genome tolerates Alu inserts better than the larger LINE-1 inserts.Citation8 However, other studies suggest about a 10-fold higher germline insertion rate for Alu than L1.Citation9 In addition, polymorphic Alu insertions are far more common than polymorphic L1 insertions.Citation10,Citation11 This is particularly intriguing as LINE-1 shows a cis-preference and preferentially mobilizes its own RNA.Citation12 Many non-autonomous elements have little to no sequence similarity to their autonomous partner or to other non-autonomous element families. This is particularly evident when comparing the sequences of non-autonomous elements driven by LINE-1. In rodents, LINE-1 has mobilized not one SINE, but several families of SINEs: B1, B2, B4/RSINE and ID.Citation13 In the human genome, LINE-1 has contributed to the accumulation of about 35,000 processed pseudogenes from a large variety of genes,Citation14-Citation16 AluCitation5 and the retroposon SVA.Citation17-Citation19 However, one feature is shared by all these parasitic elements: a 3′ oligo-A tail, also present in the LINE-1. Hence, an alternate name exists for LINES: polyA retrotransposons. As more data became available from LINE/SINE pairs, the presence of shared sequences at the 3′ end emerged. Okada's group first reported this 3′ shared sequence homology between the tortoise SINE and CR1-like LINE in turtlesCitation20 and multiple subsequent examples confirming this observation.Citation21,Citation22 The importance of having a shared 3′end became clearer with the development of a model for the retrotransposon insertion mechanism based on discoveries using the R2Bm element Bombyx mori.Citation23,Citation24 The model delineates a process of target primed reverse transcription where the cleaved DNA provides the priming site for reverse transcription by base-pairing with the 3′ end of the retrotransposon’s RNA. LINE/SINE pairs are subdivided into stringent or relaxed based on the shared sequence present at their 3′ end (). The 3′ sequence of a stringent SINE element is derived from the LINE (). A stem-loop structure at the 3′ end is commonly observed in the stringent LINE/SINE pairs. This stem-loop is important for mobilization, as sequence changes affecting structure significantly reduce retrotransposition efficiency.Citation21 The relaxed LINE/SINE pairs share a 3′ oligoA region. The polyA connection has been previously discussed.Citation25 The human LINE-1 and its diverse non-autonomous partners fit in this category (). Although a simple repetitive sequence, the absence of the polyA-tail significantly hinders retrotransposition. The presence of the 3′ polyA tail in the RNA is just as important for AluCitation26 as for L1 retrotransposition.Citation27
Figure 1. Shared 3′ end of paired autonomous and non-autonomous elements. Non-LTR elements and their non-autonomous partners (LINE/SINE pairs) can be subdivided into two subgroups based on their shared 3′ end sequence: (A) stringent and (B) relaxed, reviewed in.Citation50 Schematics of representative examples of the two subgroups are shown. The “stringent” LINE/SINE pair group (A) share the 3′ end sequence (green box). Several different LINE/SINE pairs from reptiles, fish, mammals and plants fall into this category. SINEs in this subgroup derived their 3′ end from the LINE sequence. A stem-loop structure in the 3′end is commonly observed in these LINE/SINE pairs (shown as a loop). The transcripts of the “relaxed” SINE/LINE pair group (B) share a 3′-poly(A) tail, which include the human LINE-1 element with its two open reading frames, ORF1 (purple) and ORF2 (orange). LINE-1 provides the proteins that mobilize several non-autonomous RNAs in trans: mRNAs generating processed pseudogenes and the composite retrotransposon SVA (SINE-VNTR-Alu) element which are transcribed by the RNA polymerase II (pol II); and the RNA polymerase III (pol III) transcribed SINEs that are ancestrally derived from pol III genes, such as 7SL and tRNAs. Some of these SINE transcripts form secondary structures (represented as looped lines inside the boxes) that are important for retrotransposition. One example is Alu (shown as a dimeric molecule where each non-identical side was individually derived from the 7SL gene, represented as blue boxes separated by an A-rich region represented by “AA”). “U”s at the end of the transcripts represent pol III termination and blue “N”s represent sequences preceding the pol III termination signal derived from the flanking DNA of the specific locus generating the transcript. However, the vast majority of SINEs are derived from tRNA genes (represented as a purple box). The SINEs with polyA tails can be further subdivided into two classes, class T- and class T+, depending on the presence/absence of a polyadenylation signal (pAS) and a TC-motif preceding the pol III terminator and polyA tail at the 3′ end.Citation29
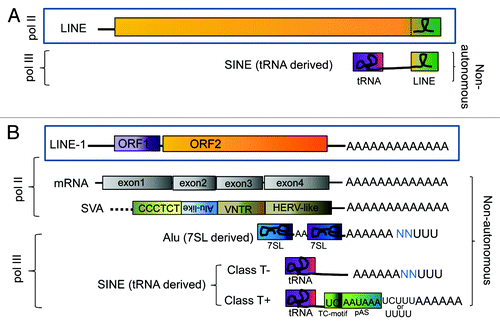
Transcripts from PolyA non-LTR retrotransposons generated by RNA polymerase II (pol II), such as LINE-1, SVA and the mRNA that gives rise to processed pseudogenes have the added advantage that the polyadenylation machinery will enzymatically add 100 to 200 adenosines to the 3′ end.Citation28 This process ensures that the transcripts of these elements will consistently have this important feature for retrotransposition. However, SINEs are transcribed by RNA polymerase III (pol III) and do not normally undergo polyadenylation as mRNAs do. Thus, most SINEs must encode an oligo(dA) sequence in their 3′ ends and are categorized as class T-. A separate group of SINEs referred to as class T+ contain a TC-motif, a polyadenylation signal (pAS) and a pol III terminator (usually 4 Ts or TCTTT) preceding the polyA tail at the 3′ end ().Citation29 Although transcribed by pol III, class T+ SINEs are proposed to be polyadenylated in an AAUAAA dependent manner. Data from studies on the rodent B2 supports this alternate route.Citation30 In this case, the pol III transcript ends at the pol III terminator downstream of the pAS (AATAAA). It is possible that the pol III transcript is recognized by the polyadenylation machinery as it may mimic a cleaved pol II mRNA. This could explain the location of the polyA-tail downstream of the pol III terminator. In contrast, the pol III termination of the class T- SINEs is localized downstream of the encoded polyA-tail. Although mechanisms vary, successful SINEs have a 3′ A-tail that serve as an important feature for SINEs to be able to parasitize polyA LINEs.
To date, all known SINEs are ancestrally derived from pol-III transcribed RNA genes (reviewed inCitation31,Citation32). The vast majority are derived from different tRNA genes and only two (Alu and the rodent B1) from the 7SL RNA gene, the ribonucleic acid component of the signal recognition particle (SRP) involved in protein secretion.Citation33 Alu elements are dimeric, composed of two non-identical 7SL derived monomers (). There are a few examples of SINEs derived from 5S.Citation34 Other SINEs, found in the genomes of the tree shrewCitation35 and the prosimian galago,Citation36 form composite elements (part 7SL, part tRNA). However, the 7SL RNA and the tRNAs do not have polyA-tails. Because the presence of an A-tail is critical for retrotransposition, it brings into question how did the first ancestral pol III transcript gain an A-tail? If the acquisition of an A-tail resulted from rare stochastic events, their emergence might be limited to a few isolated lineages. However, SINE families are widespread and most were derived independently, suggesting the possiblity of a non-random event leading to a “propensity” of tRNAs to acquire A-tails. The first hint of cellular processes being involved in the polyadenylation of pol III transcripts came from studies demonstrating that the 7SL RNA was 3′ processed by removal of the terminal Us (pol III terminator) followed by the addition of one adenosine.Citation37 Subsequent analyses revealed and this event was not the result of the canonical mRNA polyA polymerases (PAPs).Citation38
Most, if not all, pol III transcripts are essential for cell function and highly abundant. However, deregulation of pol III transcripts is detrimental, as overexpression of some pol III RNAs has been associated with cancer.Citation39 Thus, cells have well established systems for surveillance of pol III products in the nucleus (reviewed inCitation40,Citation41). Defective pol III transcripts are targeted for degradation through the 3′ poly(A) addition by the TRAMP complex (). The Trf4/Air2/Mtr4 polyadenylation complex (TRAMP) consists of a complex of three proteins that are highly conserved in eukaryotes: the RNA helicase (Mtr4p), a Zn-binding protein (Air2p or Air1p) and a non-canonical poly(A) polymerase (Trf4p or Trf5p in S. cerevisiae). In humans, the complex is composed of Rrf4p, Mtr4p and Air2p and referred to as TRAMP4. Evidence for nuclear surveillance exists for 5S rRNA,Citation42 U6 snRNA, 7SL and pre-tRNAs.Citation43 Data suggests that TRAMP preferentially polyadenylates defective copies, variants or pre-tRNAs.Citation44-Citation46 Once polyadenylated, the TRAMP complex interacts with the nuclear exosome for degradation of the pol III RNA from the 3′ end.Citation44,Citation45
Figure 2. SINE genesis model. RNA Pol III products such as tRNAs, U6, 5S rRNA and 7SL undergo nuclear surveillance. Defective or incorrectly formed pol III transcripts are targeted for destruction in the nucleus by the exosome (reviewed in ref. Citation40). One of the steps in the surveillance of the pol III RNAs occurs through the polyadenylation of the transcript by the TRAMP complex. The diagram shows TRAMP4 composed of Rrf4p, Mtr4p and Air2p in humans. It is proposed that the addition of the polyA tail at the 3′ end signals the exosome to degrade the RNA. In our model, we propose that a potential outcome is that a few of the defective polyadenylated tRNAs or other pol III RNAs escape degradation by the exosome. This will in turn allow the stochastic interaction with the LINE retrotransposition machinery (in primate lineage expect to find L1 ORF2 protein and L1 ORF1 proteins) already present in the nucleus. LINE proteins may vary in other lineages, such as plants. The retrotransposition of the polyadenylated pol III transcript into the DNA would generate the first ancestral element. Fortuitous integration in a transcriptionally supportive site could lead to the creation of an active element, i.e., the birth of a SINE. In this model, the expansion of the A-tail during insertion caused by the LINE ORF2 protein reverse transcription slippage would significantly favor the introduction of the critical A-tail length to generate a retrotranspositionally competent insert.
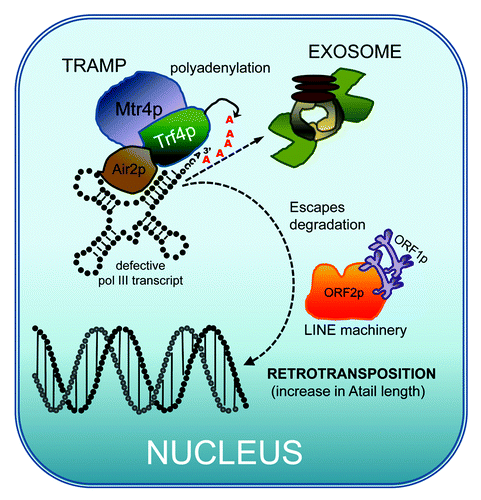
Although highly speculative, could a polyadenylated pol III transcript that escaped degradation by the exosome have become the ancestral precursor of a SINE? In other words, is the activity of TRF4 directly responsible for the genesis of SINEs? In this model (), the polyadenylated pol III transcript is already present in the nucleus. This could favor the possibility of an encounter with a LINE-1 RNP to undergo retrotransposition. However, it cannot be ruled out that the pol III transcript needs to interact with the LINE-1 RNP in the cytoplasm. The fact that most SINEs are not simple pseudogene copies of their ancestral pol III gene (tRNA or 7SL) may reflect that they were derived from defective transcripts that escaped degradation from the exosome. For example, did the Alu monomeric precursor FAM originate from a truncated or prematurely terminated 7SL transcript that was targeted for destruction? Unfortunately, this could be hard to assess as defective RNAs or ribonucleoprotein particles (RNPs) are presumably rare.
An important difference between the TRAMP complex and the canonical PAPs relates to the number of adenosines added to the transcript. Transcripts polyadenylated by TRAMP have shorter polyA-tails, peaking at around 4–5 adenosinesCitation47 and the number added depends on the modulation by the RNA helicase Mtr4.Citation48 Not only the presence but the length of the A-tail has an impact on retrotransposition, where going below a certain threshold may abolish activity of an element.Citation26,Citation49 Thus, having a short A-tail could be insufficient to generate an ancestral SINE copy that could become a source element, making this model less attractive. In other words, if the insert has no potential to generate new copies, creation of a SINE lineage would be inconceivable. However, our recent observation on Alu A-tail expansion in Wagstaff et al.Citation1 provides an insight that could alleviate this shortcoming. In our study, we adapted a tagged Alu system to easily recover de novo Alu inserts in cultured cells. By using non-A nucleotide disruptions within the encoded 3′ polyA-tail of the tagged Alu, we were able to show that newly inserted Alu elements have substantially longer A-tails than their transcripts. We proposed a model where slippage by the LINE-1 ORF2 protein during reverse transcription causes A-tail length to increase. Thus, in addition to our previously discussed impacts of A-tail expansion, there is an added effect of this feature of ORF2p where it may have played an important role in generating progenitor SINE source elements. Thus, ORF2p could have had a double role in SINE creation, by first reverse transcribing the progenitor RNA and second by providing the new insert the longer A-tail needed for retrotransposition competency and the ability to parasitize PolyA LINEs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Work in the Roy-Engel lab is supported by National Institutes of Health (NIH) P20GM103518/ P20RR020152 and R01GM079709A. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
References
- Wagstaff BJ, Hedges DJ, Derbes RS, Campos Sanchez R, Chiaromonte F, Makova KD, et al. Rescuing Alu: recovery of new inserts shows LINE-1 preserves Alu activity through A-tail expansion. PLoS Genet 2012; 8:e1002842; http://dx.doi.org/10.1371/journal.pgen.1002842; PMID: 22912586
- Han K, Xing J, Wang H, Hedges DJ, Garber RK, Cordaux R, et al. Under the genomic radar: the stealth model of Alu amplification. Genome Res 2005; 15:655 - 64; http://dx.doi.org/10.1101/gr.3492605; PMID: 15867427
- Walker JA, Konkel MK, Ullmer B, Monceaux CP, Ryder OA, Hubley R, et al. Orangutan Alu quiescence reveals possible source element: support for ancient backseat drivers. Mob DNA 2012; 3:8; http://dx.doi.org/10.1186/1759-8753-3-8; PMID: 22541534
- Kopera HC, Moldovan JB, Morrish TA, Garcia-Perez JL, Moran JV. Similarities between long interspersed element-1 (LINE-1) reverse transcriptase and telomerase. Proc Natl Acad Sci U S A 2011; 108:20345 - 50; http://dx.doi.org/10.1073/pnas.1100275108; PMID: 21940498
- Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet 2003; 35:41 - 8; http://dx.doi.org/10.1038/ng1223; PMID: 12897783
- Naito K, Cho E, Yang G, Campbell MA, Yano K, Okumoto Y, et al. Dramatic amplification of a rice transposable element during recent domestication. Proc Natl Acad Sci U S A 2006; 103:17620 - 5; http://dx.doi.org/10.1073/pnas.0605421103; PMID: 17101970
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al, International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 2001; 409:860 - 921; http://dx.doi.org/10.1038/35057062; PMID: 11237011
- Graham T, Boissinot S. The genomic distribution of L1 elements: the role of insertion bias and natural selection. J Biomed Biotechnol 2006; 2006:75327; http://dx.doi.org/10.1155/JBB/2006/75327; PMID: 16877820
- Xing J, Zhang Y, Han K, Salem AH, Sen SK, Huff CD, et al. Mobile elements create structural variation: analysis of a complete human genome. Genome Res 2009; 19:1516 - 26; http://dx.doi.org/10.1101/gr.091827.109; PMID: 19439515
- Iskow RC, McCabe MT, Mills RE, Torene S, Pittard WS, Neuwald AF, et al. Natural mutagenesis of human genomes by endogenous retrotransposons. Cell 2010; 141:1253 - 61; http://dx.doi.org/10.1016/j.cell.2010.05.020; PMID: 20603005
- Stewart C, Kural D, Strömberg MP, Walker JA, Konkel MK, Stütz AM, et al, 1000 Genomes Project. A comprehensive map of mobile element insertion polymorphisms in humans. PLoS Genet 2011; 7:e1002236; http://dx.doi.org/10.1371/journal.pgen.1002236; PMID: 21876680
- Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, et al. Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol 2001; 21:1429 - 39; http://dx.doi.org/10.1128/MCB.21.4.1429-1439.2001; PMID: 11158327
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, et al, Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature 2002; 420:520 - 62; http://dx.doi.org/10.1038/nature01262; PMID: 12466850
- Gonçalves I, Duret L, Mouchiroud D. Nature and structure of human genes that generate retropseudogenes. Genome Res 2000; 10:672 - 8; http://dx.doi.org/10.1101/gr.10.5.672; PMID: 10810090
- Ohshima K, Hattori M, Yada T, Gojobori T, Sakaki Y, Okada N. Whole-genome screening indicates a possible burst of formation of processed pseudogenes and Alu repeats by particular L1 subfamilies in ancestral primates. Genome Biol 2003; 4:R74; http://dx.doi.org/10.1186/gb-2003-4-11-r74; PMID: 14611660
- Esnault C, Maestre J, Heidmann T. Human LINE retrotransposons generate processed pseudogenes. Nat Genet 2000; 24:363 - 7; http://dx.doi.org/10.1038/74184; PMID: 10742098
- Wang H, Xing J, Grover D, Hedges DJ, Han K, Walker JA, et al. SVA elements: a hominid-specific retroposon family. J Mol Biol 2005; 354:994 - 1007; http://dx.doi.org/10.1016/j.jmb.2005.09.085; PMID: 16288912
- Hancks DC, Goodier JL, Mandal PK, Cheung LE, Kazazian HH Jr.. Retrotransposition of marked SVA elements by human L1s in cultured cells. Hum Mol Genet 2011; 20:3386 - 400; http://dx.doi.org/10.1093/hmg/ddr245; PMID: 21636526
- Raiz J, Damert A, Chira S, Held U, Klawitter S, Hamdorf M, et al. The non-autonomous retrotransposon SVA is trans-mobilized by the human LINE-1 protein machinery. Nucleic Acids Res 2011; PMID: 22053090
- Ohshima K, Hamada M, Terai Y, Okada N. The 3′ ends of tRNA-derived short interspersed repetitive elements are derived from the 3′ ends of long interspersed repetitive elements. Mol Cell Biol 1996; 16:3756 - 64; PMID: 8668192
- Kajikawa M, Okada N. LINEs mobilize SINEs in the eel through a shared 3′ sequence. Cell 2002; 111:433 - 44; http://dx.doi.org/10.1016/S0092-8674(02)01041-3; PMID: 12419252
- Terai Y, Takahashi K, Okada N. SINE cousins: the 3′-end tails of the two oldest and distantly related families of SINEs are descended from the 3′ ends of LINEs with the same genealogical origin. Mol Biol Evol 1998; 15:1460 - 71; http://dx.doi.org/10.1093/oxfordjournals.molbev.a025873; PMID: 12572609
- Luan DD, Korman MH, Jakubczak JL, Eickbush TH. Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell 1993; 72:595 - 605; http://dx.doi.org/10.1016/0092-8674(93)90078-5; PMID: 7679954
- Luan DD, Eickbush TH. RNA template requirements for target DNA-primed reverse transcription by the R2 retrotransposable element. Mol Cell Biol 1995; 15:3882 - 91; PMID: 7540721
- Boeke JD. LINEs and Alus--the polyA connection. Nat Genet 1997; 16:6 - 7; http://dx.doi.org/10.1038/ng0597-6; PMID: 9140383
- Dewannieux M, Heidmann T. Role of poly(A) tail length in Alu retrotransposition. Genomics 2005; 86:378 - 81; http://dx.doi.org/10.1016/j.ygeno.2005.05.009; PMID: 15993034
- Moran JV, Holmes SE, Naas TP, DeBerardinis RJ, Boeke JD, Kazazian HH Jr.. High frequency retrotransposition in cultured mammalian cells. Cell 1996; 87:917 - 27; http://dx.doi.org/10.1016/S0092-8674(00)81998-4; PMID: 8945518
- Keller W. No end yet to messenger RNA 3′ processing!. Cell 1995; 81:829 - 32; http://dx.doi.org/10.1016/0092-8674(95)90001-2; PMID: 7781059
- Borodulina OR, Kramerov DA. Short interspersed elements (SINEs) from insectivores. Two classes of mammalian SINEs distinguished by A-rich tail structure. Mamm Genome 2001; 12:779 - 86; http://dx.doi.org/10.1007/s003350020029; PMID: 11668393
- Borodulina OR, Kramerov DA. Transcripts synthesized by RNA polymerase III can be polyadenylated in an AAUAAA-dependent manner. RNA 2008; 14:1865 - 73; http://dx.doi.org/10.1261/rna.1006608; PMID: 18658125
- Kramerov DA, Vassetzky NS. Short retroposons in eukaryotic genomes. Int Rev Cytol 2005; 247:165 - 221; http://dx.doi.org/10.1016/S0074-7696(05)47004-7; PMID: 16344113
- Chen JM, Stenson PD, Cooper DN, Férec C. A systematic analysis of LINE-1 endonuclease-dependent retrotranspositional events causing human genetic disease. Hum Genet 2005; 117:411 - 27; http://dx.doi.org/10.1007/s00439-005-1321-0; PMID: 15983781
- Walter P, Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 1982; 299:691 - 8; http://dx.doi.org/10.1038/299691a0; PMID: 6181418
- Gogolevsky KP, Vassetzky NS, Kramerov DA. 5S rRNA-derived and tRNA-derived SINEs in fruit bats. Genomics 2009; 93:494 - 500; http://dx.doi.org/10.1016/j.ygeno.2009.02.001; PMID: 19442632
- Nishihara H, Terai Y, Okada N. Characterization of novel Alu- and tRNA-related SINEs from the tree shrew and evolutionary implications of their origins. Mol Biol Evol 2002; 19:1964 - 72; http://dx.doi.org/10.1093/oxfordjournals.molbev.a004020; PMID: 12411605
- Daniels GR, Deininger PL. Repeat sequence families derived from mammalian tRNA genes. Nature 1985; 317:819 - 22; http://dx.doi.org/10.1038/317819a0; PMID: 3851163
- Chen Y, Sinha K, Perumal K, Gu J, Reddy R. Accurate 3′ end processing and adenylation of human signal recognition particle RNA and alu RNA in vitro. J Biol Chem 1998; 273:35023 - 31; http://dx.doi.org/10.1074/jbc.273.52.35023; PMID: 9857035
- Sinha K, Perumal K, Chen Y, Reddy R. Post-transcriptional adenylation of signal recognition particle RNA is carried out by an enzyme different from mRNA Poly(A) polymerase. J Biol Chem 1999; 274:30826 - 31; http://dx.doi.org/10.1074/jbc.274.43.30826; PMID: 10521474
- White RJ. RNA polymerases I and III, non-coding RNAs and cancer. Trends Genet 2008; 24:622 - 9; http://dx.doi.org/10.1016/j.tig.2008.10.003; PMID: 18980784
- Anderson JT, Wang X. Nuclear RNA surveillance: no sign of substrates tailing off. Crit Rev Biochem Mol Biol 2009; 44:16 - 24; http://dx.doi.org/10.1080/10409230802640218; PMID: 19280429
- Houseley J, Tollervey D. The many pathways of RNA degradation. Cell 2009; 136:763 - 76; http://dx.doi.org/10.1016/j.cell.2009.01.019; PMID: 19239894
- Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA 2006; 12:508 - 21; http://dx.doi.org/10.1261/rna.2305406; PMID: 16431988
- Copela LA, Fernandez CF, Sherrer RL, Wolin SL. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA 2008; 14:1214 - 27; http://dx.doi.org/10.1261/rna.1050408; PMID: 18456844
- LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, et al. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 2005; 121:713 - 24; http://dx.doi.org/10.1016/j.cell.2005.04.029; PMID: 15935758
- Vanácová S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, et al. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 2005; 3:e189; http://dx.doi.org/10.1371/journal.pbio.0030189; PMID: 15828860
- Schneider C, Anderson JT, Tollervey D. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol Cell 2007; 27:324 - 31; http://dx.doi.org/10.1016/j.molcel.2007.06.006; PMID: 17643380
- Wlotzka W, Kudla G, Granneman S, Tollervey D. The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J 2011; 30:1790 - 803; http://dx.doi.org/10.1038/emboj.2011.97; PMID: 21460797
- Jia H, Wang X, Liu F, Guenther UP, Srinivasan S, Anderson JT, et al. The RNA helicase Mtr4p modulates polyadenylation in the TRAMP complex. Cell 2011; 145:890 - 901; http://dx.doi.org/10.1016/j.cell.2011.05.010; PMID: 21663793
- Roy-Engel AM, Salem AH, Oyeniran OO, Deininger L, Hedges DJ, Kilroy GE, et al. Active Alu element “A-tails”: size does matter. Genome Res 2002; 12:1333 - 44; http://dx.doi.org/10.1101/gr.384802; PMID: 12213770
- Okada N, Hamada M, Ogiwara I, Ohshima K. SINEs and LINEs share common 3′ sequences: a review. Gene 1997; 205:229 - 43; http://dx.doi.org/10.1016/S0378-1119(97)00409-5; PMID: 9461397