Abstract
Long interspersed nuclear elements -1 (LINEs, L1s) are retroelements occupying almost 17% of the human genome. L1 retrotransposition can cause deleterious effects on the host-cell and it is generally inhibited by suppressive mechanisms, but it can occur in some specific cells during early development as well as in some tumor cells and in the presence of several environmental factors. In a recent publication we reported that extremely low frequency pulsed magnetic field can affect L1 retrotransposition in neuroblastoma cells. In this commentary we discuss the interaction between environment and L1 activity in the light of the new emerging paradigm of host-LINE relationship.
Transposable elements (TEs) are DNA sequences able to transport themselves from one location in the genome to another using a copy/cut-and-paste mechanism. This action can result in a replicative or nonreplicative transposition. They have been defined as selfish, tricky, parasitic, junk DNA, somehow suggesting that they are “entities” which slip into host genome pillaging various substances (polymerases, repair system etc.) from the host cell with the only purpose of their own propagation and with no regard for the consequences, e.g., possible malfunctions of host genome. In this context, the relationship TEs-host has often been imagined as a battle between aggressive selfish elements in attack and host cell on the defense, and it has been suggested that after millions of years of battle, which contributed greatly to genome evolution,Citation1 the conflict is now in “cold war.” However the fact that TEs make up a significant proportion of the genomes of nearly all living organisms, and persist in populations of both asexual and sexual species is an enigmatic problem in evolutionary genetics. Indeed TEs have also been described as “the genome’s dark matter” indicating that an air of mystery surrounds their role. However, a great deal of emerging evidence indicates that TEs and host cells may have a mutually advantageous relationship.Citation2
It is well known that TE-host relationship can be disturbed by environmental conditions. Barbara McClintock was the first to propose the “genomic shock” hypothesis, stating that ”the genome response” to environmental stimuli can induce TE mobility, so that “a genome may modify itself when confronted with unfamiliar conditions.”Citation3 According to this hypothesis, by inducing TE activity, cells increase their genotypic variation, which can contribute to organism adaptation in the presence of environmental disturbance. Therefore, TEs can be a positive resource when needed. This hypothesis is supported by evidence that TE mobility from various organisms, including yeast, drosophila, plants, mammals, can be activated by various environmental stresses.Citation4,Citation5 Therefore TEs, host and environment are involved in a three-player game.
The relationship between TE mobility and disease,Citation6 including aging,Citation7 tumorigenesis and tumor progression,Citation8 is currently an emerging topic of study. As a result, interest has grown in recent years about the interaction between environmental agents and human mobile genetic elements. TEs represent more than 44% of the human genome, although only a minor number of TEs are active. Among them long interspersed nuclear elements (LINEs) are the most abundant and active family.
LINE-1s (L1s) are the most significant retroelements of the LINE class, they constitute almost 17% of human genomic DNA. Most of them are truncated elements unable to move, but approximately 100 full-length elements are retrotransposition competent (RC-L1s). Functional L1s are 6kb in length, which includes two promoters (with sense and antisense activity) in the 5′-untranslated (UTR) region, two open reading frames encoding proteins necessary for the retrotransposition (ORF1encoding a nucleic acid chaperone; ORF2 encoding an endonuclease and reverse transcriptase) and a 3′-UTR with a poly(A)tail (reviewed in refs. Citation6 and Citation9). L1 retrotransposition (L1-RTP) consists of several steps, the first occurring in the nucleous (transcription of L1 element), others occurring in the cytoplasm (translation of the two ORFs producing ORF1p and ORF2p, association of L1 transcript with ORF proteins in ribonucleic acid particles, or RNPs), the last is the return to the nucleus and reintegration into a new location of the host genome by target-site-primed reverse-transcription (TPRT). In this TPRT molecular mechanism, not yet well known, a single-stranded nick in an AT-rich target site within genomic DNA is introduced liberating a 3′-OH that can be used to prime reverse transcription of the RNA, then a DNA double strand break (DSB) occurs. Most of the L1-RTP processes produce 5′-truncated immobile elements, due to an incomplete process.Citation10 L1 activity can induce several deleterious changes in the genome, promoting insertions, deletions, transductions, exonisation, rearrangements, new splicing sites, and highly affecting neighboring gene expression (reviewed in refs Citation6, Citation9, Citation11).
Host suppressive mechanisms can affect each step of the retrotransposition process in several ways: L1 promoter DNA methylation and repressive histone modifications hinder L1 transcription, small RNA-based mechanisms, APOBEC protein activity and stress granules seem to hinder L1- RNP activity, host DNA repair system can interfere with TPRT (reviewed in refs Citation6, Citation12). Defense host strategies seem to be winning in healthy differentiated somatic cells, where in fact retrotransposition events generally do not occur. This cannot be said for other phases, i.e., during early development,Citation13,Citation14 including gametogenesis and neurogenesis,Citation15,Citation16 where retrotransposition have been shown to happen. So, an unanswered question is why the host cell becomes permissive to L1 activity in these critical stages of organism development. As for neurogenesis, L1 elements have been found to insert themselves into protein-coding genes differentially expressed in neurons, inducing a change of expression.Citation17 One hypothesis says that L1-RTP is involved in neural plasticity.Citation18
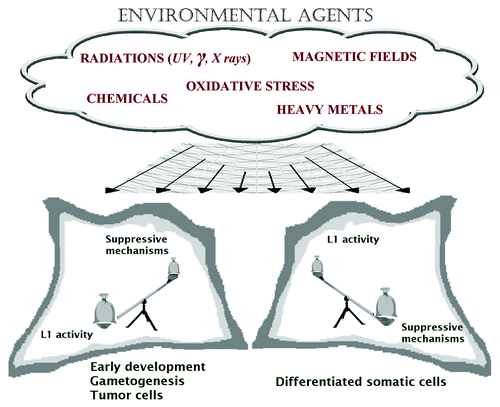
Several environmental agents have been observed to interfere with host-L1 balance (), indeed L1 mobility was increased by ionizing irradiation,Citation19 heavy metals,Citation20 benz[a]pyrene (B[a]P),Citation21 6-formylindolo[[3,2-b]carbazole (FICZ),Citation22 oxidative stress,Citation23 heterocyclic amines (HCAs),Citation24 voluntary exercise.Citation25 The molecular mechanisms behind these observations are still unclear, however they seem to differ depending on the inducing factor. L1-RTP can be triggered at the transcriptional and/or post transcriptional level, for example, X-rays and B[a]P increased L1 mRNA expression,Citation19,Citation21 whereas nickel chloride and FICZ did not.Citation20,Citation22 Different cellular factors can be involved, for example, L1-RTP induced by ionizing irradiation was affected by DNA repair pathways,Citation19 while L1-RTP induced by B[a]P and by FICZ depended, respectively, on aryl hydrocarbon receptor (AhR) and AhR nuclear translocator-1(ARNT).Citation21,Citation22 In these latter cases, the molecular mechanism involved seems to be the chromatin recruitment of ORF1 via transcription factors such as basic-Helix-Loop-Helix/Per-Arnt-Sim (BHLH/PAS) proteins (reviewed in ref. Citation26). It has been therefore suggested that bHLH/PAS proteins, inducing both cellular response to various compounds and L1-RTP, are the molecular basis of the link between L1 induction and environmental adaptation.Citation26
Figure 1. L1 activity is generally suppressed in normal differentiated somatic cells, while it sometimes occurs in some specific cells during early development, as well as in some tumor cells. Environmental agents can interfere with L1 activity control and the result could depend on cell condition and history.
A recent our study explores a particular environmental factor: the exposure to extremely low frequency magnetic fields (ELF-MF).Citation27 ELF-MF is an ubiquitous environmental stimulus in the western world and seems to cause a number of biological effects (reviewed in ref. Citation28). At present this topic is widely discussed for two main reasons: growing concern about potential hazards and second, arising possible biomedical applications. ELF-MF has been observed to alter TE activity in bacterial cells.Citation29,Citation30 Moreover, in recent years many papers showed ELF-MF effects on nerve cells.Citation31-Citation33 For its ubiquity, its ability to interfere with bacterial TE, and its effects on nervous cells, ELF-MF seemed to be as a good candidate to evaluate L1 retransposition under altered environmental conditions.
We selected as a model a neuroblastoma cell line which represents embryonic precursors of sympathetic neurons,Citation34 and which has been shown to support L1 retrotrasposition.Citation35 We used the in vitro retrotransposition assay developed by Kazazian laboratory.Citation36 Most of the advances in the knowledge of L1 biology have been achieved thanks to this useful tool. It consists in a EGFP-marked L1 vector, carrying an EGFP gene inserted in the opposite direction of the L1 transcript and interrupted by an intron that is inserted in the same transcriptional direction as the L1 transcript. So, EGFP gene can be expressed only as a consequence of a retrotransposition event, and the number of L1-RTP events can be simply evaluated by counting EGFP positive cells by a flow cytometry analysis. However this analysis detects a retrotransposition event only when the expression level of the EGFP marker gene exceeds the established threshold level of fluorescence, hence retrotransposition events occurring in not very permissive genomic locations cannot be observed. A number of studies have reported that ELF-MF exposure can affect gene transcription, although molecular mechanisms on the basis of this effect are not yet clear. Therefore currently it is not possible to exclude that gene silencing and/or genomic site accessibility can be modified under ELF-MF exposure. For this reason, we decided to not use flow cytometry analysis and evaluated the number of L1-RTP events by quantifying the inserted EGFP-L1 by quantitative Real-Time PCR analysis on genomic DNA.Citation35 Unexpectedly, a slight but significant reduction of retrotransposition events was found in the cells exposed to 50 Hz ELF pulsed MF.
This is the first report on this topic, so up to date it is not known whether the observed ELF-MF effect on L1-RTP is cell type specific or can also be observed in other cell types. It would be interesting to test pluripotent stem cells where the L1-RTP frequency is higher than other somatic cell.Citation37
Further studies are needed to elucidate the molecular mechanisms which are on the basis of the observed phenomenon. Several evidences indicate that ELF-MF can trigger the signaling pathway MAPK (Mitogen-Activated Protein Kinase) and the DNA repair system.Citation38 Moreover it can alter gene expression,Citation31,Citation33 and cellular redox status in nerve cells.Citation39 Which of these parameters could be involved in the observed reduction of L1-RTP still needs to be verified. It is well known that L1 activity depends on host repair system, since some molecules, such as ATM (ataxia telangiectasia mutated) are required for L1 integration,Citation40 whereas others reduce L1 insertion, such as the human flap endonuclease ERCC1 (excision repair cross complementing 1).Citation41 So a differential expression/activity of these molecules could be involved in the ELF-MF effect on L1-RTP.
The observed reduction of L1-RTP activity makes ELF-MF exposure different from the other agents studied, as reported above. Indeed, all these agents have been found to cause an increase of L1-RTP events. Unlike many chemical and physical agents, electromagnetic fields are not lethal for cells, at least no dose or exposure time have yet been observed to cause cell death. Most observations indicate that cells tolerate electromagnetic exposure, whose effect mainly consists in a transient modulation of biological functions which can restore the disturbed balance.Citation28
The “genomic shock hypothesis” considers that TE activity is useful for host cells in the presence of changed environmental conditions, because it increases genetic variability. Barbara McClintock’s intuition has been confirmed by many findings that provided some of the molecular mechanisms by which a biological stress response can cause TE activation (e.g., bHLH/PAS proteins). However, emerging evidence indicates that L1-RTP can be useful, if not even essential, during the neurogenesis and the early stages of the development,Citation13 regardless of changes in environmental conditions.
How L1 insertions can contribute to cellular function is not yet clear, however they are involved in the genome regulation, can induce epigenetic modification and also affect post-transcriptional control of genes.Citation2,Citation11,Citation42 Moreover, recent results of the ENCODE (Encyclopedia of DNA Elements) project, highlight the centrality of the transcript in genomic organization will surely shed new light on the role of retroelements in genome functionality.
In the emerging picture, the L1-host relationship looks less like an entirely antagonistic relationship, and more like a “gentlemen’s agreement” consisting in a specific spatio-temporal control of L1 activity. This behavior recalls that of other highly mutagen endogenous mechanisms, recruited during evolution, which occur in the immune cells in specific spatio-temporal windows such as V(D)J recombination, class switch recombination (CSR) and somatic hypermutation (SHM). Similarly, L1 activity seems to be subjected to specific control (specific cells, at certain developmental stages and specific time) during neurogenesis promoting differential gene expression. Curiously, immune and nerve cells are both involved in regulating the organism-environment interface, so perhaps they need a source of genome instability to draw from.
Therefore, in addition to the “genomic shock” effect, regarding the stress-induced TE activation in cells where TE is generally suppressed, another effect should be considered that is what an environmental stress can trigger in cells where TE activity is useful and allowed - although this usefulness remains so far elusive. ()
One might wonder whether the reduction of L1-RTP we saw in neuroblastoma cells under ELF-MF, is an advantage or a disadvantage for the organism. Currently there is no clear answer to this question, because we do not have enough knowledge of the role of L1-RTP in neural cells and tumor cells. Indeed, L1 activity could have both negative and positive effects, contributing to tumor progression on one side and playing a central role in mammal neuronal diversification on the other. In the first case new therapeutic applications of ELF-MF could be hypothesized, while in the second case concerns about ELF-MF exposure during neurogenesis process should be considered.
Barbara McClintock during her Nobel lecture said that “A goal for the future would be to determine the extent of knowledge the cell has of itself, and how it utilizes this knowledge in a “thoughtful” manner when challenged.” Perhaps LINE elements themselves are one of those means by which a cell knows itself and the LINE “round trip,” which starts and returns to the cell nucleus, could be seen as a kind of scouting journey in which the LINE nucleic acid gains experience from visiting cell cytoplasm, a very special place where at every moment in time numerous external signals are received and numerous homeostatic adjustments are performed.
Finally, considering that LINE elements are gaining importance in genome biology, that de novo retrotransposition events are involved in more than 70 diseases,Citation6 and in tumor onset and progression,Citation8 further investigation should be performed on the influence of environmental agents on L1 acitivity. Moreover it is reasonable to suggest that L1 activity evaluation be added to routine mutagenicity testing.
| Abbreviations: | ||
| TE | = | Transposable element |
| LINE-1 or L1 | = | long interspersed nuclear elements class1 |
| RC-L1 | = | retrotransposition competent |
| UTR | = | untranslated region |
| L1-RTP | = | L1 retrotransposition |
| ORF | = | open reading frame |
| TPRT | = | target-site-primed reverse-transcription |
| ELF-MF | = | extremely low frequency magnetic field |
| EGFP | = | enhanced green fluorescent protein |
Disclosure of Potential Conflicts of Interest
The authors report no conflicts of interest.
Acknowledgments
This work was supported by “Electromagnetic field effects on neuronal cells” PRIN-2007 program, from Italian MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca).
References
- Huang CR, Burns KH, Boeke JD. Active transposition in genomes. Annu Rev Genet 2012; 46:651 - 75; http://dx.doi.org/10.1146/annurev-genet-110711-155616; PMID: 23145912
- Faulkner GJ, Carninci P. Altruistic functions for selfish DNA. Cell Cycle 2009; 8:2895 - 900; http://dx.doi.org/10.4161/cc.8.18.9536; PMID: 19736519
- McClintock B. The significance of responses of the genome to challenge. Science 1984; 226:792 - 801; http://dx.doi.org/10.1126/science.15739260; PMID: 15739260
- Morales JF, Snow ET, Murnane JP. Environmental factors affecting transcription of the human L1 retrotransposon. II. Stressors. Mutagenesis 2003; 18:151 - 8; http://dx.doi.org/10.1093/mutage/18.2.151; PMID: 12621071
- Feng G, Leem YE, Levin HL. Transposon integration enhances expression of stress response genes. Nucleic Acids Res 2012; 27:1 - 15; PMID: 23193295
- Beck CR, Garcia-Perez JL, Badge RM, Moran JV. LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet 2011; 12:187 - 215; http://dx.doi.org/10.1146/annurev-genom-082509-141802; PMID: 21801021
- St Laurent G 3rd, Hammell N, McCaffrey TAA. A LINE-1 component to human aging: do LINE elements exact a longevity cost for evolutionary advantage?. Mech Ageing Dev 2010; 131:299 - 305; http://dx.doi.org/10.1016/j.mad.2010.03.008; PMID: 20346965
- Lee E, Iskow R, Yang L, Gokcumen O, Haseley P, Luquette LJ 3rd, et al, Cancer Genome Atlas Research Network. Landscape of somatic retrotransposition in human cancers. Science 2012; 337:967 - 71; http://dx.doi.org/10.1126/science.1222077; PMID: 22745252
- Goodier JL, Kazazian HH Jr.. Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 2008; 135:23 - 35; http://dx.doi.org/10.1016/j.cell.2008.09.022; PMID: 18854152
- Ostertag EM, Kazazian HH Jr.. Twin priming: a proposed mechanism for the creation of inversions in L1 retrotransposition. Genome Res 2001; 11:2059 - 65; http://dx.doi.org/10.1101/gr.205701; PMID: 11731496
- Kines KJ, Belancio VP. Expressing genes do not forget their LINEs: transposable elements and gene expression. Front Biosci 2012; 17:1329 - 44; http://dx.doi.org/10.2741/3990; PMID: 22201807
- Levin HL, Moran JV. Dynamic interactions between transposable elements and their hosts. Nat Rev Genet 2011; 12:615 - 27; http://dx.doi.org/10.1038/nrg3030; PMID: 21850042
- Vitullo P, Sciamanna I, Baiocchi M, Sinibaldi-Vallebona P, Spadafora C. LINE-1 retrotransposon copies are amplified during murine early embryo development. Mol Reprod Dev 2012; 79:118 - 27; http://dx.doi.org/10.1002/mrd.22003; PMID: 22139884
- Garcia-Perez JL, Marchetto MC, Muotri AR, Coufal NG, Gage FH, O’Shea KS, et al. LINE-1 retrotransposition in human embryonic stem cells. Hum Mol Genet 2007; 16:1569 - 77; http://dx.doi.org/10.1093/hmg/ddm105; PMID: 17468180
- Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature 2005; 435:903 - 10; http://dx.doi.org/10.1038/nature03663; PMID: 15959507
- Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, et al. L1 retrotransposition in human neural progenitor cells. Nature 2009; 460:1127 - 31; http://dx.doi.org/10.1038/nature08248; PMID: 19657334
- Baillie JK, Barnett MW, Upton KR, Gerhardt DJ, Richmond TA, De Sapio F, et al. Somatic retrotransposition alters the genetic landscape of the human brain. Nature 2011; 479:534 - 7; http://dx.doi.org/10.1038/nature10531; PMID: 22037309
- Singer T, McConnell MJ, Marchetto MC, Coufal NG, Gage FH. LINE-1 retrotransposons: mediators of somatic variation in neuronal genomes?. Trends Neurosci 2010; 33:345 - 54; http://dx.doi.org/10.1016/j.tins.2010.04.001; PMID: 20471112
- Tanaka A, Nakatani Y, Hamada N, Jinno-Oue A, Shimizu N, Wada S, et al. Ionising irradiation alters the dynamics of human long interspersed nuclear elements 1 (LINE1) retrotransposon. Mutagenesis 2012; 27:599 - 607; http://dx.doi.org/10.1093/mutage/ges025; PMID: 22547343
- Kale SP, Moore L, Deininger PL, Roy-Engel AM. Heavy metals stimulate human LINE-1 retrotransposition. Int J Environ Res Public Health 2005; 2:14 - 23; http://dx.doi.org/10.3390/ijerph2005010014; PMID: 16705797
- Stribinskis V, Ramos KS. Activation of human long interspersed nuclear element 1 retrotransposition by benzo(a)pyrene, an ubiquitous environmental carcinogen. Cancer Res 2006; 66:2616 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-05-3478; PMID: 16510580
- Okudaira N, Iijima K, Koyama T, Minemoto Y, Kano S, Mimori A, et al. Induction of long interspersed nucleotide element-1 (L1) retrotransposition by 6-formylindolo[3,2-b]carbazole (FICZ), a tryptophan photoproduct. Proc Natl Acad Sci U S A 2010; 107:18487 - 92; http://dx.doi.org/10.1073/pnas.1001252107; PMID: 20852066
- Giorgi G, Marcantonio P, Del Re B. LINE-1 retrotransposition in human neuroblastoma cells is affected by oxidative stress. Cell Tissue Res 2011; 346:383 - 91; http://dx.doi.org/10.1007/s00441-011-1289-0; PMID: 22160459
- Okudaira N, Okamura T, Tamura M, Iijma K, Goto M, Matsunaga A, et al. Long interspersed element-1 is differentially regulated by food-borne carcinogens via the aryl hydrocarbon receptor. Oncogene 2012; •••:1 - 12; PMID: 23208499
- Muotri AR, Zhao C, Marchetto MC, Gage FH. Environmental influence on L1 retrotransposons in the adult hippocampus. Hippocampus 2009; 19:1002 - 7; http://dx.doi.org/10.1002/hipo.20564; PMID: 19771587
- Ishizaka Y, Okudaira N, Tamura M, Iijima K, Shimura M, Goto M, et al. Modes of retrotransposition of long interspersed element-1 by environmental factors. Front Microbiol 2012; 3:191; http://dx.doi.org/10.3389/fmicb.2012.00191; PMID: 22666219
- Del Re B, Marcantonio P, Gavoçi E, Bersani F, Giorgi G. Assessing LINE-1 retrotransposition activity in neuroblastoma cells exposed to extremely low-frequency pulsed magnetic fields. Mutat Res 2012; 749:76 - 81; http://dx.doi.org/10.1016/j.mrgentox.2012.07.004; PMID: 22981769
- Santini MT, Rainaldi G, Indovina PL. Cellular effects of extremely low frequency (ELF) electromagnetic fields. Int J Radiat Biol 2009; 85:294 - 313; http://dx.doi.org/10.1080/09553000902781097; PMID: 19399675
- Potenza L, Ubaldi L, De Sanctis R, De Bellis R, Cucchiarini L, Dachà M. Effects of a static magnetic field on cell growth and gene expression in Escherichia coli. Mutat Res 2004; 561:53 - 62; http://dx.doi.org/10.1016/j.mrgentox.2004.03.009; PMID: 15238230
- Giorgi G, Marcantonio P, Bersani F, Gavoçi E, Del Re B. Effect of extremely low frequency magnetic field exposure on DNA transposition in relation to frequency, wave shape and exposure time. Int J Radiat Biol 2011; 87:601 - 8; http://dx.doi.org/10.3109/09553002.2011.570855; PMID: 21504343
- Nikolova T, Czyz J, Rolletschek A, Blyszczuk P, Fuchs J, Jovtchev G, et al. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J 2005; 19:1686 - 8; PMID: 16116041
- Capone F, Dileone M, Profice P, Pilato F, Musumeci G, Minicuci G, et al. Does exposure to extremely low frequency magnetic fields produce functional changes in human brain?. J Neural Transm 2009; 116:257 - 65; http://dx.doi.org/10.1007/s00702-009-0184-2; PMID: 19189041
- Marcantonio P, Del Re B, Franceschini A, Capri M, Lukas S, Bersani F, et al. Synergic effect of retinoic acid and extremely low frequency magnetic field exposure on human neuroblastoma cell line BE(2)C. Bioelectromagnetics 2010; 31:425 - 33; PMID: 20564173
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 1978; 38:3751 - 7; PMID: 29704
- Del Re B, Marcantonio P, Capri M, Giorgi G. Evaluation of LINE-1 mobility in neuroblastoma cells by in vitro retrotransposition reporter assay: FACS analysis can detect only the tip of the iceberg of the inserted L1 elements. Exp Cell Res 2010; 316:3358 - 67; http://dx.doi.org/10.1016/j.yexcr.2010.06.024; PMID: 20620136
- Ostertag EM, Prak ETL, DeBerardinis RJ, Moran JV, Kazazian HH Jr.. Determination of L1 retrotransposition kinetics in cultured cells. Nucleic Acids Res 2000; 28:1418 - 23; http://dx.doi.org/10.1093/nar/28.6.1418; PMID: 10684937
- Wissing S, Muñoz-Lopez M, Macia A, Yang Z, Montano M, Collins W, et al. Reprogramming somatic cells into iPS cells activates LINE-1 retroelement mobility. Hum Mol Genet 2012; 21:208 - 18; http://dx.doi.org/10.1093/hmg/ddr455; PMID: 21989055
- Luukkonen J, Liimatainen A, Höytö A, Juutilainen J, Naarala J. Pre-exposure to 50 Hz magnetic fields modifies menadione-induced genotoxic effects in human SH-SY5Y neuroblastoma cells. PLoS One 2011; 6:e18021; http://dx.doi.org/10.1371/journal.pone.0018021; PMID: 21448285
- Sulpizio M, Falone S, Amicarelli F, Marchisio M, Di Giuseppe F, Eleuterio E, et al. Molecular basis underlying the biological effects elicited by extremely low-frequency magnetic field (ELF-MF) on neuroblastoma cells. J Cell Biochem 2011; 112:3797 - 806; http://dx.doi.org/10.1002/jcb.23310; PMID: 21826706
- Gasior SL, Wakeman TP, Xu B, Deininger PL. The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol 2006; 357:1383 - 93; http://dx.doi.org/10.1016/j.jmb.2006.01.089; PMID: 16490214
- Gasior SL, Roy-Engel AM, Deininger PL. ERCC1/XPF limits L1 retrotransposition. DNA Repair (Amst) 2008; 7:983 - 9; http://dx.doi.org/10.1016/j.dnarep.2008.02.006; PMID: 18396111
- Lee KJ, Conley AB, Lunyak VV, Jordan IK. Do human transposable element small RNAs serve primarily as genome defenders or genome regulators?. Mob Genet Elements 2012; 2:19 - 25; http://dx.doi.org/10.4161/mge.19031; PMID: 22754749